Congressional Justification 2010
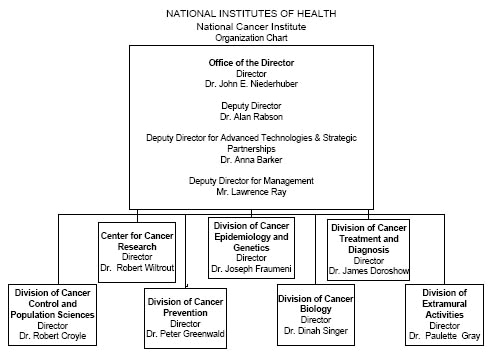
Organization Chart
Top
Appropriation Language
For carrying out Section 301 and title IV of the Public Health Service Act with respect to cancer, [$4,968,973,000] $5,150,170,000, of which up to $8,000,000 may be used for facilities repairs and improvements at the National Cancer Institute-Frederick Federally Funded Research and Development Center in Frederick, Maryland (Department of Health and Human Services Appropriation Act, 2009).
Top
Amounts Available for Obligation
1
| Source of Funding |
FY 2008 Actual |
FY 2009 Estimate |
FY 2010 Estimate |
| Appropriation |
$4,890,525,000 |
$4,968,973,000 |
$5,150,170,000 |
| Rescission |
-85,437,000 |
0 |
0 |
| Supplemental |
25,559,000 |
0 |
0 |
| Subtotal, adjusted appropriation |
4,830,647,000 |
4,968,973,000 |
5,150,170,000 |
| Real transfer under Director's one-percent transfer authority (GEI) |
-3,091,000 |
0 |
0 |
| Comparative transfer under Director's one-percent transfer authority (GEI) |
3,091,000 |
0 |
0 |
| Subtotal, adjusted budget authority |
4,830,647,000 |
4,968,973,000 |
5,150,170,000 |
| Unobligated balance, start of year |
0 |
0 |
0 |
| Unobligated balance, end of year |
0 |
0 |
0 |
| Subtotal, adjusted budget authority |
4,830,647,000 |
4,968,973,000 |
5,150,170,000 |
| Unobligated balance lapsing |
-4,000 |
0 |
0 |
| Total obligations |
4,830,643,000 |
4,968,973,000 |
5,150,170,000 |
Top
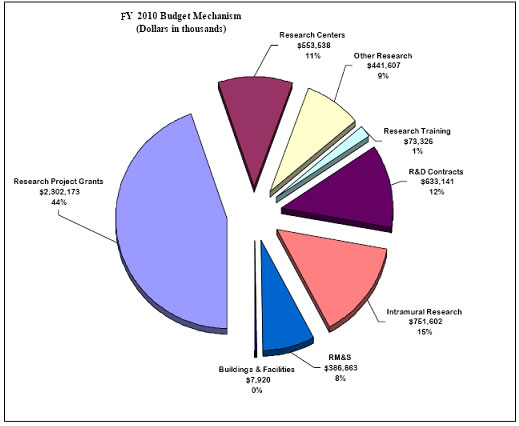
Budget Mechanism Table
| MECHANISM |
FY 2008
Actual |
FY 2009
Estimate |
FY 2010
Estimate |
Change |
| Research Grants: |
No. |
Amount |
No. |
Amount |
No. |
Amount |
No. |
Amount |
| Research Projects: |
|
| Noncompeting |
3,876 |
$1,569,207 |
3,653 |
$1,573,380 |
3,751 |
$1,593,306 |
98 |
$19,926 |
| Administrative supplements |
(266) |
22,665 |
(276) |
27,000 |
(280) |
29,500 |
(4) |
2,500 |
| Competing: |
|
| Renewal |
303 |
161,691 |
303 |
154,494 |
339 |
173,033 |
36 |
18,539 |
| New |
962 |
293,319 |
1,108 |
362,796 |
1,217 |
408,831 |
109 |
46,035 |
| Supplements |
19 |
1,634 |
1 |
57 |
1 |
64 |
0 |
7 |
| Competing |
1,284 |
456,644 |
1,412 |
517,347 |
1,557 |
581,928 |
145 |
64,581 |
| Subtotal, RPGs |
5,160 |
2,048,516 |
5,065 |
2,117,727 |
5,308 |
2,204,734 |
243 |
87,007 |
| SBIR/STTR |
312 |
97,439 |
284 |
91,439 |
297 |
97,439 |
13 |
6,000 |
| Subtotal, RPGs |
5,472 |
2,145,955 |
5,349 |
2,209,166 |
5,605 |
2,302,173 |
256 |
93,007 |
| Research Centers: |
|
| Specialized/comprehensive |
195 |
512,290 |
200 |
528,590 |
208 |
552,590 |
8 |
24,000 |
| Clinical research |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Biotechnology |
0 |
948 |
0 |
948 |
0 |
948 |
0 |
0 |
| Comparative medicine |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Research Centers in Minority Institutions |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Subtotal, Centers |
195 |
513,238 |
200 |
529,538 |
208 |
553,538 |
8 |
24,000 |
| Other Research: |
|
| Research careers |
525 |
79,528 |
525 |
81,928 |
532 |
85,178 |
7 |
3,250 |
| Cancer education |
82 |
30,089 |
82 |
30,989 |
84 |
32,239 |
2 |
1,250 |
| Cooperative clinical research |
133 |
235,832 |
133 |
242,832 |
135 |
252,832 |
2 |
10,000 |
| Biomedical research support |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| Minority biomedical research support |
0 |
1,360 |
0 |
1,400 |
0 |
1,440 |
0 |
40 |
| Other |
162 |
64,933 |
162 |
66,848 |
168 |
69,918 |
6 |
3,070 |
| Subtotal, Other Research |
902 |
411,742 |
902 |
423,997 |
919 |
441,607 |
17 |
17,610 |
| Total Research Grants |
6,569 |
3,070,935 |
6,451 |
3,162,701 |
6,732 |
3,297,318 |
281 |
134,617 |
| Research Training: |
FTTPs |
FTTPs |
FTTPs |
|
| Individual awards |
233 |
10,020 |
238 |
10,320 |
242 |
10,530 |
4 |
210 |
| Institutional awards |
1,281 |
59,881 |
1,306 |
61,581 |
1,330 |
62,796 |
24 |
1,215 |
| Total, Training |
1,514 |
69,901 |
1,544 |
71,901 |
1,572 |
73,326 |
28 |
1,425 |
| Research & development contracts |
460 |
586,776 |
470 |
605,636 |
488 |
633,141 |
18 |
27,505 |
| (SBIR/STTR) |
(38) |
(7,757) |
(80) |
(16,500) |
(80) |
(16,500) |
(0) |
(0) |
| |
FTEs |
FTEs |
FTEs |
FTEs |
| Intramural research |
1,841 |
723,602 |
1,874 |
740,602 |
1,912 |
751,602 |
38 |
11,000 |
| Research management and support |
1,041 |
371,513 |
1,059 |
380,213 |
1,080 |
386,863 |
21 |
6,650 |
| Construction |
0 |
0 |
0 |
0 |
| Buildings and Facilities |
7,920 |
7,920 |
7,920 |
0 |
| Total, NCI |
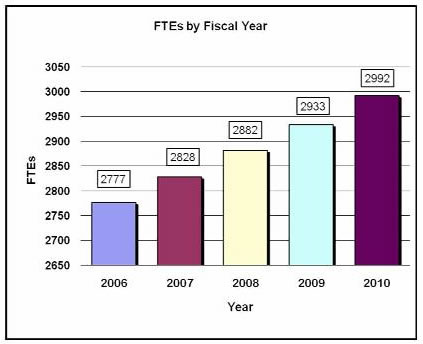
2,882 |
4,830,647 |
2,933 |
4,968,973 |
2,992 |
5,150,170 |
59 |
181,197 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research.
