Forms/Instructions
Considerations for iPSC Research
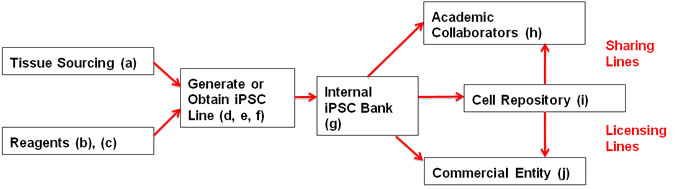
- NIH CRM Template Consent Form — Reference the NIH CRM template consent when collecting tissue for use in iPSC research to ensure that iPSC lines can be freely transferred among collaborators, used in future research, banked in a repository, etc. All materials need to be approved by your Institutional Review Board (IRB).
- Limited Use Label License (LULL) — Review all restrictions placed on cells derived using each reagent and ensure they do not overly restrict the future use of your work.
- Material Transfer Agreement (MTA) — Carefully review all MTAs to ensure you relevant issues are considered when obtaining iPSC lines and/or reagents used to generate/manipulate iPSCs.
- iPSC Checklist — Required registration document for IRP investigators that generate or obtain iPSC lines.
- Obtain Office of Human Subjects Research (OHSR) — OHSR review request form, If OHSR exemption is not an option, you will need to get IRB approval.
- Import Documentation — If a non-U.S. provider, fill in appropriate documentation to import biological materials — submit to NIH Quarantine Permit Service Office (Permit to Import Biological Agents or Vectors of Human Disease into the United States)
- NIH CRM Cell Deposit Form — For collection of information necessary when working with, sharing, and/or licensing iPSC lines.
- NIH CRM Material Transfer Agreement (MTA) — Reference when developing documentation for sharing your lines with academic/government/not-for-profit collaborators.
- NIH CRM Deposit Agreement — Reference when developing documentation for depositing lines at a cell bank/repository.
- NIH Biological Materials License Agreement (BLMA) — Reference when developing documentation licensing iPSC lines to commercial entities from the NIH intramural program.

