FY 2013 President's Budget Request
DEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
National Institute of Diabetes and Digestive and Kidney Diseases
FY 2013 Budget
Print Version (PDF 465 KB, 28 pages)
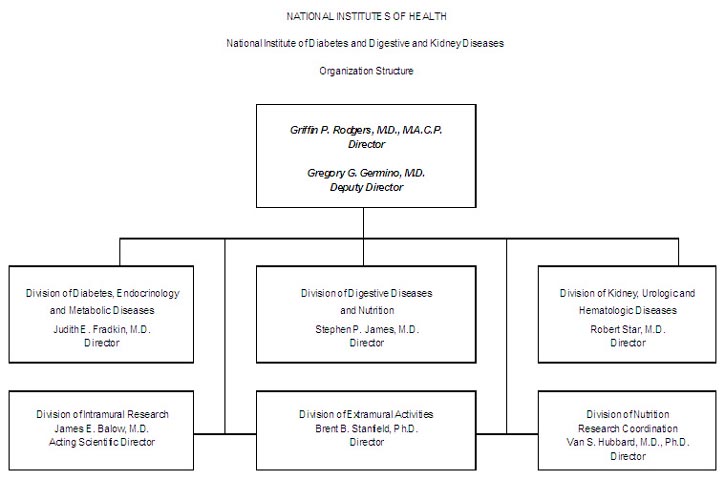
NATIONAL INSTITUTES OF HEALTH
National Institute of Diabetes and Digestive and Kidney Diseases
For carrying out section 301 and title IV of the PHS Act with respect to diabetes and digestive and kidney diseases, [$1,800,447,000] $1,792,107,000. (Department of Health and Human Services Appropriations Act, 2012.)
Amounts Available for Obligation 1/
(Dollars in thousands)
| Source of Funding |
FY 2011 Actual |
FY 2012 Enacted |
FY 2013 PB |
| Appropriation |
1,808,100 |
1,800,447 |
1,792,107 |
| Type 1 Diabetes 2/ |
150,000 |
150,000 |
150,000 |
| Rescission |
-15,876 |
-3,403 |
0 |
| Subtotal, adjusted appropriation |
1,942,224 |
1,947,044 |
1,942,107 |
| Real transfer under Secretary's transfer authority |
0 |
-512 |
0 |
| Comparative Transfers for NCATS reorganization |
0 |
0 |
0 |
| Comparative Transfers to NCATS for Therapeutics and Rare and Neglected Diseases (TRND) |
-1,476 |
0 |
0 |
| Comparative Transfers to NLM for NCBI and Public Access |
-1,538 |
-1,627 |
0 |
| Subtotal, adjusted budget authority |
1,939,210 |
1,944,905 |
1,942,107 |
| Unobligated balance lapsing |
-69 |
0 |
0 |
| Total obligations |
1,939,141 |
1,944,905 |
1,942,107 |
1/ Excludes the following amounts for reimbursable activities carried out by this account:
FY 2011 - $3,982 FY 2012 - $6,000 FY 2013 - $6,000
2/ Type 1 Diabetes Special Statutory Authority in Accordance with P.L. 107-360, P.L. 110-275, and P.L. 111-309.
Budget Mechanism - Total 1/
(Dollars in thousands)
| MECHANISM |
FY 2011 Actual No. |
FY 2011 Actual Amount |
FY 2012 Enacted No. |
FY 2012 Enacted Amount |
FY 2013 PB No. |
FY 2013 PB Amount |
Change vs. FY 2012 No. |
Change vs. FY 2012 Amount |
| Research Project Grants: Noncompeting |
2,196 |
$878,721 |
2,178 |
$911,756 |
2,096 |
$859,981 |
-82 |
-$51,775 |
| Research Project Grants: Administrative Supplements |
105 |
12,711 |
104 |
11,986 |
105 |
14,000 |
1 |
2,014 |
| Research Project Grants: Competing - Renewal |
224 |
105,657 |
229 |
103,091 |
242 |
107,573 |
13 |
4,482 |
| Research Project Grants: Competing - New |
425 |
267,859 |
420 |
230,824 |
450 |
260,181 |
30 |
29,357 |
| Research Project Grants: Competing - Supplements |
1 |
244 |
1 |
240 |
1 |
250 |
0 |
10 |
| Subtotal, Research Project Grants: Competing |
650 |
$373,760 |
650 |
$334,155 |
693 |
$368,004 |
43 |
$33,849 |
| Subtotal, Research Project Grants (RPGs) |
2,846 |
$1,265,192 |
2,828 |
$1,257,897 |
2,789 |
$1,241,985 |
-39 |
-$15,912 |
| Research Project Grants: SBIR/STTR |
100 |
$47,186 |
105 |
$49,810 |
109 |
$51,317 |
4 |
$1,507 |
| Research Project Grants |
2,946 |
$1,312,378 |
2,933 |
$1,307,707 |
2,898 |
$1,293,302 |
-35 |
-$14,405 |
| Research Centers: Specialized/Comprehensive |
93 |
$100,064 |
93 |
$100,345 |
95 |
$100,345 |
2 |
$0 |
| Research Centers in Clinical Research |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Research Centers in Biotechnology |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Research Centers in Comparative Medicine |
0 |
548 |
0 |
548 |
0 |
548 |
0 |
0 |
| Research Centers in Minority Institutions |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Other Research: Research Careers |
499 |
$76,324 |
499 |
$76,484 |
499 |
$76,484 |
0 |
$0 |
| Other Research: Cancer Education |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Other Research: Cooperative Clinical Research |
0 |
4,584 |
0 |
4,584 |
0 |
4,584 |
0 |
0 |
| Other Research: Biomedical Research Support |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Other Research: Minority Biomedical Research Support |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Other Research: Other |
114 |
52,929 |
121 |
56,078 |
122 |
56,078 |
1 |
0 |
| Other Research |
613 |
$133,837 |
620 |
$137,146 |
621 |
$137,146 |
1 |
$0 |
| Total Research Grants |
3,652 |
$1,546,827 |
3,646 |
$1,545,746 |
3,614 |
$1,531,341 |
-32 |
-$14,405 |
| Research Training: Individual Awards |
268 (FTTPs) |
$12,309 |
263 (FTTPs) |
$12,330 |
262 (FTTPs) |
$12,459 |
-1 (FTTPs) |
$129 |
| Research Training: Institutional Awards |
930 (FTTPs) |
46,302 |
907 (FTTPs) |
46,302 |
895 (FTTPs) |
47,091 |
-12 (FTTPs) |
789 |
| Total Research Training (FTTPs) |
1,198 (FTTPs) |
$58,611 |
1,170 (FTTPs) |
$58,632 |
1,157 (FTTPs) |
$59,550 |
-13 (FTTPs) |
$918 |
| Research & Development Contracts |
117 |
$88,301 |
125 |
$95,056 |
124 |
$105,745 |
-1 |
$10,689 |
| Research & Development Contracts: SBIR/STTR |
3 |
$71 |
5 |
$300 |
5 |
$300 |
0 |
$0 |
| Intramural Research |
352 (FTEs) |
$179,593 |
352 (FTEs) |
$179,593 |
349 (FTEs) |
$179,593 |
-3 (FTEs) |
$0 |
| Research Management and Support |
285 (FTEs) |
65,878 |
285 (FTEs) |
65,878 |
282 (FTEs) |
65,878 |
-3 (FTEs) |
0 |
| Construction |
|
0 |
|
0 |
|
0 |
|
0 |
| Buildings and Facilities |
|
0 |
|
0 |
|
0 |
|
0 |
| Total, NIDDK (FTEs) |
637 (FTEs) |
$1,939,210 |
637 (FTEs) |
$1,944,905 |
631 (FTEs) |
$1,942,107 |
-6 (FTEs) |
-$2,798 |
1/ All items in italics are "non-adds"
Budget Mechanism - Type 1 Diabetes 1/
(Dollars in thousands)
| MECHANISM |
FY 2011 No. |
FY 2011 Amount |
FY 2012 Enacted No. |
FY 2012 Enacted Amount |
FY 2013 PB No. |
FY 2013 PB Amount |
Change vs. FY 2012 No. |
Change vs. FY 2012 Amount |
| Research Project Grants: Noncompeting |
37 |
$30,394 |