Top
Budget Authority by Activity
NCI BA by Program
(Dollars in Thousands)
| |
FY 2006
Actual |
FY 2007
Actual |
FY 2008
Actual |
FY 2008
Comparable |
FY 2009
Estimate |
FY 2010
Estimate |
Change |
Extramural Research
Detail: |
FTEs |
Amount |
FTEs |
Amount |
FTEs |
Amount |
FTEs |
Amount |
FTEs |
Amount |
FTEs |
Amount |
FTEs |
Amount |
| Understand the Mechanisms of Cancer |
|
$795,035 |
|
$796,449 |
|
$810,147 |
|
$813,298 |
|
$855,160 |
|
$904,314 |
|
$49,154 |
| Understand the Causes of Cancer |
|
1,085,449 |
|
1,094,106 |
|
1,082,098 |
|
1,082,098 |
|
1,115,146 |
|
1,150,016 |
|
34,870 |
| Improve Early Detection and Diagnosis |
|
406,767 |
|
410,618 |
|
387,125 |
|
387,125 |
|
381,767 |
|
390,239 |
|
8,472 |
| Develop Effective and Efficient Treatments |
|
1,123,239 |
|
1,146,272 |
|
1,186,919 |
|
1,186,919 |
|
1,238,275 |
|
1,291,095 |
|
52,820 |
| Cancer Prevention and Control |
|
350,619 |
|
333,224 |
|
325,222 |
|
325,222 |
|
317,060 |
|
318,277 |
|
1,217 |
Cancer Centers, Specialized Centers and
SPOREs |
|
463,860 |
|
471,669 |
|
477,034 |
|
477,034 |
|
488,614 |
|
510,703 |
|
22,089 |
| Research Workforce Development |
|
182,015 |
|
179,155 |
|
179,518 |
|
179,518 |
|
184,818 |
|
190,743 |
|
5,925 |
| Buildings and Facilities |
|
7,920 |
|
7,920 |
|
7,920 |
|
7,920 |
|
7,920 |
|
7,920 |
|
|
| Subtotal, Extramural* |
|
4,414,904 |
|
4,439,413 |
|
4,455,983 |
|
4,459,134 |
|
4,588,760 |
|
4,763,307 |
|
174,547 |
| Intramural research (non-add) |
1,766 |
691,721 |
1,811 |
706,179 |
1841 |
724,086 |
1,841 |
723,602 |
1,874 |
740,602 |
1,912 |
751,602 |
38 |
11,000 |
|
Res. management & support |
1,011 |
339,221 |
1,017 |
353,211 |
1041 |
371,573 |
1,041 |
371,513 |
1,059 |
380,213 |
1,080 |
386,863 |
21 |
6,650 |
|
TOTAL |
2,777 |
4,754,125 |
2,828 |
4,792,624 |
2,882 |
4,827,556 |
2,882 |
4,830,647 |
2,933 |
4,968,973 |
2,992 |
5,150,170 |
59 |
181,197 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research.
*The detail programs listed above include both extramural and intramural funding.
Top
Major Changes in Budget Request
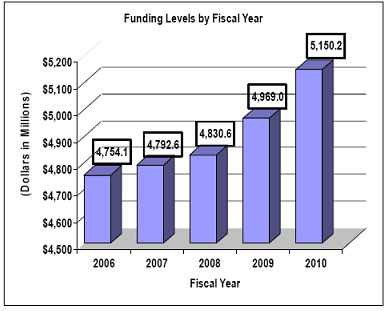
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2010 budget request for NCI, which is $181.197 million more than the FY 2009 Estimate, for a total of $5,150.170 million.
Research Project Grants (+$87.007 million, total $2,204.734 million). NCI will support a total of 5,308 Research Project Grant (RPG) awards (not including SBIR/STTR) in FY 2010. Noncompeting RPGs will increase by 98 awards and $19.926 million. Competing RPGs will increase by 145 awards and $64.581 million. The NIH budget policy for RPGs in FY 2010 is a 2% inflationary increase in noncompeting awards and 2% increase in average cost for competing RPGs.
Understand the Mechanisms of Cancer. NCI will increase its Understanding the Mechanisms of Cancer program by $49.154 million to $904.314 million, a 5.7 percent increase above the FY 2009 estimate. To support the program goals, high priority will be given to expanding the TCGA from the pilot phase targeting brain, ovarian and lung cancer to include other tumor types.
Develop Effective and Efficient Treatments. NCI will increase its Developing Effective and Efficient Treatments program by $52.820 million to $1,291.095 million, a 4.3 percent increase above the FY 2009 estimate. A high priority will be placed on developing an integrated Drug Development Platform that will use the knowledge of the genomic and functional changes associated with tumors to identify specific targets for further development.
Top
Summary of Changes
| FY 2009 estimate |
$4,968,973,000
|
| FY 2010 estimated budget authority |
5,150,170,000 |
Net change |
181,197,000 |
| |
2009 Current
Estimate Base |
Change from Base |
| CHANGES |
FTEs |
Budget
Authority |
FTEs |
Budget
Authority |
| A. Built-in: |
|
| 1. Intramural research: |
|
| a. Annualization of January 2009 pay increase |
|
$302,365,000 |
|
$3,613,000 |
| b. January FY 2010 pay increase |
|
302,365,000 |
|
4,535,000 |
| c. Zero less days of pay |
|
302,365,000 |
|
0 |
| d. Payment for centrally furnished services |
|
109,589,000 |
|
2,192,000 |
| e. Increased cost of laboratory supplies, materials, and other expenses |
|
328,648,000 |
|
5,264,000 |
| Subtotal |
|
15,604,000 |
| 2. Research management and support: |
|
| a. Annualization of January 2009 pay increase |
|
$153,271,000 |
|
$1,832,000 |
| b. January FY 2010 pay increase |
|
153,271,000 |
|
2,299,000 |
| c. Zero less days of pay |
|
153,271,000 |
|
0 |
| d. Payment for centrally furnished services |
|
28,516,000 |
|
570,000 |
| e. Increased cost of laboratory supplies, materials, and other expenses |
|
198,426,000 |
|
3,237,000 |
| Subtotal |
|
7,938,000 |
| |
| Subtotal, Built-in |
|
23,542,000 |
| |
2009 Current
Estimate Base |
Change from Base |
| CHANGES |
No. |
Amount |
No. |
Amount |
| B. Program: |
|
| 1. Research project grants: |
|
| a. Noncompeting |
3,653 |
$1,600,380,000
|
98 |
$22,426,000 |
| b. Competing |
1,412 |
517,347,000 |
145 |
64,581,000 |
| c. SBIR/STTR |
284 |
91,439,000 |
13 |
6,000,000 |
| Total |
5,349 |
2,209,166,000 |
256 |
93,007,000 |
| 2. Research centers |
200 |
529,538,000 |
8 |
24,000,000 |
| 3. Other research |
902 |
423,997,000 |
17 |
17,610,000 |
| 4. Research training |
1,544 |
71,901,000 |
28 |
1,425,000 |
| 5. Research and development contracts |
470 |
605,636,000 |
18 |
27,505,000 |
| Subtotal, extramural |
|
163,547,000 |
| |
FTEs |
|
FTEs |
|
| 6. Intramural research |
1,874 |
740,602,000 |
38 |
(4,747,000) |
| 7. Research management and support |
1,059 |
380,213,000 |
21 |
(1,288,000) |
| 8. Construction |
|
0 |
|
0 |
| 9. Buildings and Facilities |
|
7,920,000 |
|
0 |
| Subtotal, program |
|
4,968,973,000 |
|
157,512,000 |
| Total changes |
2,933 |
|
59 |
181,054,000 |
Top
Budget Graphs
Fiscal Year 2010 Budget Graphs
History of Budget Authority and FTEs:
Distribution by Mechanism:
Change by Selected Mechanism:
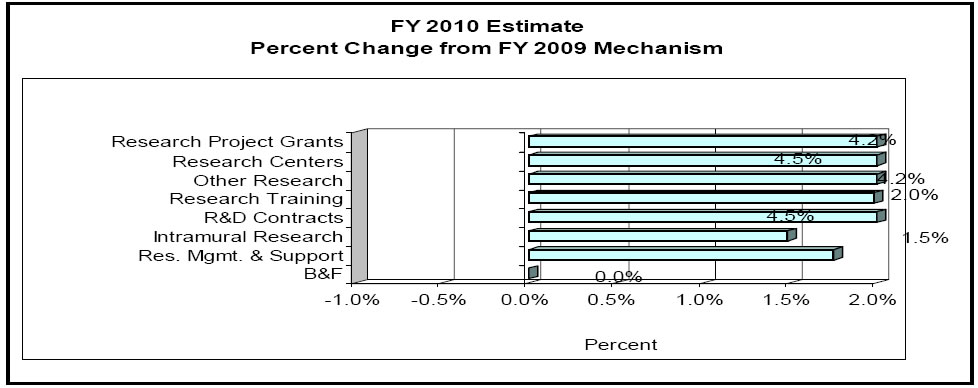
Top
Justification Narrative
National Cancer Institute
Authorizing Legislation: Section 301 and Title IV of the Public Health Service Act, as amended.
Budget Authority
|
FY 2008
Appropriation
______________ |
FY 2009
Omnibus
______________ |
FY 2009
Recovery Act
