Downloading Content for Analysis
This page is recommended for advanced users. It explains how to download study record data in Extensible Markup Language (XML) a machine-readable format, and in other data formats.
The structure of study records in XML is defined by this XML Schema.
Use of ClinicalTrials.gov data is subject to these Terms and Conditions.
Contents
- Download Study Information from a Search Results List
- Use URL Parameters to Display and Save XML Data
- Download Oracle Extracts From the Clinical Trials Transformation Initiative (CTTI) Database for Aggregate Analysis of ClinicalTrials.gov (AACT)
Download Study Information from a Search Results List
The Download option on the search results page is an advanced feature that allows you to download information about some or all of the study records shown in the search results:

Click on the Download link located in the upper right of the search results List tab. A form will appear, displaying the following download options:
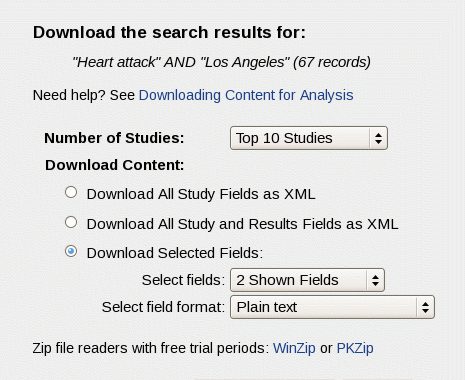
Number of Studies: Using the drop-down list, you can choose the number of records to download. Your options are:
- The first or top 10, 100, or 1000 studies retrieved by your search. These options will be not be available when the number of studies found for your search is less than the option. For example, if your search finds fewer than 1000 studies, the "Top 1000 Studies" option will not be available.
- The studies displayed on the current Search Results page, usually "20 Shown Studies"
- All studies retrieved by your search. For example, if your search finds 67 studies, the option will be "67 Found Studies".
Download Content:
Download Multiple Study Records in XML
Choose one of these options to download a separate XML file for each study:
- Download All Study Fields as XML. Choose this option to download only the protocol information (shown on the Full Text View tab on the study record page).
- Download All Study and Results Fields as XML. Some completed studies have results information posted on ClinicalTrials.gov (shown on the Study Results tab on the study record page). Choose this option to download both protocol and study results information when available. Note that many studies may not contain results information because this feature was added in September 2008, and results information is required only for some completed studies.
Download a List of Records
Choose this option to download a single list of records. You can decide what information about each study is included for each record.
- Download Selected Fields.
- Select Fields. Use the drop-down list to choose the study fields that you want to download from your search results list. You can choose to download either the Shown Fields on the screen or the 20 Available Fields. The default study fields shown are Condition and Intervention. To change which fields are shown in your search results, close the window you are in, click on the Show Display Options link (located above the list of search results), and then add or remove fields by marking the check-boxes.
- Select File Format. Use the drop-down list to select one of the following formats for your saved file:
- Plain text. Unformatted text that can be read in a simple editor, such as Notepad.
- Tab- and comma-separated values. Each study record is saved as a separate line in the file, with tabs or commas as delimiters, or spacers, between each field. These formats are useful for importing study information into spreadsheets or databases.
- XML. XML is a machine-readable format that will be most useful to advanced users.
The study records or list file will be downloaded as a Zip file or compressed package. Click on the Download Zip File button to save this file to your computer. (Sample Zip file readers with free trial periods: WinZip or PKZip)
Note: It may take several minutes to download a large number of studies.
Use URL Parameters to Display and Save XML Data
Display a Single Record in XML
To display an individual study protocol record in your browser in XML, add the URL parameter "displayxml=true" to the end of a "show study" URL:
Some completed studies have results information posted on ClinicalTrials.gov (See How to Find Results of Studies.) To include both protocol and results information, choose a study with posted results and use the URL parameter "resultsxml=true":
The resultsxml output is a superset of the protocol and study results XML. Do not include both the displayxml and resultsxml parameters in the URL.
Download Multiple Records in XML
Add the URL parameter "studyxml=true" to the end of a "search request" URL to immediately begin downloading protocol records in XML:
Some completed studies have results information posted on ClinicalTrials.gov (See How to Find Results of Studies.) To include both protocol and results information, use the URL parameter "resultsxml=true":
The resultsxml output is a superset of the protocol and study results XML. Do not include both the studyxml and resultsxml parameters in the URL.
Note: It may take several minutes to download a large number of studies.
Download Oracle Extracts From the Clinical Trials Transformation Initiative (CTTI) Database for Aggregate Analysis of ClinicalTrials.gov (AACT)
CTTI, a public-private partnership, has restructured and reformatted ClinicalTrials.gov data into a relational database under its Improving the Public Interface for Use of Aggregate Data in ClinicalTrials.gov project.
AACT provides Oracle extracts in two formats:
- dmp
- pipe-delimited text
Supporting documents, such as the High Level Data Dictionary and a document outlining points to consider, are also available on the AACT site.