77 |
$63,014 |
55 |
$42,836 |
-22 |
-$20,178 |
| Research Project Grants: Administrative Supplements |
4 |
1,986 |
4 |
1,986 |
5 |
2,000 |
1 |
14 |
| Research Project Grants: Competing Renewal |
0 |
2,798 |
0 |
1,961 |
0 |
2,493 |
0 |
532 |
| Research Project Grants: Competing New |
16 |
103,230 |
11 |
72,364 |
14 |
91,996 |
3 |
19,632 |
| Research Project Grants: Competing Supplements |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Subtotal, Research Project Grants: Competing |
16 |
$106,028 |
11 |
$74,325 |
14 |
$94,489 |
3 |
$20,164 |
| Subtotal, Research Project Grants (RPGs) |
53 |
$138,408 |
88 |
$139,325 |
69 |
$139,325 |
-19 |
$0 |
| Research Project Grants: SBIR/STTR |
5 |
$4,167 |
5 |
$4,167 |
5 |
$4,167 |
0 |
$0 |
| Research Project Grants |
58 |
$142,575 |
93 |
$143,492 |
74 |
$143,492 |
-19 |
$0 |
| Research Centers: Specialized/Comprehensive |
0 |
$0 |
0 |
$0 |
0 |
$0 |
0 |
$0 |
| Research Centers in Clinical Research |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Research Centers in Biotechnology |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Research Centers in Comparative Medicine |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Research Centers in Minority Institutions |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Research Centers |
0 |
$0 |
0 |
$0 |
0 |
$0 |
0 |
$0 |
| Other Research: Research Careers |
5 |
$1,924 |
5 |
$1,924 |
5 |
$1,924 |
0 |
$0 |
| Other Research: Cancer Education |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Other Research: Cooperative Clinical Research |
0 |
4,584 |
0 |
4,584 |
0 |
4,584 |
0 |
0 |
| Other Research: Biomedical Research Support |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Other Research: Minority Biomedical Research Support |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Other Research: Other |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Other Research |
5 |
$6,508 |
5 |
$6,508 |
5 |
$6,508 |
0 |
$0 |
| Total Research Grants |
63 |
$149,083 |
98 |
$150,000 |
79 |
$150,000 |
-19 |
$0 |
| Research Training: Individual Awards |
0 (FTTPs) |
$0 |
0 (FTTPs) |
$0 |
0 (FTTPs) |
$0 |
0 |
$0 |
| Research Training: Institutional Awards |
0 (FTTPs) |
0 |
0 (FTTPs) |
0 |
0 (FTTPs) |
0 |
0 |
0 |
| Total Research Training(FTTPs) |
0(FTTPs) |
$0 |
0 (FTTPs) |
$0 |
0 (FTTPs) |
$0 |
0 |
$0 |
| Research & Development Contracts |
3 |
$917 |
0 |
$0 |
0 |
$0 |
0 |
$0 |
| SBIR/STTR |
0 |
$0 |
0 |
$0 |
0 |
$0 |
0 |
$0 |
| Intramural Research |
0 (FTEs) |
$0 |
0 (FTEs) |
$0 |
0 (FTEs) |
$0 |
0 (FTEs) |
$0 |
| Research Management and Support |
0 (FTEs) |
0 |
0 (FTEs) |
0 |
0 (FTEs) |
0 |
0 (FTEs) |
0 |
| Construction |
|
0 |
|
0 |
|
0 |
|
0 |
| Buildings and Facilities |
|
0 |
|
0 |
|
0 |
|
0 |
| Total, Type 1 Diabetes |
0 |
$150,000 |
0 |
$150,000 |
0 |
$150,000 |
0 |
$0 |
1/ All items in italics are "non-adds"
Major Changes in the Fiscal Year 2013 President's Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2013 budget request for NIDDK, which is $2.798 million less than the FY 2012 level, for a total of $1,942.107 million.
Research Project Grants (RPGs; -$14.405 million; total $1,293.302 million): NIDDK will continue to support competing RPGs—693 awards in FY 2013, an increase of 43 from FY 2012. About 2,096 noncompeting RPGs, totaling $874.0 million will also be made in FY 2013. NIH budget policy for RPGs in FY 2013 discontinues inflationary allowances and reduces the average cost of noncompeting and competing RPGs by one percent below the FY 2012 level.
CER Study Toward Establishing Personalized Therapy for Type 2 Diabetes (+$23.0 million; total $23.0 million): A cooperative agreement will support a new, multicenter study of the comparative effectiveness of five common drugs used for therapy of type 2 diabetes.
Causes and Consequences of Cystic-Fibrosis Related Diabetes (+1.5million; total $1.5 million): This new initiative will support studies of the causes and progression of CFRD in humans and animal models, and studies to understand the increased morbidity in patients with CFRD.
Vitamin D for Type 2 Diabetes (D2D Trial) (+5.0 million; total $5.0 million): A cooperative agreement would support a new, multicenter study to test whether vitamin D can prevent or delay progression to type 2 diabetes in adults at high risk of developing the disease.
Drug-Induced Liver Injury Network (DILIN) (-3.25 million; total $0 million): This nationwide study to collect and analyze cases of severe liver injury caused by prescription drugs, over-the-counter drugs, and alternative medicines, is ending in FY 2013 as scheduled.
Studies of Drug-Induced Liver Injury (+3.25 million; total $3.25 million): A new research consortium will be created to study drug-induced liver injury.
Stimulating-Hematology Investigation: New Endeavors (SHINE) (+1.0 million; total $2.0 million): This initiative will continue to support investigator-initiated research project grants in areas of basic and translational hematology research.
Advancing Translational and Clinical Research in Primary Glomerular Disease (+4.0 million; total $4.0 million): This new initiative will support research to identify and test agents that treat or reduce progression of primary (non-diabetic) glomerulopathies.
Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN) (+2.7 million; total $4.0 million): This new initiative will support a cooperative research network to improve measurement of symptoms in both men and women to advance clinical studies.