______________ |
FY 2010 President's
Budget
______________ |
FY 2010 +/- 2009
Omnibus
______________ |
| BA |
$4,830,647,000 |
$4,968,973,000 |
$1,256,517,000 |
$5,150,170,000 |
$181,197,000 |
| FTE |
2,882 |
2,933 |
0 |
2,992 |
59 |
This document provides justification for the Fiscal Year (FY) 2010 activities of the National Cancer Institute (NCI), including HIV/AIDS activities. Details of the FY 2010 HIV/AIDS activities are in the “Office of AIDS Research (OAR)” Section of the Overview. Details on the Common Fund are located in the Overview, Volume One. Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
In FY 2009, a total of $1,256,517,000 American Recovery and Reinvestment Act (ARRA) funds were transferred from the Office of the Director. These funds will be used to support scientific research opportunities that help support the goals of the ARRA. The ARRA allows NIH to execute these funds via any NIH funding mechanism. Funds are available until September 30, 2010. These funds are not included in the FY 2009 Omnibus amounts reflected in this document.
DIRECTOR'S OVERVIEW
As we gain a greater understanding of the genomic changes that underlie the development and metastasis of cancer, we have grown increasingly aware of the immense complexity of cancer and the considerable barriers that must be overcome in order to cure or control its progression. This continuous expansion of knowledge is giving us deeper insight into the relationship of a tumor to its microenvironment, the role of cancer stem cells, the intra- and inter-cellular communication pathways and the mechanisms driving metastasis. This expansion of information is increasingly informing new means by which cancer can be prevented, diagnosed and treated.
The NCI, as the leader of the National Cancer Program, is investing in an extensive strategic program based upon a drug development platform that takes our improved understanding of the cancer process and moves our discoveries as rapidly as possible into the carefully characterized and specifically selected patient. The progress that is being made is opening up a vision for a future of personalized cancer medicine; a time when doctors will determine risk for disease, prognosis if disease occurs, and treatment options that are pharmacogenetically based on understanding each patient’s unique genetic makeup and the genetic aberrations that have led to his or her cancer. The opportunities for significant advances based upon the increased understanding of the genomic changes have never been greater.
NCI is focusing to link research teams, not just biologists but also chemists and physicists, to create new bioinformatics infrastructures that will allow researchers to share data, resources and tools; to employ advanced technologies like subcellular imaging to map molecular interactions and pathways associated with disease; and to bring the cutting-edge cancer advances to the patients in the communities where they live and work.
NCI is enabling the transition of knowledge from the laboratory to a patient by rapidly identifying those cancer-specific genetic changes that are the best targets for drug development, by optimizing these drugs to be more effective, less toxic, and by moving the most promising agents into first in man studies. The NCI’s unique contribution rests not only in its capacity to conduct pioneering research, but in translating those findings to patient benefit at the NIH Clinical Research Center and through its unique national Cancer Centers Program.
NCI plays a unique role, not only as the leader, but as the unifier by fostering innovative partnerships and programs, bringing together the public, private and academic sectors, diverse scientific disciplines, and stakeholders, to hasten the pace of our scientific progress and dramatically alter the impact of cancer.
Program Activities in 2009:
- Empowering Cancer Research. Advances in our understanding of the molecular causes of cancer and the enhanced ability to translate that knowledge into clinical practice have opened up a future of personalized cancer medicine when doctors will determine prognosis and treatment options by understanding each patient's unique genetic makeup that have led to his or her cancer. This future is evident in today's efforts around the discovery of biomarkers to detect and measure changes in protein and cellular function associated with specific cancers and to use these profiles for prognosis and therapy.
- Reaching All Communities Touched by Cancer. NCI worked to make significant progress to lessen the cancer care disparity gap and bring the latest scientific advances and the highest level of multi-specialty care to a much larger population of cancer patients through launch of the NCI Community Cancer Centers Program (NCCCP).
- Realizing the Promise of Prevention and Early Detection. For most cancers there exists a mix of inherited genetic alterations and potential environmental influences that challenge simple prevention strategies. Part of the prevention solution involves identifying biological markers predicting risk of developing cancer through genome-wide association studies such as the Cancer Genetic Markers of Susceptibility (CGEMs) initiative.
FY 2010 Justification by Activity Detail
The FY 2010 Budget reflects the President’s prioritization of biomedical research supported by NIH. The Budget is the first year of an eight-year strategy to double the NIH-wide cancer research budget and includes over $6 billion for this purpose. The Budget balances the President’s commitment to cancer research with that of research in other areas.
NIH’s FY 2010 Budget will build upon the unprecedented $10 billion provided in the American Recovery and Reinvestment Act of 2009, which will support new NIH research on a wide array of diseases, condition, and disorders in 2009 and 2010.
Because cancer research involves the dissection and understanding of perhaps the most basic functions of human cell growth and differentiation, cancer research will always produce many serendipitous discoveries. Such discoveries involving the most basic properties of human cells have historically contributed to our understanding of the basic biology underlying almost all diseases.
