Highlights |
In situ neutron diffraction study of CO clathrate hydrate
- The structure of a CO clathrate hydrate has been studied for the first time using high-P low-T neutron diffraction.
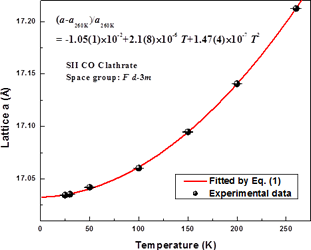
- Rietveld analysis shows that lattice parameter a (SII cubic clathrate structure) increases with increasing temperature.
- CO molecules are positionally disordered and off-centered in both large and small cages.
- Each large cage is occupied by two CO molecules while each small cage is occupied by one CO.
 |
 |
|
A representative neutron diffraction pattern of SII CO clathrate hydrate. |
Variation of lattice parameter a of CO SII clathrate hydrate as a function of temperature. |
|
|
1) Clathrate hydrates are ice-like solids in which gas molecules are physically trapped in the frameworks. These phases occur on the deep ocean floor (a potential energy resource) and as permafrost, and can form inside gas pipelines (causing plug problems for the petroleum industry). Thus studying their structures and formation kinetics is important.
2) A carbon monoxide clathrate hydrate (deuterated) has been studied for the first time using neutron diffraction. Structural parameters of this phase, which has a cubic SII clathrate structure, from 25 to 260 K were determined from Rietveld analysis of neutron diffraction data.
3) Lattice parameter a of CO SII clathrate increases with increasing temperature, which can be fitted to a two-order polynomial thermal expansion relation.
4) CO molecules are positionally disordered and off-centered in both large and small cages. Each large cage is occupied by two CO molecules while each small cage is occupied by one CO. In addition, the CO molecules in small cages appear to be more confined/localized around the cage centers.


 large cage
large cage small cage
small cage