Summary of Changes
(Dollars in thousands)
FY 2012 Enacted : 1,944,905
FY 2013 President’s Budget: 1,942,107
Net Change: -$2,798
| Built-in CHANGES |
2013 President’s Budget FTEs |
2013 President’s Budget Authority |
Change from FY 2012 FTEs |
Change from FY 2012 Budget Authority |
| Intramural Research: Annualization of January 2012 pay increase and benefits |
|
$72,382 |
|
$5 |
| Intramural Research: January FY 2013 pay increase and benefits |
|
72,382 |
|
225 |
| Intramural Research: One more day of pay |
|
72,382 |
|
271 |
| Intramural Research: Annualization of PY net hires |
|
72,382 |
|
0 |
| Intramural Research: Payment for centrally furnished services |
|
31,584 |
|
0 |
| Intramural Research: Increased cost of laboratory supplies, materials other expenses, and non-recurring costs |
|
75,627 |
|
0 |
| Subtotal |
|
|
|
$501 |
| Research Management and Support: Annualization of January 2012 pay increase and benefits |
|
$39,661 |
|
$3 |
| Research Management and Support: January FY 2013 pay increase and benefits |
|
39,661 |
|
122 |
| Research Management and Support: One more day of pay |
|
39,661 |
|
149 |
| Research Management and Support: Annualization of PY net hires |
|
39,661 |
|
0 |
| Research Management and Support: Payment for centrally furnished services |
|
2,536 |
|
0 |
| Increased cost of laboratory supplies, materials, other expenses, and non-recurring costs |
|
23,681 |
|
0 |
| Subtotal |
|
|
|
$274 |
| Subtotal, Built-in |
|
|
|
$775 |
Summary of Changes... continued
| Program CHANGES |
2013 President’s Budget No. |
2013 President’s Budget Amount |
Change from FY 2012 No. |
Change from FY 2012 Amount |
| 1. Research Project Grants: |
|
|
|
|
| Research Project Grants: Noncompeting |
2,096 |
$873,981 |
-82 |
-$49,761 |
| Research Project Grants: Competing |
693 |
368,004 |
43 |
33,849 |
| Research Project Grants: SBIR/STTR |
109 |
51,317 |
4 |
1,507 |
| Research Project Grants: Total |
2,898 |
$1,293,302 |
-35 |
-$14,405 |
| 2. Research Centers |
95 |
$100,893 |
2 |
$0 |
| 3. Other Research |
621 |
137,146 |
1 |
0 |
| 4. Research Training |
1,157 |
59,550 |
-13 |
918 |
| 5. Research and development contracts |
124 |
105,745 |
-1 |
10,689 |
| Subtotal, Extramural |
|
$1,696,636 |
|
-$2,798 |
| 6. Intramural Research |
349(FTEs) |
$179,593 |
-3(FTEs) |
-$501 |
| 7. Research Management and Support |
282(FTEs) |
65,878 |
-3(FTEs) |
-274 |
| 8. Construction |
|
0 |
|
0 |
| 9. Buildings and Facilities |
|
0 |
|
0 |
| Subtotal, program |
631 |
$1,942,107 |
-6 |
-$3,573 |
| Total changes |
|
|
|
-$2,798 |
History of Budget Authority and FTEs: 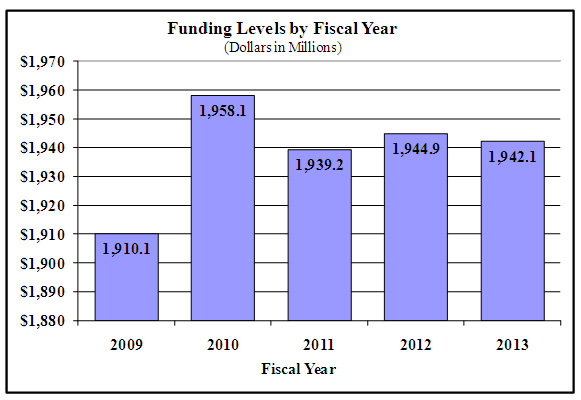
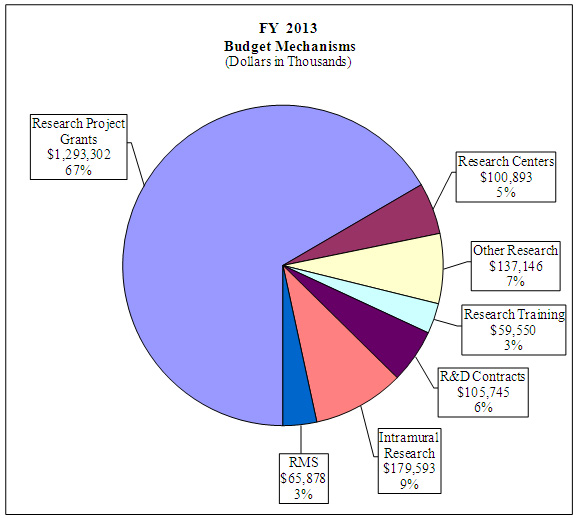
 Distribution by Mechanism
Distribution by Mechanism:
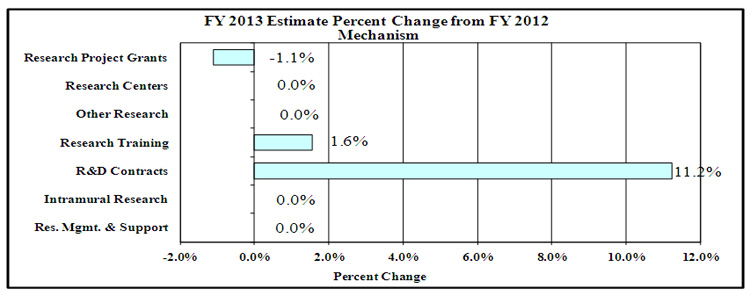
 Percent Change by Mechanism
Percent Change by Mechanism:
Budget Authority by Activity
(Dollars in thousands)
| Detail: Extramural Research |
FY 2011 Actual 1/ FTEs |
FY 2011 Actual 1/ Amount |
FY 2012 Enacted FTEs |
FY 2012 Enacted Amount |
FY 2013 PB FTEs |
FY 2013
PB Amount |
Change vs. FY 2012 Enacted FTEs |
Change vs. FY 2012 Enacted Amount |
| Diabetes, Endocrinology, and Metabolic Diseases |
|
$628,390 |
|
$635,268 |
|
$634,121 |
|
-1,147 |
| Digestive Diseases and Nutrition |
|
491,160 |
|
495,819 |
|
494,924 |
|
-895 |
| Kidney, Urologic, and Hematologic Diseases |
|
424,189 |
|
418,347 |
|
417,591 |
|
-756 |
| Type 1 Diabetes |
|
150,000 |
|
150,000 |
|
150,000 |
|
0 |
| Subtotal, Extramural |
|
$1,693,739 |
|
$1,699,434 |
|
$1,696,636 |
|
-$2,798 |
| Intramural Research |
352 |
$179,593 |
352 |
$179,593 |
349 |
$179,593 |
-3 |
$0 |
| Research Management and Support |
285 |
$65,878 |
285 |
$65,878 |
282 |
$65,878 |
-3 |
$0 |
| TOTAL |
637 |
$1,939,210 |
637 |
$1,944,905 |
631 |
$1,942,107 |
-6 |
-$2,798 |
1/ .Includes FTEs which are reimbursed from the NIH Common Fund.
2/ Includes Real Transfers and Comparable Adjustments as detailed in the "Amounts Available for Obligation" table.
Appropriations History 1/
| Fiscal Year |
Budget Estimate to Congress |
House Allowance |
Senate Allowance |
Appropriation |
| 2004 |
$1,820,007,000 |
$1,820,007,000 |
$1,833,007,000 |
$1,821,240,000 |
|
| Rescission |
|
|
|
-$10,654,000 |
|
| 2005 |
$1,876,196,000 |
$1,876,196,000 |
$1,889,100,000 |
$1,863,584,000 |
|
| Rescission |
|
|
|
-$14,112,000 |
|
| 2006 |
$1,872,146,000 |
$1,872,146,000 |
$1,917,919,000 |
$1,854,925,000 |
|
| Rescission |
|
|
|
-$17,221,000 |
|
| 2007 |
$1,844,298,000 |
$1,844,298,000 |
$1,857,753,000 |
$1,855,868,000 |
|
| Rescission |
|
|
|
$0 |
|
| 2008 |
$1,858,045,000 |
$1,881,893,000 |
$1,897,784,000 |
$1,855,868,000 |
|
| Rescission |
|
|
|
$0 |
|
| Supplemental |
|
|
|
$9,077,000 |
|
| 2009 |
$1,858,487,000 |
$1,767,071,000 |
$1,755,881,000 |
$1,911,338,000 |
|
| Rescission |
|
|
|
$0 |
|
| 2010 |
$1,931,494,000 |
$1,974,251,000 |
$1,940,518,000 |
$1,958,100,000 |
|
| Rescission |
|
|
|
$0 |
|
| 2011 |
$2,007,589,000 |
|
$2,004,674,000 |
$1,958,100,000 |
|
| Rescission |
|
|
|
-$15,876,196 |
|
| 2012 |
$1,987,957,000 |
$1,987,957,000 |
$1,922,045,000 |
$1,950,447,000 |
|
| Rescission |
|
|
|
-$3,402,845 |
|
| 2013 |
$1,942,107,000 |
|
|
|
|
1/ Includes Type 1 Diabetes Special Statutory Authority Funds.