In addition, cancer research also involves technology development that will benefit research in a number of disease areas. For example, cancer research includes a major effort to understand the complete genetic alterations that result in abnormal cell growth. This effort in whole genome sequencing is a major driver in the development of sequencing technology that we believe will lead to our ability in the next 2-3 years to perform whole genome sequencing in a matter of hours for less than $1,000. This effort in cancer will completely change the way all chronic diseases are diagnosed and managed. NCI’s efforts to generate the cancer electronic health record will be a model for the national plan to computerize all health records. In addition, the development of advanced imaging technologies to look into subcellular spaces to identify and actually visualize protein–protein interactions will have a major impact on drug development. Other efforts in whole cell imaging will refine diagnosis and, therefore tailor treatments. Such advances in imaging technology will benefit research on many diseases including Alzheimer’s Disease and Parkinson’s, just to name a few.
Research supported by the NCI Alliance for Nanotechnology in Cancer also involves developing technologies for targeted treatments that involve micro-systems that deliver drugs only to the disease site. Once again, the development of such targeted therapy modalities, including systems that can penetrate the blood-brain barrier to deliver drugs to brain tumors as well as other brain cells involved in a range of neurological disorders, will benefit the development of treatment strategies and possibilities for numerous diseases.
Numerous other Institutes and Centers contribute their expertise to fundamental research on biological processes, technologies, and tools, and work collaboratively with NCI to fund important research in cancer. For example, much of what has been learned at NCI in controlling tobacco usage is now being applied to study and address the growing health burden of obesity. NIH will work to ensure that cancer research resources are allocated responsibly, effectively, in accordance with peer review principles, and on the basis of sound science and cancer relevance.
Program Descriptions and Accomplishments
NCI will lead the NIH-wide effort to double cancer research over eight years. NCI will also play a leading role in the effort to draft a corresponding strategic plan identifying how NIH-wide cancer resources will be allocated--carefully monitored for cancer relevance and transparently report as currently done at NCI. As such, NCI’s 2010 priorities will be factored into this strategic and overarching effort. While decisions regarding the strategic plan and its corresponding priorities are not yet finalized, examples of projects NCI has prioritized in 2010 include:
- Drug Development Platform. Knowledge of the molecular mechanisms underlying cancer will certainly result in therapeutic solutions aimed at targets from the genomic and functional changes associated with specific tumors. With expansion of genome-wide association studies, The Cancer Genome Atlas (TCGA), and the Therapeutically Applicable Research to Generate Effective Treatments (TARGET) for childhood cancers, identification of these potential targets will be greatly accelerated. With initiatives such as the Chemical Biology Consortium (CBC) and the Rapid Access to Intervention Development (RAID) program, NCI is ideally positioned to enable a new drug development paradigm. Working across the entire drug development process, NCI is seeking to ensure that scientific discoveries are rapidly translated into improved patient outcomes. To accelerate this process of new treatments NCI is seeking to develop partnerships across the research community.
- New Clinical Trials Structure. The conduct of clinical research has remained mostly unchanged for the last 50 years. NCI will invest in redesigning the clinical trial infrastructure to harmonize and standardize clinical protocols to enable data sharing across studies and centralizing approval procedures to increase patient accrual and accelerate studies. The ability to characterize individual tumors will be facilitated by establishment of national certified tumor characterization labs.
- BIG HealthTM. By extending the process for managing data and information, adding procedures to accommodate live patient data, and expanding the community of users to consumers, payers, and local physicians, the BIG HealthTM consortium will initiate a new era and bring together, in a novel organizational framework, a continuum of information management empowering personalized medicine.
- Physical Sciences Oncology Centers. Establishment of these centers will build for the first time, new teams and fields of study in which physical scientists working closely with basic and clinical cancer researchers will catalyze the fundamental understanding of the physical and chemical forces, such as thermodynamics and chemical gradients, that govern the behavior of cancer at all levels.
Overall Budget Policy: NCI’s highest priorities for FY 2010 are to create an extensive strategic program based upon patient characterization and a drug development platform that takes our rapidly expanding knowledge of the genomic changes that underlie the development and metastasis of cancer and moves those discoveries into preventive, diagnostic and therapeutic interventions for the cancer patient. The progress that is being made is opening up a vision and creating an opportunity for personalized cancer medicine; a time when doctors will determine risk for disease, prognosis if disease occurs, and treatment options that are pharmacogenetically based on the understanding of each individual patient’s unique genetic makeup and the genetic aberrations that have led to his or her cancer. At the same time, NCI will continue to support a critical need for our country--the training and mentoring of new investigators, who will invigorate our country’s scientific capacity for years to come.
The major portion of NCI’s budget is allocated to support scientists within the nation’s research community at universities, teaching hospitals, and cancer centers dispersed throughout the country. These extramural investigators submit proposals that contain their best scientific thinking that address all aspects of the cancer research continuum. The NCI Intramural Research Program also encompasses laboratory investigation, epidemiologic and genetics studies, translational research, and clinical research. The excellence of NCI scientists and the intramural infrastructure enable the Institute to conduct high-risk and distinctive research, broadly distribute advanced technologies, and forge partnerships with public, private and academic institutions. Cancer patients benefit from access to clinical research protocols and treatments conducted at the Sen. Mark Hatfield NIH Clinical Research Center.
NCI will continue to support new investigators and to maintain an adequate number of competing RPGs. NCI is providing a 2 percent inflationary increase for non-competing and competing grants. Intramural Research and Research Management and Support receive an increase to help cover the cost of pay and other increases.
Understand the Mechanisms of Cancer
Expanding our knowledge of the genomic changes that underlie the development and metastasis of cancer is a keystone of NCI’s research mission. This unparalleled explosion of information is increasingly informing new means by which cancer can be prevented, diagnosed and treated. Emerging technologies that enable comprehensive molecular analysis of tumors and the tumor environment will help researchers understand the processes involved in the initiation and progression of specific types of cancer. Research to unravel the molecular complexities of cancer demands a multidisciplinary approach that draws on the strengths of both the intramural and extramural research communities.
Significant findings came from the first tumor type studied in The Cancer Genome Atlas (TCGA), glioblastoma. Three previously unrecognized mutations that occur with significant frequency were discovered, as well as the delineation of pathways disrupted in this type of brain cancer. One of the most exciting results is an unexpected observation that points to a potential mechanism of resistance to a common chemotherapy drug used for brain cancer.
Budget Policy: The FY 2010 budget estimate for the Understanding the Mechanisms of Cancer program is $904.314 million, an increase of $49.154 million or 5.7 percent above the FY 2009 estimate. To support the program goals, high priority will be given to expanding the TCGA from the pilot phase targeting brain, ovarian and lung cancer to include other tumor types. A new initiative will study stem cell biology as it relates to cancer. The initiative involves developing methods for isolating and culturing cancer stem cells as well as developing technologies such as imaging relevant to study stem cell biology. NCI is also planning to establish Physical Sciences Oncology Centers that will build new teams and fields of study in which physical scientists working closely with basic and clinical cancer researchers. will catalyze the fundamental understanding of the physical and chemical forces that govern the behavior of cancer at all levels.
Other activities include: (1) Tumor Microenvironment Network (TMEN) initiative designed to gain a comprehensive understanding of the stroma (the supporting connective tissue of an organ), in normal tissues as well as its role in tumorigenesis; (2) Developing high-resolution electronic microscopic imaging to look at the subcellular molecular mechanisms involved in cancer biology; (3) Establishing a patient characterization center to coordinate complex analysis on biospecimens, creating patient profiles to inform disease management; and (4) The Integrative Cancer Biology Program (ICBP) which uses a systems approach focusing on the analysis of cancer as a complex biological system using computational models of processes relevant to cancer biology.
