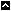National Toxicology Program (NTP) Division
The National Institute of Environmental Health Sciences (NIEHS) is home to the National Toxicology Program , an interagency program dedicated to testing and evaluating substances in our environment. The National Toxicology Program Division oversees and carries out the activities of the NTP.
The Division is responsible for carrying out the goals of NTP including:
- Providing toxicological evaluations on substances of public health concern
- Developing and validating improved toxicology methods (more sensitive, specific, and rapid)
- Developing approaches and generating data to strengthen the science base for risk assessments
- Communicating results with all stakeholders
As Director of NIEHS, Linda Birnbaum, Ph.D. is also the Director of the NTP. John Bucher, Ph.D, serves as Associate Director and provides daily oversight and guidance to the Program.
The Associate Director provides policy, analytical, and scientific leadership for the National Toxicology Program (NTP) and for planning, managing, and coordinating all of the NTP’s internal and external activities.
-

-
John R. Bucher, Ph.D. (http://www.niehs.nih.gov/research/atniehs/dntp/index.cfm)
Associate Director, National Toxicology Program
Director, National Toxicology Program Division -
Tel (919) 541-4532
Fax (919) 541-4255
bucher@niehs.nih.govCurriculum Vitae (245KB) -
P.O. Box 12233
Mail Drop K2-02
Research Triangle Park, North Carolina 27709
Delivery Instructions
In addition to holding the position of NTP associate director, John Bucher, Ph.D. serves as director of the National Toxicology Program Division.
Bucher joined the NTP as a toxicologist in 1983 and since then has played a key role in shaping the program’s research and policies. In 2007, he was named NTP Associate Director("/Rhythmyx/assembler/render?sys_contentid=17319&sys_revision=1&sys_variantid=610&sys_context=0&sys_authtype=0&sys_siteid=&sys_folderid=" sys_dependentvariantid="610" sys_dependentid="17319" inlinetype="rxhyperlink" rxinlineslot="103" sys_dependentid="17319" sys_siteid="" sys_folderid="") where he began to oversee the day to day operations of the program. Bucher has been instrumental in preparing the program to meet the needs of the 21th century. For example, in 2008 he coauthored a paper (249KB) in the journal, Science, which describes a way to transform toxicology by taking advantage of new technologies and shifting protocols for toxicity assessments from laboratory animal studies to more cell-based tests.
Bucher also played a major role in developing the NTP Vision and Roadmap for the 21st Century , a plan for toxicology research to advance as a predictive science, building on the knowledge gained from traditional single-agent studies primarily in rodents.
Bucher is an internationally recognized expert in the design and interpretation of cancer bioassays, and has authored a number of important publications examining critical issues in dose selection for toxicology and cancer studies. He holds a doctorate in pharmacology from the University of Iowa, a Masters of Science in biochemistry from the University of North Carolina at Chapel Hill, and a Bachelor of Arts in biology from Knox College in Galesburg, Illinois.
Branches and Laboratories
Biomolecular Screening Branch ("/Rhythmyx/assembler/render?sys_contentid=33796&sys_revision=14&sys_variantid=1278&sys_context=0&sys_authtype=0&sys_siteid=&sys_folderid=" sys_dependentvariantid="1278" sys_dependentid="33796" inlinetype="rxhyperlink" rxinlineslot="103" sys_dependentid="33796" sys_siteid="" sys_folderid="")
- Develops research and testing activities in high and medium throughput screening assays for rapid detection of biological activities of significance to toxicology and carcinogenesis
- Carries out automated screening assays with Caenorhabditis elegans
- Develops analysis tools and approaches to allow an integrated assessment of high throughput screening endpoints and associations with findings from traditional toxicology and cancer models
- Develops assays and approaches to understand the genetic and epigenetic bases for differences in susceptibility
Cellular and Molecular Pathology Branch
- Responsible for managing, evaluating, reviewing, and reporting all pathology data generated through conduct of NTP toxicity and carcinogenicity studies
- Establishes standards, terminology, and diagnostic criteria for rodent pathology
- Providing laboratory animal medicine support for NTP and the NIEHS Intramural Research Division
- Maintains the NTP Archives
- Manages pathology, toxicology, and other contracts to support NTP and DIR investigators. Staff veterinary scientists provide collaborative pathology diagnostic support for DIR investigators and mentoring/training in toxicologic pathology and laboratory animal medicine.