Justification of Budget Request
National Institute of Diabetes and Digestive and Kidney Diseases
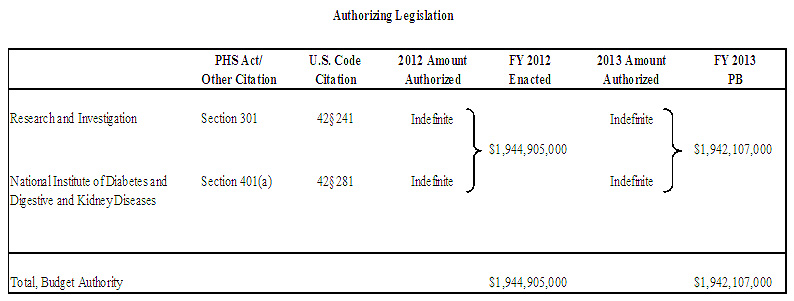
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
Budget Authority
|
FY 2011
Actual |
FY 2012
Enacted |
FY 2013
President's
Budget |
FY 2013 +/-
FY 2012 |
| BA |
$1,939,210,000 |
$1,944,905,000 |
$1,942,107,000 |
-$2,798,000 |
| FTEs |
637 |
637 |
631 |
-6 |
| Type 1 Diabetes |
-$150,000,000 |
-$150,000,000 |
-$150,000,000 |
|
| Labor/HHS: |
$1,789,210,000 |
$1,794,905,000 |
$1,792,107,000 |
|
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director’s Overview
The mission of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) is to support and conduct research to combat diabetes and other endocrine and metabolic diseases, liver and other digestive diseases, nutritional disorders, obesity, and kidney, urologic, and hematologic diseases. These diseases are chronic, common, costly, and consequential for patients, their families, and our Nation. Diabetes afflicts an estimated 25.8 million people in the U.S., greatly increasing the risk for many serious complications, such as heart disease and kidney failure.[1] Estimates of chronic kidney disease (CKD) show that more than 23 million Americans are affected, and over 550,000 have irreversible kidney failure.[2] Many urologic diseases are also highly prevalent.[3] Digestive diseases account for an estimated 104.7 million visits to ambulatory care centers and 13.5 million hospitalizations per year.[4] Obesity affects approximately one-third of U.S. adults and about 17 percent of children and adolescents.[5] Obesity is a strong risk factor for type 2 diabetes, nonalcoholic steatohepatitis (NASH), and many other diseases. Cystic fibrosis and other genetic diseases within NIDDK’s purview are less widespread, but still devastating in their impacts. Building on emerging opportunities from past research investments, NIDDK will continue to pursue basic, clinical, and translational research; research training and career development; and health information dissemination, with continued focus on preserving balance in its investigator-initiated research portfolio.
Support of NIH Director’s Themes:
Overall Theme: Extraordinary Scientific Opportunities
The enormous progress made in understanding and treating chronic diseases now enables us to think boldly and beyond sustained treatment. We are beginning to identify points in the natural history of diseases when efforts to arrest and reverse progression are likely to succeed. For example, NIDDK’s Diabetes Prevention Program and its follow-up study showed that type 2 diabetes can be prevented or delayed in high-risk individuals by the oral diabetes drug metformin or lifestyle intervention, and promising pilot studies suggest that translating the lifestyle intervention to lower cost group settings is effective. Already these results are being implemented in YMCAs in many states. An ongoing trial of lifestyle intervention to prevent complications of type 2 diabetes has shown improved control of glucose, blood pressure, and lipids with less use of medications.[6] Research revealed that people with CKD who have elevated levels of a novel biomarker, FGF-23, are at higher risk for kidney failure, cardiovascular disease, and death.[7] Studies showed improvements in aspects of adult and pediatric NASH in response to 2-year therapy with vitamin E.[8] These findings all support identifying windows of opportunity to develop new strategies and discover novel therapeutic targets. By stabilizing and reversing the disease process, we could defer or eliminate the enormous personal and economic costs associated with disease complications. Moreover, we could achieve better medical management of chronic disease until patients could benefit from emerging advances in pharmacology, regenerative medicine, genetics, and other fields. Such efforts complement NIDDK’s existing research programs aimed at primary prevention and optimal treatment of these diseases.
- Theme 1: Investing in Basic Research
Recent technological advances in analyzing the human genome aided discovery of genetic risk factors for several disorders and diseases in NIDDK’s mission, including diabetes, inflammatory bowel diseases, body weight, nonalcoholic fatty liver disease, and focal segmental glomerulosclerosis (FSGS)—one of the leading causes of kidney failure in children and adults and characterized by scarring in scattered regions of the kidney. Capitalizing on such findings can accelerate development of new and personalized diagnostic, therapeutic, and preventive approaches; for example, the discovery of the cystic fibrosis gene led to an effective therapy to slow progression of the disease.[9]
- Theme 2: Accelerating Discovery Through Technology
NIDDK-supported researchers also utilized cutting-edge genetic technologies for pioneering studies of the gut microbial effects on conditions such as obesity and digestive diseases. Recent advances in microbiome research elucidated how helpful bacteria can survive in the digestive tract without triggering an immune attack, determined that different diets are associated with distinct collections of gut bacteria which fluctuate in response to changes in diet, and found that certain mixes of intestinal bacteria are associated with pediatric irritable bowel syndrome[10]. In addition, NIDDK metabolic research and studies of obesity led to the development of mathematical models of human metabolism to simulate more accurately the body’s response to physical activity and various diets[11].
- Theme 3: Advancing Translational Sciences
NIDDK is advancing the development of new and better diagnostics, therapeutics, and preventive approaches to improve public health. Research in the emerging field of metabolomics revealed a pattern associated with a four-fold increase in future risk for developing type 2 diabetes.[12] Identification of novel factors that may cause FSGS, or cardiovascular disease in people with CKD, may yield new molecular targets for therapeutic development.[13] Basic research is driving discovery of new potential therapeutics; NIDDK-supported researchers identified experimental drugs and a natural product that improve metabolic abnormalities associated with type 2 diabetes in mice.[14] Scientists also identified a class of drugs that corrects a defect in cells from people with a rare genetic disease called Neimann-Pick type C.[15] Clinical trials evaluated natural compounds as therapeutics; for example, researchers found that vitamin E is effective in treatment of some adults and children with fatty liver disease; vitamin D may prevent or delay progression to type 2 diabetes in people at high risk; and a dietary supplement boosts response to therapy in people infected with a type of hepatitis C virus that typically does not respond well to therapy.[16]
- Theme 4: Encouraging New Investigators and New Ideas
In 2011, to encourage and facilitate participation of underrepresented racial and ethnic minority groups in the conduct of biomedical research, NIDDK held its 9th annual Network of Minority Research Investigators workshop. NIDDK also supported the Short-Term Education Program for Underrepresented Persons (STEP-UP) to provide summer research opportunities for underrepresented high school and undergraduate students. NIDDK programs are also helping to attract young physician scientists, behavioral scientists, and bioengineers to study diabetes, and NIDDK’s new investigator workshops aim to support current work and enhance future success.
NIDDK also disseminates science-based health information through its Weight-control Information Network; the National Diabetes Education Program, co-sponsored with CDC; the National Kidney Disease Education Program; and information clearinghouses. NIDDK seeks external strategic planning and resource allocation advice from investigators, professional organizations, patient advocates, the public, NIDDK’s National Advisory Council, intra-/inter-agency coordinating bodies, ad hoc planning groups, and scientific meetings.
Overall IC Budget Policy: The FY 2013 President's Budget request for NIDDK is $1,942.107 million, a decrease of $2.798 million or 0.14 percent below the FY 2012 Enacted level. NIDDK has targeted a portion of funds for competing research project grants to support high-priority projects outside the payline, including awards to new and early-stage investigators. NIDDK also seeks to balance support for solicitations to the extramural community and funding for investigator-initiated projects. In FY 2013, NIDDK will support new investigators on R01 equivalent awards at success rates equal to those of established investigators submitting new R01 equivalent applications. NIH will provide an increase of 2 percent for stipend levels under the Ruth L. Kirschstein National Research Service Award training program to continue efforts to attain the stipend levels recommended by the National Academy of Sciences. This will build on the 2 percent increase in stipend levels for FY 2012 Enacted level. Stipend levels were largely flat for several years, and the requested increase will help to sustain the development of a highly qualified biomedical research workforce. Intramural Research and Research Management and Support will stay the same as the FY 2012 Enacted level. NIDDK will continue to support research consistent with the NIH Director’s themes. Funds are included in R&D contracts to support trans-NIH initiatives, such as the Basic Behavioral and Social Sciences Opportunity Network (OppNet) as well as increased support for other HHS agencies through the program evaluation set-aside.
Program Descriptions and Accomplishments
Diabetes, Endocrinology, and Metabolic Diseases: The goals of this program are to increase understanding of diabetes and other endocrine and metabolic disorders and to develop and test prevention and treatment strategies. This program supports basic, clinical, and translational research, as well as research training, in the areas of type 1 and type 2 diabetes, cystic fibrosis, obesity, energy balance, and endocrinology. Knowledge from this research is communicated to patients, health professionals, and the public through the National Diabetes Information Clearinghouse and the National Diabetes Education Program.