Physical Sciences-Oncology Centers
FY 2009 Level: $0
FY 2010 Level: $18 million
Change: $18 million
Through a series of very successful workshops, the NCI is exploring new and innovative approaches to better understand and control cancer through initiatives that enable the convergence of the physical sciences with cancer biology. Building on the progress in molecular biology, rapidly advancing technologies that enable whole genome sequencing in real time, and new applied advanced technologies, NCI will develop innovative hypotheses and new fields of study based on the application of theoretical physics, advanced applied mathematics, physical chemistry and bioengineering to address questions in cancer research. NCI plans to join these often disparate areas of science and establish trans-disciplinary teams through the award of research grants to Physical Sciences-Oncology Centers to better understand the physical laws and principles that shape and govern the emergence and behavior of cancer at all scales.
Cancer research has moved from disrupting a single gene, protein or pathway to studying the disease in a systems biology approach, yet the underlying physics driving these perturbations remains virtually unexplored or understood. Given the complexity of cancer, it is becoming increasingly clear that external forces and physical laws have profound influences on cancer behavior at all levels--initiation, invasion and metastasis. Novel conceptual approaches using thermodynamics and biomechanics, principles of sub-molecular information coding, and evolutionary concepts hold a promise to produce a comprehensive understanding of cancer.
Working in close conjunction with the cancer research communities, new scientific teams will test novel cancer concepts by challenging ‘accepted’ dogma, generating physical measurements and integrating that information with existing knowledge, and developing dynamic theoretical physic constructs that are predictive of cancer across multiple scales of energy, space and time. These new Centers will be integrated with NCI’s very successful centers in nanobiology, systems biology and proteomics. The research will be used to generate new perspective strategies for cancer treatment, diagnosis and prevention.
Understand the Causes of Cancer
NCI’s etiology research focuses on genetic, environmental, and lifestyle factors, which contribute to cancer development. The study of genetic and environmental risk factors for cancer has advanced considerably in the past year. An important opportunity has been created through NCI’s sustained investment in collaborative population studies that help researchers exploit the potential of genetics and identify gene variants that affect cancer risk, diagnosis, and prognosis.
A recent discovery from the Genome-Wide Association Studies (GWAS) showed a region of chromosome 15 may contain genetic risk factors for lung cancer. The results from the first GWAS identify the genetic component of disease that is closely associated with a strong environmental cause. The study provides strong evidence for a link between DNA variants on chromosome 15 and lung cancer that now allow further exploration.
Budget Policy: The FY2010 budget estimate for the Understanding the Causes of Cancer program is $1,150.016 million, an increase of $34.870 million or 3.1 percent above the FY 2009 estimate. To support the program goals, high priority will be given to expanding programs to identify those genetic changes associated with cancer susceptibility. Under the auspices of the NIH Genes and Environment Initiative (GEI), a GWAS will be conducted to investigate the genetic determinants of lung cancer risk and identify genes that contribute to smoking persistence and different lung cancer outcomes.
Other activities include: (1) Evaluating the role of genetic susceptibility, environmental exposures, and gene-environment interactions in cancer risk through international consortia, including the Cohort Consortium, InterLymph, and the Childhood Cancer Survivor Study; (2) Supporting novel molecular research through the Epigenetic Approaches in Cancer Epidemiology Initiative, with the key objective of using population studies to evaluate cancer risk associated with DNA methylation 1; and (3) Conducting innovative research in genetics, imaging, and cancer molecular signatures to better understand the relationships between aging and the development and progression of cancer.
Improve Early Detection and Diagnosis
Accurate tools for detecting and diagnosing tumors can markedly improve the likelihood of a cancer patient’s successful treatment and survival. These tools are of greatest benefit early in the disease process, before the tumor becomes invasive. Validated biomarkers are needed for cancer detection and diagnosis, prognosis, and treatment monitoring.
A new model for calculating invasive breast cancer risk, called the CARE model, has been found to give better estimates of the number of breast cancers that would develop in African American women 50 to 79 years of age than an earlier model which was based primarily on data from white women.
Budget Policy: The FY 2010 budget estimate for the Improving Early Detection and Diagnosis program is $390.239 million, an increase of $8.472 million or 2.2 percent above the FY 2009 estimate. NCI expects molecular imaging will profoundly affect the everyday lives of many cancer patients by detection of cancer in its earliest stages and providing information to inform diagnosis and response to therapy. This technology can reveal functional and molecular information about how a cell transforms from normal to cancerous. In addition, to support the program goals, the NCI plans to establish a national system of standardized biospecimens (the current human biobank, or caHUB) using best practices that ensure the highest quality of biospecimens are available for cancer research. The NCI is a leader in the field of biospecimen resource practices, recognizing that the quality of biospecimens is the foundation of personalized medicine. The Early Detection Research Network (EDRN) will launch validation studies of
biomarkers for early detection of bladder cancer and bladder cancer recurrence. This is based on a non-invasive test to detect abnormal epigenetic patterns in urine sediment of bladder cancer patients. Preliminary data suggest that such markers can predict cancer as well as or better than the current standard of care (cytology and cystoscopy) for early detection and recurrence of superficial bladder cancer.
Other activities include: (1) The Lymphoma/Leukemia Molecular Profiling Project which defines the profiles of gene expression 2 associated with all types of human lymphoid malignancies to redefine the classification of these malignancies in molecular terms. The project also seeks to define molecular correlates of clinical parameters which can be used in prognosis and in the selection of appropriate therapy for these patients; (2) The Clinical Proteomic Technologies for Cancer (CPTC) will develop accurate and reliable proteomic technologies and identify issues of variability; (3) The Trans-NCI Breast Premalignancy Program, through which a number of institute initiatives are focused on understanding and detecting the early biological events that lead to breast cancer. A recent initiative will support research studies on the biology of breast premalignancy through multidisciplinary efforts targeted on the characterization of the genetic, molecular, and/or cellular changes of pre-malignancy states of human breast cancer; (4) Leveraging data warehousing technology to host and integrate clinical and functional genomics data from clinical trials involving patients with gliomas, a type of brain cancer, through the Repository for Molecular Brain Neoplasia Data (REMBRANDT); and (5) Determining whether certain cancer screening tests reduce deaths for example through the Prostate, Lung, Colorectal, Ovarian Screening Study (PLCO). Pre-diagnostic samples from PLCO and the NIH Women’s Health Initiative are being used to study the lead-time before clinical diagnosis of ovarian cancer, narrowing the list of candidate biomarkers and potentially leading to an ovarian cancer diagnostic test within the next few years.
Develop Effective and Efficient Treatments
Developing more efficient and effective cancer treatments that leave healthy tissues unharmed is a primary mission of NCI’s research agenda. Knowledge of the unique molecular signature of patients’ tumors is used to classify cancer patients into defined populations – those who will respond best to one type of therapy versus another. The outcome is tailored cancer treatment for patients.
A large national clinical trial for non-small cell lung cancer was launched to validate whether a biomarker can predict clinical benefit in the treatment of this disease. The biomarker, the epidermal growth factor receptor (EGFR), can be increased in some lung cancers due to the presence of extra copies of its coding gene. These extra copies can result in activation of tumor growth, so drugs that block this activation could have a significant impact on lung cancer treatment. This study is called MARVEL (Marker Validation for Erlotinib in Lung Cancer) and is proposed to definitively establish the future value of selecting patients for treatment based on the presence or absence of EGFR activation.