Program Operations Branch
- Provides recommendations to the NTP for scientific, administrative, and fiscal procedures and requirements by which NTP goals may be accomplished through in-house and contract activities
- Provides resources for analytical chemistry, toxicokinetics, and evaluations of bioavailability and biotransformation
- Initiates the contract award process and participates with NIEHS contracts office in the review and awarding of contracts to support NTP research and testing activities
- Manages toxicity and carcinogenicity studies performed under contract and monitors them for technical and fiscal performance
- Manages the receipt, maintenance, tracking, and dissemination of NTP documents and data
Toxicology Branch
- Responsible for the design, interpretation, review, and reporting of general toxicology and carcinogenicity studies usually in rodent models, as well as studies to evaluate targeted effects on the immune system, reproduction, development, and interference with chromosomes and DNA for substances studied by the NTP
- Integrates information derived from studies of absorption, metabolism, distribution, and excretion of test substances within the body and develops mathematical models that utilize this information in the extrapolation and prediction of findings across different species and exposure conditions
- Oversees analysis and development of mathematical models using information derived from studies of gene expression in different tissues
- Incorporates systems biology approaches
- Develops new methodologies for toxicological assessments
- Provides guidance on the proper utilization of toxicology information in hazard identification, hazard characterization, and regulatory decision-making
NTP Laboratory
- Provides laboratory capabilities and support for the performance of specific, targeted research on substances nominated to the NTP, issues of central importance to NTP programs, and the development of new methods for NTP research and testing
Recent Stories about NTP Activities
- NTP board gives go ahead for PAH research and systematic review (http://www.niehs.nih.gov/news/newsletter/2013/1/science-ntp/index.htm) - January 2013
- NIEHS/NTP celebrates 20-year partnership with FDA (http://www.niehs.nih.gov/news/newsletter/2012/12/spotlight-FDA/index.htm) - December 2012
- NIEHS pharmacologist Larry Hart remembered (http://www.niehs.nih.gov/news/newsletter/2012/12/spotlight-larryhart/index.htm) - December 2012
- NTP panel reviews outcomes of women treated for cancer while pregnant (http://www.niehs.nih.gov/news/newsletter/2012/11/science-ntp/index.htm) - November 2012
- NTP fellow wins 1st Place Young Investigator Award (http://www.niehs.nih.gov/news/newsletter/2012/8/spotlight-ntpfellow/index.htm) - August 2012
- NTP board supports systematic review, new carcinogen concepts (http://www.niehs.nih.gov/news/newsletter/2012/7/science-ntp/index.htm) - July 2012
- Enthusiasm for science reigns at SOT (http://www.niehs.nih.gov/news/newsletter/2012/4/spotlight-sot/index.htm) - April 2012
- Science showcases grantee and NIEHS/NTP tox efforts (http://www.niehs.nih.gov/news/newsletter/2012/4/science-science/index.htm) - April 2012
- Interagency Mineral Fibers Group Meets at NIEHS (http://www.niehs.nih.gov/news/newsletter/2012/3/science-mineral-fibers/index.htm) - March 2012
- NIEHS and NTP to lead events at 51st SOT meeting (http://www.niehs.nih.gov/news/newsletter/2012/3/spotight-sot/index.htm) - March 2012
- Panel peer reviews and approves seven NTP technical reports (http://www.niehs.nih.gov/news/newsletter/2012/3/science-ntp/index.htm) - March 2012
- NTP toxicologist Kamal Abdo remembered (http://www.niehs.nih.gov/news/newsletter/2012/2/spotlight-abdo/index.htm) - February 2012
- NTP advisor named as fellow of the Collegium Ramazzini - January 2012
- NTP board moves initiatives forward - January 2012
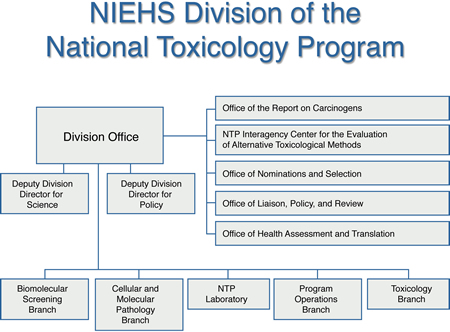
What is the NTP?
The National Toxicology Program (NTP) is an interagency program established in 1978 to coordinate toxicology research and testing across the Department of Health and Human Services (HHS). The program was also created to strengthen the science base in toxicology, develop and validate improved testing methods, and provide information about potentially toxic chemicals to health regulatory and research agencies, scientific and medical communities, and the public.
NTP is headquartered at the National Institute of Environmental Health Sciences (NIEHS). NIEHS is one of three core agencies that provide support for NTP activities. The other two agencies include:
- The U.S. Food and Drug Administration, primarily through its National Center for Toxicological Research (NCTR) .
- The Centers for Disease Control and Prevention, via the National Institute for Occupational Safety and Health (NIOSH) .
NIEHS provides leadership for the NTP. The NIEHS Director, Linda Birnbaum, Ph.D. ("/Rhythmyx/assembler/render?sys_contentid=1840&sys_revision=15&sys_variantid=639&sys_context=0&sys_authtype=0&sys_siteid=&sys_folderid=" sys_dependentvariantid="639" sys_dependentid="1840" inlinetype="rxhyperlink" rxinlineslot="103" sys_dependentid="1840" sys_siteid="" sys_folderid=""), also serves as the NTP Director. John Bucher, Ph.D. serves as the NTP Associate Director. To fulfill its goals, NTP employs a highly integrated, cooperative research and testing program that is accomlished through in-house research, research and development contracts, cooperative agreements, and other support mechanisms. NTP also has a strong training component in toxicology and pathology.
Toxicology Liaison
-

-
Christopher Weis, Ph.D., D.A.B.T. (http://www.niehs.nih.gov/about/od/advisor/weis/index.cfm)
Toxicology Liaison -
Tel (301) 496-3511
Fax (301) 496-0563
Christopher.Weis@nih.gov