In 2011, NIDDK completed the first major trial of type 2 diabetes management in children and adolescents, a newly emerging problem, and demonstrated that intensive glucose control in people with type 1 diabetes can reduce rates of chronic kidney disease and end-stage renal disease by 50 percent 22 years later.[17] Trials investigating bariatric surgery as treatment for diabetes continued, as well as experimental studies of bariatric surgery in animals. NIDDK supported planning grants for a comparative effectiveness clinical trial testing different medications, in combination with the oral diabetes drug metformin, for type 2 diabetes treatment, and for a clinical trial testing vitamin D in prevention of type 2 diabetes based on a promising pilot study.[18] New initiatives are fostering research toward preserving function of insulin-producing beta cells early in the course of type 2 diabetes, and a new consortium was launched to study approaches to prevent gestational diabetes. The Beta Cell Biology Consortium identified a potential new strategy to induce beta cell regeneration to replace lost beta cells and reverse aging-associated decline in beta cell growth.[19] In addition, NIDDK continues efforts to understand and ameliorate disparities in diabetes with research to identify gene regions conferring type 2 diabetes risk in multiple ethnic groups, translational research to bring scientific discoveries to all who can benefit, and a clinical trial of type 2 diabetes management including minority youth and adolescents.
Budget Policy: The FY 2013 President's Budget request for this program is $634.121 million, a decrease of $1.147 million or 0.18 percent below the FY 2012 Enacted level. With FY 2013 resources, NIDDK will continue major diabetes clinical trials and encourage and support development of major new investigator-initiated clinical studies. FY 2013 funds will also support research capitalizing on new opportunities to identify diabetes risk genes in minority populations, to advance progress toward developing new therapeutic approaches, and to support comparative effectiveness research. New research efforts to study the causes and consequences of cystic fibrosis related-diabetes will be supported. NIDDK will also continue to fund translational research in FY 2013 and support health information dissemination activities to bring scientific discoveries in diabetes and obesity to real-world medical practice and other community settings. In FY 2013, NIDDK will continue an initiative encouraging collaborative, multidisciplinary research teams to work on complex biomedical problems in diabetes, endocrinology, and metabolic diseases. NIDDK will also continue funding for research centers to advance research relevant to diabetes and to cystic fibrosis and other genetic metabolic diseases. NIDDK plans for FY 2013 include capitalizing on new findings relevant to brown fat and gestational diabetes and pursuing other efforts as part of an overall balanced research program.
Program Portrait: The Environmental Determinants of Diabetes in the Young (TEDDY)
| FY 2012 Level: |
$ 5.6 million |
| FY 2013 Level: |
$44.0 million |
| Change: |
+$38.4 million |
The ambitious, long-term TEDDY study aims to discover the infectious agents, dietary factors, or other environmental conditions that trigger type 1 diabetes in genetically susceptible individuals. Investigators screened over 425,000 newborns to identify infants with an increased risk of type 1 diabetes. Over 8,000 high-risk infants are now being followed from birth through 15 years of age. For a decade and a half, investigators will regularly collect information on the child’s diet, illnesses, vaccinations, and psychosocial stresses; biological samples, including blood, stool, and toenail clippings, will be collected and made available to researchers worldwide through the NIDDK Central Repositories. In addition, TEDDY children are frequently tested for evidence of the autoimmune process that characterizes type 1 diabetes, enabling researchers to study the very early stages in the development of the disease. Investigators are now beginning pilot studies to identify environmental triggers. Children enrolled in the study are developing autoimmunity and type 1 diabetes at the predicted rates, indicating that those at risk can be accurately identified and that the study is on track to make a major contribution. The substantial investment in TEDDY can reap major rewards and revolutionize the ability to prevent type 1 diabetes. Identification of a dietary or infectious cause of type 1 diabetes could have an enormously positive impact on public health through a diet change or vaccine for disease prevention. Because celiac disease and type 1 diabetes share some genetic susceptibility factors, newborns studied in TEDDY are also at high risk for celiac disease and investigators are studying the development of this disease as well. |
Digestive Diseases and Nutrition: The goals of this program are to increase understanding of digestive diseases, nutrition, and obesity, and to develop and test strategies for disease prevention and treatment. This program supports basic, clinical, and translational research, as well as research training, encompassing fundamental studies of the digestive system; disease-targeted research involving the esophagus, stomach, small intestine, large intestine and anorectum, liver and biliary system, and pancreas; studies relevant to nutrition; and research on obesity. Insights gleaned from scientific efforts are communicated to patients, health professionals, and the public through NIDDK’s National Digestive Diseases Information Clearinghouse and Weight-control Information Network. NIDDK supports the Hepatitis B Research Network, which will test treatments in at-risk populations, such as Asian Americans and Pacific Islanders. CHILDREN, a network conducting studies on severe forms of childhood liver injury, will include a new focus on liver disease associated with cystic fibrosis. NIDDK supports A2ALL, a study of risk and benefits associated with adult-to-adult living donor transplantation. NIDDK will continue CORI, a clinical outcomes research initiative and the largest national database on gastrointestinal endoscopy. The Nonalcoholic Steatohepatitis Clinical Research Network will continue to complete clinical trials in adults and children and collection of biospecimens and data. NIDDK supports the Gastrointestinal Stem Cell Consortium to improve understanding of intestinal biology and function, and aid therapeutic development through research on gastrointestinal progenitor cells. Studies by the Acute Liver Failure Study Group were key to developing new regulations regarding acetaminophen and showing the benefits of N-acetylcysteine for treating acute liver failure due to other causes.[20] Support of Look AHEAD, a major long-term study that aims to determine whether intensive lifestyle measures involving diet and exercise improve morbidity and mortality from cardiovascular diseases in people with diabetes, will continue.
Budget Policy: The FY 2013 President's Budget request for this program is $494.924 million, a decrease of $0.895 million or 0.18 percent below the FY 2012 Enacted level. In FY 2013, NIDDK will continue major clinical research networks to help understand and treat liver diseases, including hepatitis B and nonalcoholic steatohepatitis. Among its obesity-related efforts in FY 2013, NIDDK will support major ongoing observational studies to assess the health risks and benefits of weight-loss surgery in extremely obese adults and adolescents, as well as an ongoing trial evaluating the long-term health effects of weight loss in obese adults with type 2 diabetes (Look AHEAD). NIDDK will also use FY 2013 funds to support Digestive Diseases Research Core Centers, and to sustain a consortium that is conducting cutting-edge genetic research on inflammatory bowel diseases. Research on intestinal stem cells that can benefit a variety of digestive diseases will continue in FY 2013, along with other efforts as part of an overall balanced research program.
Program Portrait: Lifestyle Intervention in Overweight and Obese Pregnant Women
| FY 2012 Level: |
$3.0 million |
| FY 2013 Level: |
$3.0 million |
| Change: |
$0.0 million |
Numerous observational studies have linked overweight/obesity, and/or excessive gestational weight gain during pregnancy to short-term and long-term adverse health consequences in both mothers and offspring, but additional research is needed to identify effective interventions that will improve weight, glucose, and other pregnancy-related outcomes in mothers and determine whether these interventions affect obesity and metabolic abnormalities in the offspring. In FY 2013, NIDDK will continue clinical studies testing behavioral/lifestyle interventions in overweight and obese pregnant women. These studies include testing a novel lifestyle intervention given to obese, socioeconomically disadvantaged African American women through an existing national home visiting program; a personalized gestational weight management program for overweight and obese women delivered in person or remotely via a Smartphone; and an intensive lifestyle intervention provided through weekly counseling sessions during pregnancy and group counseling after delivery. All studies include follow up post-partum to determine weight and/or metabolic outcomes in both mothers and offspring. A Research Coordinating Unit facilitates collaboration, coordination, and exchange of scientific information among the investigators. Results from these studies could lead to an effective lifestyle intervention for overweight/obese women during pregnancy, reducing post-partum weight retention; incidence of gestational diabetes and subsequent development of type 2 diabetes in the mother; and the risk for obesity, metabolic abnormalities, and adverse cardiovascular outcomes in both mother and offspring. |
Kidney, Urologic, and Hematologic Diseases: The goals of this program are to increase the understanding of diseases and disorders of the kidneys, urinary tract, and blood (hematologic), and to develop and test prevention and treatment strategies. Basic, clinical, and translational research, as well as research training, is supported in the areas of chronic kidney disease (CKD), diabetic kidney disease, end-stage renal disease (ESRD or kidney failure), polycystic kidney disease, and many other kidney diseases; urinary incontinence, benign prostatic hyperplasia, interstitial cystitis/painful bladder syndrome, stones, impotence, congenital urologic disorders, and urinary tract infections; and disorders of the blood and blood-forming organs, including sickle cell disease, Cooley’s anemia, hemochromatosis, and the anemia of inflammation and of chronic disease. Science-based information is communicated to patients, health professionals, and the public through NIDDK’s National Kidney and Urologic Diseases Information Clearinghouse and National Kidney Disease Education Program (NKDEP). NIDDK is actively pursuing a range of research avenues related to kidney disease, acute kidney injury, CKD in children, hemodialysis, and other areas. Research efforts identified a novel biomarker associated with increased risk of kidney failure, cardiovascular disease (CVD), and death among people with CKD. Elevated levels of the biomarker are associated with increased risk of an adverse heart condition—change in the size of the heart’s left ventricle.[21] Studies in animal models suggest that this biomarker may actually cause CVD, indicating that strategies to interrupt the biomarker’s action could prevent or lessen damage to the heart in people with CKD. Translating knowledge into practice, NIDDK has funded five research groups to test the effectiveness of interventions for the prevention, treatment, and management of CKD that have a high likelihood of being widely adopted, and of being sustained in a wide range of health care settings and in individuals and communities at highest risk.