Budget Policy: The FY 2010 budget estimate for the Developing Effective and Efficient Treatments program is $1,291.095 million, an increase of $52.820 million or 4.3 percent above the FY 2009 estimate. A high priority will be placed on developing an integrated Drug Development Platform that will use the knowledge of the genomic and functional changes associated with tumors to identify specific targets for further development. With expansion of GWAS, TCGA, and TARGET, identification of these potential targets will be greatly accelerated. NCI has launched the Chemical Biology Consortium (CBC), a network of institutions that have formal agreements to enable biologists and chemists to be linked to collaborate early in the development process. With other existing drug development programs such as the Rapid Access to Intervention Development (RAID) program, NCI is ideally positioned to enable a new drug development paradigm. Working across the entire drug development process, NCI is seeking to ensure that scientific discoveries are rapidly translated into improved patient outcomes. To accelerate this process of new treatments, NCI will develop partnerships across the research community.
Other activities include: (1) Moving potential new agents through pre-clinical studies, evaluating outcomes, and quickly assessing whether experimental agents are reaching their target to produce the desired effects in humans before committing to large-scale development through the NCI Experimental Therapeutics Program (NExT), an intramural/extramural collaboration; (2) Expanding Phase 0, or first in human studies. Phase 0 trials expose the patient to less toxicity, and can be performed in less time, with fewer patients, than traditional phase I trials. By conducting a phase 0 trial researchers can weed out the drugs that aren't producing the desired effects much more quickly, and can avoid moving those drugs onto Phase I, II and III trials.
Drug Development Platform
FY 2009 Level: $18.00 million
FY 2010 Level: $23.00 million
Change: +$5.00 million
Building on the success of the human genome project, scientists are now able to dissect the way cancer and other diseases develop at the cellular and molecular level. This ability to catalogue the entire human genome has resulted in a cascade of genomic-wide association studies to identify common genetic variations associated with cancer risk and disease progression. Another project, the Cancer Genome Atlas is sequencing patient tumors and finding new gene alterations associated with a specific cancer’s development. Knowledge of these molecular changes is providing starting points for development of targeted therapies aimed at the unique characteristics of specific tumor types.
The Drug Development Program will utilize advances in chemical genomics, high throughput screening and chemical biology for early identification of lead compounds. The most promising agents will undergo enhanced formulation, pre-clinical testing, toxicology studies, and final production of small quantities of clinical grade drug. The program’s aim is to get targeted therapeutics into phase 0 human trials involving small numbers of highly characterized patients and to move the most promising drugs into larger phase I clinical trials.
Cancer Prevention and Control
Prevention is our first line of defense against cancer. Preventing cancer focuses on understanding and modifying behaviors that increase risk, mitigating the influence of genetic and environmental risk factors, and interrupting carcinogenesis through early intervention. National studies have shown that cancer healthcare delivery is inadequate or lacking for large numbers of patients and disadvantaged groups. NCI supports and conducts cancer control research to better understand factors that influence cancer outcomes, improve the quality of care, improve the quality of life for cancer survivors and their families, and overcome cancer health disparities.
Results from a Phase III clinical trial indicate that low doses of two chemopreventive agents, Sulindac, an anti-inflammatory and difluoromethyornithine (DFMO), an experimental compound, are highly effective at preventing the recurrence of the lesions that are often precursors to colorectal cancer.
Budget Policy: The FY2010 budget estimate for the Cancer Prevention and Control program is $318.277 million, an increase of $1.217 million or 0.4 percent above the FY 2009 estimate. Ongoing NCI studies are examining the HPV vaccine’s long-term safety and the extent and duration of protection. NCI and collaborators are also working on therapeutic HPV vaccines that would prevent the development of cancer among women previously exposed to HPV. NCI is also engaged in the development of novel vaccines for cancer immunotherapy and HIV/AIDS.
NCI will continue to study Energy Balance 3 as a way to control cancer incidence. Recently, the Department of Health and Human Services identified overweight and obesity as a public health priority. To address this problem, NCI created the Centers for Transdisciplinary Research on Energetics and Cancer (TREC). TRECs foster collaboration among transdisciplinary teams of scientists to accelerate progress toward reducing cancer incidence, morbidity, and mortality associated with obesity, low levels of physical activity, and poor diet. These activities are components of NCI’s larger energy balance research focus, which complements the Trans-NIH Obesity Task Force.
Other activities include the following: (1) Conducting research on cancer prevention, early detection, treatment, long-term care and surveillance, through the Health Maintenance Organization (HMO) Cancer Research Network (CRN). This network consists of the research programs, enrolled populations, and data systems of 14 HMOs nationwide; (2) Researching safety and efficacy of breast cancer chemoprevention agents through the Breast Cancer Prevention Program; (3) Researching the benefit of prophylactic oophorectomy, the removal of the ovaries to reduce the risk of ovarian cancer, through the Study of Prophylactic Oophorectomy to Prevent Breast/Ovarian Cancer in High Risk Women; and (4) Communicating science both at the individual level, as well as at the community, societal, and population levels (through traditional mass media and emerging diverse electronic channels). The purpose of the CECCRs program (Centers of Excellence in Cancer Communication Research) is to fund transdisciplinary research in the area of cancer communication, with the purpose of contributing directly to positive health outcomes and quality of life for individuals;
Cancer Centers, Specialized Centers and Specialized Programs of Research Excellence (SPORE) Programs
New research paradigms hinge on interdisciplinary science, strategic partnerships, rapid application of new technologies, optimal information sharing, and close links to health care delivery systems. NCI cancer centers, specialized centers, and SPOREs comprise a model framework that supports team science.
The NCI Community Cancer Centers Program has concluded the first of three years as a pilot program to study how community hospitals nationwide could most effectively bring the latest scientific advances and multi-specialty care to a much larger population of cancer patients. These community hospitals have entered into new collaborations with NCI-designated Cancer Centers located at major research institutions around the country and expanded their relationships with local private medical practice oncology physicians. Through these connections, NCI is extending the reach of its research programs into rural, inner-city and underserved communities.
Budget Policy: The FY 2010 budget estimate for the Cancer Centers, Specialized Centers and SPORE programs is $510.703 million, an increase of $22.089 million or 4.5 percent above the FY2009 estimate. NCI has launched a BIG HealthTM initiative to extend the infrastructure for managing data and information, adding procedures to accommodate live patient data and expanding the community of users to consumers, payers and local physicians. The BIG HealthTM consortium will bring together, in a novel organizational framework, a continuum of information management empowering personalized medicine. The NCI-designated Cancer Centers integrate multidisciplinary research within and across institutions nationwide, and also provide clinical and educational services to their local communities. The Cancer Centers bring together the best of basic, translational, and population research to achieve improved cancer prevention, diagnosis, and treatment, while also stimulating innovative pilot projects in new investigational areas. Specialized Programs of Research Excellence (SPOREs) comprise a model framework that supports team science. Specialized centers, such as the Integrative Cancer Biology Program Centers, Tumor Microenvironment Centers, Translational Research on Energetics and Cancer Centers, and Nanotechnology Centers, will focus on key research areas to reduce cancer morbidity and mortality, whereas SPOREs focus entirely on discovery-to-delivery research dedicated to specific cancers.
Other activities include: (1) NCI is developing an integrated biological view of cancer through the development and application of systems biology programs and predictive computational modeling to understand the complexity of cancer. The primary effort in this area is the Integrated Cancer Biology Program that supports nine centers that use multidisciplinary approaches to link biology, bioinformatics, and mathematical modeling across the spectrum of cancer biology and translational research; (2) The Minority Institution-Cancer Center Program is designed to build and stabilize independent competitive cancer research capacity at minority-serving institutions and create stable, long-term collaborative relationships between the Minority Serving Institution (MSI) and the Cancer Center in all areas of cancer research, training, education and outreach. This partnership also seeks to improve the effectiveness of the NCI-designated Cancer Center activities to address cancer disparities in underserved populations and improve minority accrual to cancer clinical trials.
Community-Based Research
FY 2009 Level: $6 million
FY 2010 Level: $6 million
Change: $0
The pace of research in understanding of cancer has accelerated in recent years, raising the need for more immediate and effective ways of translating this new knowledge into patient care in their local communities. Approximately 85% of all cancer patients are cared for in community hospitals and physicians’ offices. To that end NCI initiated a pilot program, the NCI Community Cancer Centers Program (NCCCP). Each of the 16 participating community hospitals has taken significant steps to accelerate cancer clinical research, including work in behavioral sciences, and in so doing to raise the quality of care with a special emphasis on minority and underserved patients.
National studies have shown that cancer healthcare delivery is inadequate, most often fragmented and even lacking for a large numbers of patients. There may come a time in the near future when the greatest mortality risk for cancer patients will be their limited access to optimal care.
The program was designed to complement other NCI initiatives in seeking to achieve four major goals: expand participation in clinical trials; reduce cancer healthcare disparities; collect, store, and share blood and tissue samples needed for research at our major academic centers; and explore the utility of a national database of electronic medical records.
The NCCCP pilot will be an important step in learning how to transfer the latest scientific advances to community hospitals, raising the quality of care and acquainting community physicians with state-of-the-art cancer care management. Pilot sites are sharing best practices and refining the overall concept as a prelude to launching a new national network of research-driven cancer care at the community level.
Research Workforce Development
Developments in molecular biology and translational medicine have broadened the scope of cancer research and presented new challenges for training future cancer researchers. NCI career development opportunities prepare the next generation of cancer researchers to meet the challenges of multidisciplinary research. NCI provides cancer research training and career entree to high school, undergraduate and graduate students, postdoctoral fellows, and physicians across the United States.
Budget Policy: The FY 2010 budget estimate for the Research Workforce Development program is $190.743 million, an increase of $5.925 million or 3.2 percent above the FY 2009 estimate. To support the program goals, high priority will be given to continue the Interagency Oncology Taskforce (IOTF), a partnership with the FDA. Training opportunities have arisen from this relationship, including the Fellowship Program in Research and Regulatory Review. This program provides training for a cadre of researchers to bridge the varied research and regulatory processes that range from scientific discovery through clinical development and regulatory review of new oncology products. Fellows also will learn how to bring state-of-the art knowledge and technology to bear on the design, conduct, and review of clinical trials. In addition, the Principal Investigator 101 training for the Rapid Access to Intervention Development program is organized through the IOTF. Through the Integrative Cancer Biology Program (ICBP) or the Tumor Microenvironment Network (TMEN) Centers, the inter-center training program provides opportunities to gain a broader understanding of the novel approaches to the multi and diverse disciplines which comprise the fields of systems biology or are pertinent to tumor microenvironment.
Other activities include: (1) Supporting medical school training for individuals through the Uniformed Services University/NCI Training Program; (2) Increasing the number of doctors and Oncology Registered Nurses in clinical and translational research through career awards for clinical oncology; (3) Training and mentoring physician scientists to expand their expertise in laboratory or clinical translational research through the Physician Scientist Training Program; and (4) paying special attention to funding training and fellowship awards to young investigators to ensure that the future of biomedical research maintains its high level during future decades.
Buildings and Facilities
The renovation and improvement funds for the facilities at the NCI-Frederick campus, located in Frederick, Maryland, were budgeted as facilities funds beginning in FY 2005. The funds are necessary to maintain the operation of these facilities for the scientific missions of NCI, NIH, other government agencies, and the extramural community.
Budget Policy: The FY 2010 budget estimate for the Building and Facilities program is $7.920 million, even with the FY 2009 estimate. NCI is evaluating and prioritizing the specific projects to be funded in FY 2010.
Research Management and Support (RMS)
NCI RMS activities provide support for the review, award, and monitoring of technical and administrative services. These services include central administration, overall program direction, grant and contract administration, human resources, program coordination, and financial management. NCI regularly engages in business planning activities to streamline administrative functions.
Budget Policy: The FY 2010 budget estimate for RMS is $386.863 million, an increase of $6.650 million or 1.75 percent above the FY 2009 estimate.
Footnotes:
Top
Budget Authority by Object
| |
FY 2009
Estimate |
FY 2010
Estimate |
Increase or
Decrease |
Percent
Change |
| Total compensable workyears: |
|
| Full-time employment |
2,933 |
2,992 |
59 |
2.0 |
| Full-time equivalent of overtime and holiday hours |