Budget Policy: The FY 2013 President's Budget request for this program is $417.591 million, a decrease of $0.756 million or 0.18 percent below the FY Enacted 2012 level. In FY 2013, NIDDK will continue support for ongoing major clinical studies of CKD in adults and children and fund new research to identify and validate biomarkers and risk assessment tools for patients with this condition. NIDDK also plans to continue to sponsor planning grants to conduct translational research on the effectiveness of interventions shown in clinical trials to prevent, treat, and manage CKD, and will continue to sponsor studies to improve adherence to medical therapy in adolescents with CKD. In FY 2013, NIDDK will continue studies to improve measurements of outcomes in lower urinary tract disorders of the prostate and urinary bladder. NIDDK will continue treatment trials for polycystic kidney disease (HALT-PKD study) and continue support for the Consortium for Radiologic Imaging Studies of PKD; results of these studies will help to define measures of kidney disease progression. Centers focused on kidney, urologic, and hematologic research will receive continued funding, as will research on acute kidney injury and a study of arteriovenous fistulas. NIDDK will also continue support for the Systolic Blood Pressure Intervention Trial (led by NHLBI) and for other efforts as part of an overall balanced research portfolio.
Program Portrait: The Chronic Renal Insufficiency Cohort (CRIC) Study
| FY 2012 Level: |
$7.9 million |
| FY 2013 Level: |
$7.9 million |
| Change: |
$0.0 million |
In addition to being at high risk for kidney failure or end-stage renal disease, people with chronic kidney disease (CKD) have an increased risk of cardiovascular disease (CVD). In 2001, NIDDK established the Chronic Renal Insufficiency Cohort (CRIC) Study to identify risk factors that predict loss of kidney function, as well as the development and worsening of CVD, in people with CKD. CRIC has recruited and is following nearly 3,900 racially diverse participants with CKD at 13 U.S. clinical sites. Because African Americans are at increased risk for kidney disease, as are people with diabetes, these groups are well-represented in the study. A wide range of studies is being conducted, including accurate assessment of kidney function, measurement of coronary calcium, retinal photographs, echocardiograms, genetic investigation, and assessment of dietary intake, among others. Study investigators are carefully collecting information about cardiovascular, peripheral vascular, cerebrovascular, and kidney-disease related events, as well as death. Data and specimens from the study will be stored in the NIDDK Central Repositories and serve as a national resource for other investigators who are interested in studying CKD and CVD. Recently, investigators, using data from CRIC, demonstrated that high levels of a hormone that regulates phosphate metabolism are associated with increased risk of kidney failure, CVD, and death among people with CKD. Results from CRIC studies, and other studies to identify biological "markers", can allow physicians to better predict how various diseases are likely to progress in different patients and thereby personalize treatments to improve their health. |
Special Statutory Funding Program for Type 1 Diabetes Research: Complementing efforts of the Diabetes, Endocrinology, and Metabolic Disease program, the Special Program’s goal is to foster improved treatment, prevention, and cure of type 1 diabetes, and its complications through basic, clinical, and translational research around six scientific goals: 1) identifying genetic and environmental causes of type 1 diabetes ($44 million); 2) preventing or reversing the disease ($ 13 million); 3) developing cell replacement therapy ($25 million); 4) improving management and care ($5 million); 5) preventing or reducing diabetes complications ($54 million); and 6) attracting new talent and applying new technologies to research ($9 million) (FY 2013 estimate dollars). Although focused on type 1 diabetes, aspects of this research are relevant to other autoimmune disorders, as well as type 2 diabetes. Both type 1 and type 2 diabetes share impaired function of insulin-producing beta cells of the pancreas along with potential complications, such as heart disease, stroke, blindness, kidney failure, nerve damage, and lower limb amputations. In 2011, NIDDK funded bold and creative research projects that confront major challenges in type 1 diabetes research, including autoimmunity, diabetes complications, and development of artificial pancreas technologies. SmartCells, Inc., which received support from NIDDK small business grants and made substantial progress in preclinical development of a new formulation of insulin, was recently acquired by Merck & Co, Inc., enabling this technology to be developed to its fullest potential. To bring new investigators to type 1 diabetes research, NIDDK re-launched a program to foster research training and career development for pediatricians specializing in childhood diabetes. NIDDK is also creating a new training program to support and develop behavioral scientists and bioengineers in careers in diabetes research.
Budget Policy: The FY 2013 President's Budget request for the Special Statutory Funding Program for Type 1 Diabetes Research is $150 million, the same as FY 2012 Enacted level. NIDDK administers the program, but because of its trans-HHS nature, the resources are disbursed among multiple NIH Institutes and Centers as well as the CDC. Building on planning grants awarded in FY2012, clinical trials to develop practical approaches to improve the health of people with type 1 diabetes and prevent diabetes complications will be implemented in FY2013 ($50 million). Among ongoing efforts that will continue with FY 2013 funds are studies to identify environmental causes of type 1 diabetes in genetically susceptible individuals as a basis for the development of a vaccine or other preventative intervention and trials to test approaches to prevent or slow the onset of the disease. ($47 million), the Clinical Islet Transplantation Consortium ($3.2 million), and the Beta Cell Biology Consortium, which aims to regenerate or replace the insulin-producing cells of the pancreas ($19 million). FY 2013 funds will also support fundamental research to uncover the etiology and pathogenesis of type 1 diabetes ($15.3 million). Research on development of artificial pancreas technologies will continue in FY 2013 through initiatives funding small business research to develop new therapeutics and monitoring technologies for type 1 diabetes ($4.2 million) and clinical research on closed-loop technologies ($4.2 million). FY 2013 funds will also foster research and early career development for pediatric endocrinologists, behavioral scientists, and bioengineers studying new approaches to treat, prevent, and cure type 1 diabetes ($7.1 million).
Intramural Research: The goal of the Intramural Research Program (IRP) is to conduct basic, translational, and clinical biomedical research related to diabetes and other endocrine and metabolic diseases; digestive diseases, including liver diseases and nutritional disorders; obesity; kidney diseases; and hematologic diseases. Intramural research is conducted in the Institute’s laboratories and clinical facilities in Bethesda, Maryland, as well as in Phoenix, Arizona, where a long-standing research relationship with the Pima Indians in the region, who have the highest rate of diabetes in the world, has led to important scientific advances in type 2 diabetes and obesity. Research training is also an integral component of the IRP. Recently, the NIDDK IRP developed a mathematical model that more accurately predicts weight loss for adults, revealing that weight loss in adults happens slowly over longer periods of time than previously expected.[22] NIDDK IRP research found that African Americans with two copies of certain genetic variants in the APOL1 gene are at increased risk of developing kidney disease, particularly focal segmental glomerulosclerosis and kidney disease related to infection with the human immunodeficiency virus.[23] Recent IRP studies identified a small molecule that inhibits the unregulated cellular mechanism that gives rise to Graves’ disease,[24] and showed that a dietary supplement, S-adenosylmethionine, safely and effectively boosts response to standard therapy in people infected with a type of hepatitis C virus that typically does not respond well to therapy.[25] Other IRP research determined the detailed structure of the A2A adenosine receptor which has the potential to speed drug discovery in many disease areas,[26] and discovered key features of hotspots associated with genetic rearrangement that leads to genetic diversity which has the potential to improve detection of genes linked to disease and help understand the causes of genetic abnormalities.[27]
Budget Policy: The FY 2013 President's Budget request for this program is $179.593 million, the same as FY 2012 Enacted level. With FY 2013 funds, the NIDDK IRP will continue a broad spectrum of research studies to strengthen understanding of basic biology and disease mechanism, and evaluate potential therapeutics approaches. For example, in FY 2013, intramural scientists will continue research on obesity in the trans-NIH Metabolic Clinical Research Unit, as well as research relevant to diabetes; digestive diseases, including liver disease; kidney disease; and hematologic disease. The program will also continue to support research training.
Research Management and Support (RMS): RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also encompass strategic planning, coordination, and evaluation of the Institute’s programs, regulatory compliance, international coordination, and liaison with other federal agencies, Congress, and the public. Through its RMS activities, NIDDK has continued to fund meritorious basic, clinical, and translational research and research training efforts, and also continued its health information dissemination and education/outreach activities. Additionally, NIDDK’s strategic planning, evaluation, and other activities have continued.
Budget Policy: The FY 2013 President's Budget request for RMS is $65.878 million, the same as FY 2012 Enacted level. NIDDK will continue effective research management and support so as to deploy research resources to the most meritorious and promising areas, and to communicate research opportunities and findings to investigators, health professionals, and the public.
Budget Authority by Object
(Dollars in thousands)
| Total compensable workyears |
FY 2012 Enacted |
FY 2013 PB |
Increase or Decrease |
| Full-time employment |
637 |
631 |
-6 |
| Full-time equivalent of overtime and holiday hours |
1 |
1 |
0 |
| Average ES salary (in dollars) |
$0 |
$0 |
$0 |
| Average GM/GS grade |
12 |
12 |
0 |
| Average GM/GS salary (in dollars) |
$95,743 |
$96,102 |
$359 |
| Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) (in dollars) |
$0 |
$0 |
$0 |
| Average salary of ungraded positions (in dollars) |
93,544 |
95,321 |
1,777 |
Budget Authority by Object
(Dollars in thousands)
| Personnel Compensation |
FY 2012 Enacted |
FY 2013 PB |
Increase or Decrease |
| Full-time permanent (11.1) |
$37,300 |
$37,928 |
$628 |
| Other than full-time permanent (11.3) |
33,457 |
34,396 |
939 |
| Other personnel compensation (11.5) |
1,905 |
1,956 |
51 |
| Military personnel (11.7) |
1,979 |
2,064 |
85 |
| Special personnel services payments (11.8) |
11,988 |
12,334 |
346 |
| Total, Personnel Compensation |
$86,629 |
$88,678 |
$2,049 |
| Personnel benefits (12.0) |
$21,213 |
$21,726 |
$513 |
| Military personnel benefits (12.2) |
1,601 |
1,639 |
38 |
| Benefits for former personnel (13.0) |
0 |
0 |
0 |
| Subtotal, Pay Costs |
$109,443 |
$112,043 |
$2,600 |
| Travel and transportation of persons (21.0) |
$2,068 |
$1,944 |
-$124 |
| Transportation of things (22.0) |
214 |
179 |
-35 |
| Rental payments to GSA (23.1) |
0 |
0 |
0 |
| Rental payments to others (23.2) |
0 |
0 |
0 |
| Communications, utilities and miscellaneous charges (23.3) |
862 |
809 |
-53 |
| Printing and reproduction (24.0) |
444 |
421 |
-23 |
| Consulting services (25.1) |
1,051 |
1,051 |
0 |
| Other services (25.2) |
11,888 |
11,888 |
0 |
| Purchase of goods and services from government accounts (25.3) |
169,538 |
188,010 |
18,472 |
| Operation and maintenance of facilities (25.4) |
5,132 |
4,477 |
-655 |
| Research and development contracts (25.5) |
24,961 |
27,242 |
2,281 |
| Medical care (25.6) |
635 |
633 |
-2 |
| Operation and maintenance of equipment (25.7) |
3,770 |
3,591 |
-179 |
| Subsistence and support of persons (25.8) |
0 |
0 |
0 |
| Subtotal, Other Contractual Services (25.0) |
$216,975 |
$236,892 |
$19,917 |
| Supplies and materials (26.0) |
$14,578 |
$14,212 |
-$366 |
| Equipment (31.0) |
4,443 |
4,216 |
-227 |
| Land and structures (32.0) |
0 |
0 |
0 |
| Investments and loans (33.0) |
0 |
0 |
0 |
| Grants, subsidies and contributions (41.0) |
1,595,878 |
1,571,391 |
-24,487 |
| Insurance claims and indemnities (42.0) |
0 |
0 |
0 |
| Interest and dividends (43.0) |
0 |
0 |
0 |
| Refunds (44.0) |
0 |
0 |
0 |
| Subtotal, Non-Pay Costs |
$1,835,462 |
$1,830,064 |
-$5,398 |
| * Total Budget Authority by Object |
$1,944,905 |
$1,942,107 |
-$2,798 |
* Includes FTEs which are reimbursed from the NIH Common Fund.
Salaries and Expenses
(Dollars in thousands)
| OBJECT CLASSES |
FY 2012 Enacted |
FY 2013 PB |
Increase or Decrease |
| Personnel Compensation: Full-time permanent (11.1) |
$37,300 |
$37,928 |
$628 |
| Personnel Compensation: Other than full-time permanent (11.3) |
33,457 |
34,396 |
939 |
| Personnel Compensation: Other personnel compensation (11.5) |
1,905 |
1,956 |
51 |
| Personnel Compensation: Military personnel (11.7) |
1,979 |
2,064 |
85 |
| Personnel Compensation: Special personnel services payments (11.8) |
11,988 |
12,334 |
346 |
| Total Personnel Compensation (11.9) |
$86,629 |
$88,678 |
$2,049 |
| Civilian personnel benefits (12.1) |
$21,213 |
$21,726 |
$513 |
| Military personnel benefits (12.2) |
1,601 |
1,639 |
38 |
| Benefits to former personnel (13.0) |
0 |
0 |
0 |
| Subtotal, Pay Costs |
$109,443 |
$112,043 |
$2,600 |
| Travel (21.0) |
$2,068 |
$1,944 |
-$124 |
| Transportation of things (22.0) |
214 |
179 |
-35 |
| Rental payments to others (23.2) |
0 |
0 |
0 |
| Communications, utilities and miscellaneous charges (23.3) |
862 |
809 |
-53 |
| Printing and reproduction (24.0) |
444 |
421 |
-23 |
| Other Contractual Services: Advisory and assistance services (25.1) |
1,051 |
1,051 |
0 |
| Other Contractual Services: Other services (25.2) |
11,888 |
11,888 |
0 |
| Other Contractual Services: Purchases from government accounts (25.3) |
102,675 |
103,873 |
1,198 |
| Other Contractual Services: Operation and maintenance of facilities (25.4) |
5,132 |
4,477 |
-655 |
| Other Contractual Services: Operation and maintenance of equipment (25.7) |
3,770 |
3,591 |
-179 |
| Other Contractual Services: Subsistence and support of persons (25.8) |
0 |
0 |
0 |
| Subtotal Other Contractual Services |
$124,516 |
$124,880 |
$364 |
| Supplies and materials (26.0) |
$14,566 |
$14,200 |
-$366 |
| Subtotal, Non-Pay Costs |
$142,670 |
$142,433 |
-$237 |
| Total, Administrative Costs |
$252,113 |
$254,476 |
$2,363 |
Details of Full-Time Equivalent Employment (FTEs)
| OFFICE/DIVISION |
FY 2011 Actual Civilian |
FY 2011 Actual Military |
FY 2011 Actual Total |
FY 2012 Enacted Civilian |
FY 2012 Enacted Military |
FY 2012 Enacted Total |
FY 2013 PB Civilian |
FY 2013 PB Military |
FY 2013 PB Total |
| Office of the Director: Direct |
150 |
0 |
150 |
150 |
0 |
150 |
148 |
0 |
148 |
| Office of the Director: Reimbursable |
1 |
0 |
1 |
1 |
0 |
1 |
1 |
0 |
1 |
| Office of the Director: Total |
151 |
0 |
151 |
151 |
0 |
151 |
149 |
0 |
149 |
| Division of Diabetes, Endocrinology, and Metabolic Diseases: Direct |
27 |
2 |
29 |
27 |
2 |
29 |
27 |
2 |
29 |
| Division of Diabetes, Endocrinology, and Metabolic Diseases: Reimbursable |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Division of Diabetes, Endocrinology, and Metabolic Diseases: Total |
27 |
2 |
29 |
27 |
2 |
29 |
27 |
2 |
29 |
| Division of Digestive Diseases and Nutrition: Direct |
19 |
3 |
22 |
19 |
3 |
22 |
19 |
3 |
22 |
| Division of Digestive Diseases and Nutrition: Reimbursable |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Division of Digestive Diseases and Nutrition: Total |
19 |
3 |
22 |
19 |
3 |
22 |
19 |
3 |
22 |
| Division of Kidney, Urologic, and Hematologic Diseases: Direct |
20 |
0 |
20 |
20 |
0 |
20 |
20 |
0 |
20 |
| Division of Kidney, Urologic, and Hematologic Diseases: Reimbursable |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Division of Kidney, Urologic, and Hematologic Diseases: Total |
20 |
0 |
20 |
20 |
0 |
20 |
20 |
0 |
20 |
| Division of Nutrition Research Coordination: Direct |
8 |
0 |
8 |
8 |
0 |
8 |
8 |
0 |
8 |
| Division of Nutrition Research Coordination: Reimbursable |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Division of Nutrition Research Coordination: Total |
8 |
0 |
8 |
8 |
0 |
8 |
8 |
0 |
8 |
| Division of Extramural Activities: Direct |
53 |
2 |
55 |
53 |
2 |
55 |
52 |
2 |
54 |
| Division of Extramural Activities: Reimbursable |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Division of Extramural Activities: Total |
53 |
2 |
55 |
53 |
2 |
55 |
52 |
2 |
54 |
| Division of Intramural Research Programs: Direct |
331 |
12 |
343 |
331 |
12 |
343 |
328 |
12 |
340 |
| Division of Intramural Research Programs: Reimbursable |
9 |
0 |
9 |
9 |
0 |
9 |
9 |
0 |
9 |
| Division of Intramural Research Programs: Total |
340 |
12 |
352 |
340 |
12 |
352 |
337 |
12 |
349 |
| FTEs supported by funds from Cooperative Research and Development Agreements |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| * Office/Division Total |
618 |
19 |
637 |
618 |
19 |
637 |
612 |
19 |
631 |
* Includes FTEs which are reimbursed from the NIH Common Fund.
Average GM/GS Grade by Year
| FISCAL YEAR |
Average GS Grade |
| 2009 |
11.8 |
| 2010 |
11.9 |
| 2011 |
12 |
| 2012 |
12 |
| 2013 |
12 |
Detail of Positions
|
| GRADE |
FY 2011 Actual |
FY 2012 Enacted |
FY 2013 PB |
| Total, ES Positions |
0 |
0 |
0 |
| Total, ES Salary |
0 |
0 |
0 |
| GM/GS-15 |
41 |
41 |
41 |
| GM/GS-14 |
68 |
70 |
70 |
| GM/GS-13 |
87 |
85 |
84 |
| GS-12 |
65 |
68 |
71 |
| GS-11 |
39 |
42 |
36 |
| GS-10 |
0 |
0 |
0 |
| GS-9 |
30 |
27 |
32 |
| GS-8 |
24 |
24 |
22 |
| GS-7 |
16 |
15 |
11 |
| GS-6 |
2 |
2 |
2 |
| GS-5 |
5 |
3 |
2 |
| GS-4 |
0 |
0 |
0 |
| GS-3 |
0 |
0 |
0 |
| GS-2 |
0 |
0 |
0 |
| GS-1 |
0 |
0 |
0 |
| Subtotal |
377 |
377 |
371 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): Assistant Surgeon General |
0 |
0 |
0 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): Director Grade |
10 |
10 |
10 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): Senior Grade |
3 |
3 |
3 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): Full Grade |
5 |
5 |
5 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): Senior Assistant Grade |
1 |
1 |
1 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): Assistant Grade |
0 |
0 |
0 |
| Subtotal, Grades established by Act of July 1, 1944 (42 U.S.C. 207) |
19 |
19 |
19 |
| Ungraded |
248 |
248 |
248 |
| Total permanent positions |
392 |
392 |
386 |
| Total positions, end of year |
644 |
644 |
638 |
| * Total full-time equivalent (FTE) employment, end of year |
637 |
637 |
631 |
| Average ES salary |
0 |
0 |
0 |
| Average GM/GS grade |
12.0 |
12.0 |
12.0 |
| Average GM/GS salary |
95,743 |
95,743 |
96,102 |
* Includes FTEs which are reimbursed from the NIH Common Fund.
[1]Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, 2011.
[2] Levey AS, et al.
Ann Intern Med 150: 604-612, 2009.; U.S. Renal Data System, USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2011.
[3] NIDDK, NIH/DHHS. Kidney and urologic diseases statistics
(http://kidney.niddk.nih.gov/statistics/), 2010.
[4] Everhart JE, ed.
The Burden of Digestive Diseases in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Dept of Health and Human Services; 2008. NIH Pub 09-6433.
[5] Flegal KM, et al.
JAMA 303: 235-241, 2010.; Ogden CL, et al.
JAMA 303: 242-249, 2010.
[6] The Look AHEAD Research Group.
Arch Intern Med 17: 1566-1575, 2010.
[7] Isakova T, et al.
JAMA 305: 2432-2439, 2011.
[8] Sanyal AJ, et al.
New Engl J Med 362: 1675-1685, 2010.
[9] Ramsey BW, et al.
NEJM 365: 1663-1672, 2011.
[10] Round et al.
Science 332: 974-977, 2011.; Vaishnava S, et al.
Science 334: 255-258, 2011.; Muegge BD, et al.
Science 332:970-974, 2011.; Faith JJ, et al.
Science 333:101-104, 2011.; Saulneier DM, et al.
Gastroenterology 141: 1782-1791, 2011.
[11] Hall KD, et al.
Lancet 378: 826-837, 2011.
[12] Wang TJ, et al.
Nat Med 17: 448-453, 2011.
[13] Faul C, et al.
J Clin Invest 121: 4393-4408, 2011.; Wei C, et al.
Nat Med 17: 952-960, 2011.
[14] Choi JH, et al.
Nature 477: 477-481, 2011.; Lee JM, et al.
Nature 474: 506-510, 2011.
[15] Pipalia NH, et al.
PNAS 108: 5620-5625, 2011.
[16] Lavine JE, et al.
JAMA 305: 1659-1668, 2011.; Mitri J, et al.
Am J Clin Nutr 94: 486-494, 2011.; Feld JJ, et al.
Gastroenterology 140: 830-839, 2011.
[17] The DCCT/EDIC Research Group.
NEJM,
in press.
[18] Mitri J, et al.
Am J Clin Nutr 94: 486-494, 2011.
[19] Chen H, et al.
Nature 478: 349-355, 2011.
[20] Lee WM, et al.
Gastroenterology 137: 856-864, 2009.
[21] Faul C, et al.
J Clin Invest 121: 4393-4408, 2011.
[22] Hall KD, et al.
Lancet 378: 826-837, 2011.
[23] Kopp JB, et al.
J Am Soc Nephrol 22: 2129-2137, 2011.
[24] Neumann S, et al.
J Clin Endocrinol Metab 96: 548-554, 2011.
[25] Feld JJ, et al.
Gastroenterology 140: 830-839, 2011.
[26] Xu F, et al.
Science 332: 322-327, 2011.
[27] Smagulova F, et al.
Nature 473: 375-378, 2011.
Page last updated: February 13, 2012