6 |
6 |
0 |
0.0 |
| Average ES salary |
$165,991 |
$170,805 |
$4,814 |
2.9 |
| Average GM/GS grade |
12.0 |
12.0 |
0.0 |
0.0 |
| Average GM/GS salary |
$85,263 |
$87,736 |
$2,473 |
2.9 |
| Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) |
$87,147 |
$89,675 |
$2,528 |
2.9 |
| Average salary of ungraded positions |
129,136 |
132,881 |
3,745 |
2.9 |
| OBJECT CLASSES |
FY 2009
Estimate |
FY 2010
Estimate |
Increase or
Decrease |
Percent
Change |
| Personnel Compensation: |
|
| 11.1 Full-time permanent |
$196,770,000 |
$206,453,000 |
$9,683,000 |
4.9 |
| 11.3 Other than full-time permanent |
98,490,000 |
103,337,000 |
4,847,000 |
4.9 |
| 11.5 Other personnel compensation |
10,869,000 |
11,377,000 |
508,000 |
4.7 |
| 11.7 Military personnel |
7,048,000 |
7,377,000 |
329,000 |
4.7 |
| 11.8 Special personnel services payments |
53,908,000 |
55,811,000 |
1,903,000 |
3.5 |
| Total, Personnel Compensation |
367,085,000 |
384,355,000 |
17,270,000 |
4.7 |
| 12.0 Personnel benefits |
84,209,000 |
88,389,000 |
4,180,000 |
5.0 |
| 12.2 Military personnel benefits |
4,342,000 |
4,544,000 |
202,000 |
4.7 |
| 13.0 Benefits for former personnel |
0 |
0 |
0 |
0.0 |
| Subtotal, Pay Costs |
455,636,000 |
477,288,000 |
21,652,000 |
4.8 |
| 21.0 Travel and transportation of persons |
16,147,000 |
16,359,000 |
212,000 |
1.3 |
| 22.0 Transportation of things |
941,000 |
953,000 |
12,000 |
1.3 |
| 23.1 Rental payments to GSA |
7,000 |
7,000 |
0 |
0.0 |
| 23.2 Rental payments to others |
150,000 |
150,000 |
0 |
0.0 |
| 23.3 Communications, utilities and miscellaneous charges |
7,513,000 |
7,593,000 |
80,000 |
1.1 |
| 24.0 Printing and reproduction |
2,460,000 |
2,486,000 |
26,000 |
1.1 |
| 25.1 Consulting services |
24,961,000 |
24,961,000 |
0 |
0.0 |
| 25.2 Other services |
155,756,000 |
156,910,000 |
1,154,000 |
0.7 |
| 25.3 Purchase of goods and services from government accounts |
502,977,000 |
510,247,000 |
7,270,000 |
1.4 |
| 25.4 Operation and maintenance of facilities |
144,273,000 |
142,956,000 |
(1,317,000) |
-0.9 |
| 25.5 Research and development contracts |
413,965,000 |
429,316,000 |
15,351,000 |
3.7 |
| 25.6 Medical care |
4,470,000 |
4,470,000 |
0 |
0.0 |
| 25.7 Operation and maintenance of equipment |
10,329,000 |
10,329,000 |
0 |
0.0 |
| 25.8 Subsistence and support of persons |
0 |
0 |
0 |
0.0 |
| 25.0 Subtotal, Other Contractual Services |
1,256,731,000 |
1,279,189,000 |
22,458,000 |
1.8 |
| 26.0 Supplies and materials |
47,881,000 |
48,596,000 |
715,000 |
1.5 |
| 31.0 Equipment |
20,597,000 |
20,597,000 |
0 |
0.0 |
| 32.0 Land and structures |
0 |
0 |
0 |
0.0 |
| 33.0 Investments and loans |
0 |
0 |
0 |
0.0 |
| 41.0 Grants, subsidies and contributions |
3,160,868,000 |
3,296,910,000 |
136,042,000 |
4.3 |
| 42.0 Insurance claims and indemnities |
4,000 |
4,000 |
0 |
0.0 |
| 43.0 Interest and dividends |
38,000 |
38,000 |
0 |
0.0 |
| 44.0 Refunds |
0 |
0 |
0 |
0.0 |
| Subtotal, Non-Pay Costs |
4,513,337,000 |
4,672,882,000 |
159,545,000 |
3.5 |
| Total Budget Authority by Object |
4,968,973,000 |
5,150,170,000 |
181,197,000 |
3.6 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research
Top
Salaries and Expenses
| OBJECT CLASSES |
FY 2009
Estimate |
FY 2010
Estimate |
Increase or
Decrease |
Percent
Change |
| Personnel Compensation: |
|
| Full-time permanent (11.1) |
$196,770,000 |
$206,453,000 |
$9,683,000 |
4.9 |
| Other than full-time permanent (11.3) |
98,490,000 |
103,337,000 |
4,847,000 |
4.9 |
| Other personnel compensation (11.5) |
10,869,000 |
11,377,000 |
508,000 |
4.7 |
| Military personnel (11.7) |
7,048,000 |
7,377,000 |
329,000 |
4.7 |
| Special personnel services payments (11.8) |
53,908,000 |
55,811,000 |
1,903,000 |
3.5 |
| Total Personnel Compensation (11.9) |
367,085,000 |
384,355,000 |
17,270,000 |
4.7 |
| Civilian personnel benefits (12.1) |
84,209,000 |
88,389,000 |
4,180,000 |
5.0 |
| Military personnel benefits (12.2) |
4,342,000 |
4,544,000 |
202,000 |
4.7 |
| Benefits to former personnel (13.0) |
0 |
0 |
0 |
0.0 |
| Subtotal, Pay Costs |
455,636,000 |
477,288,000 |
21,652,000 |
4.8 |
| Travel (21.0) |
16,147,000 |
16,359,000 |
212,000 |
1.3 |
| Transportation of things (22.0) |
941,000 |
953,000 |
12,000 |
1.3 |
| Rental payments to others (23.2) |
150,000 |
150,000 |
0 |
0.0 |
| Communications, utilities and miscellaneous charges (23.3) |
7,513,000 |
7,593,000 |
80,000 |
1.1 |
| Printing and reproduction (24.0) |
2,460,000 |
2,486,000 |
26,000 |
1.1 |
| Other Contractual Services: |
|
| Advisory and assistance services (25.1) |
24,961,000 |
24,961,000 |
0 |
0.0 |
| Other services (25.2) |
155,756,000 |
156,910,000 |
1,154,000 |
0.7 |
| Purchases from government accounts (25.3) |
383,748,000 |
391,018,000 |
7,270,000 |
1.9 |
| Operation and maintenance of facilities (25.4) |
31,656,000 |
27,656,000 |
(4,000,000) |
-12.6 |
| Operation and maintenance of equipment (25.7) |
10,329,000 |
10,329,000 |
0 |
0.0 |
| Subsistence and support of persons (25.8) |
0 |
0 |
0 |
0.0 |
| Subtotal Other Contractual Services |
606,450,000 |
610,874,000 |
4,424,000 |
0.7 |
| Supplies and materials (26.0) |
47,676,000 |
48,391,000 |
715,000 |
1.5 |
| Subtotal, Non-Pay Costs |
681,337,000 |
686,806,000 |
5,469,000 |
0.8 |
| Total, Administrative Costs |
1,136,973,000 |
1,164,094,000 |
27,121,000 |
2.4 |
Top
Authorizing Legislation
| |
PHS Act/
Other Citation |
U.S. Code
Citation |
2009 Amount
Authorized |
FY 2009
Estimate |
2010 Amount
Authorized |
FY 2010
Estimate |
| Research and Investigation |
Section 301 |
42§241 |
Indefinite |
|
Indefinite |
|
| National Cancer Institute |
Section 402(a) |
42§281 |
Indefinite |
|
Indefinite |
|
| Total, Budget Authority |
|
|
|
4,968,973,000 |
|
5,150,170,000 |
Top
Appropriations History
| Fiscal Year |
Budget Estimate to Congress |
House Allowance |
Senate Allowance |
Appropriation1 |
| 2001 |
3,249,730,0002 |
3,505,072,000 |
3,804,084 |
3,754,456,000 |
| Rescission |
|
(2,005,000) |
| 2002 |
4,177,203,000 |
4,146,291,000 |
4,258,516,000 |
4,190,405,000 |
| Rescission |
|
(9,172,000) |
| 2003 |
4,673,510,000 |
4,673,510,000 |
4,642,394,000 |
4,622,394,000 |
| Rescission |
|
(30,046,000) |
| 2004 |
4,770,519,000 |
4,770,519,000 |
4,770,519,000 |
4,770,519,000 |
| Rescission |
|
(31,264,000) |
| 2005 |
4,870,025,000 |
4,870,025,000 |
4,894,900,000 |
4,865,525,000 |
| Rescission |
|
(40,267,000) |
| 2006 |
4,841,774,000 |
4,841,774,000 |
4,960,828,000 |
4,841,774,000 |
| Rescission |
|
(48,418,000) |
| 2007 |
4,753,609,000 |
4,753,609,000 |
4,799,063,000 |
4,797,639,000 |
| Rescission |
|
0 |
| 2008 |
4,782,114,000 |
4,870,382,000 |
4,910,160,000 |
4,890,525,000 |
| Rescission |
|
(85,437,000) |
| Supplemental |
|
25,559,000 |
| 2009 |
4,809,819,000 |
4,975,039,000 |
4,958,594,000 |
4,968,973,000 |
| Rescission |
|
0 |
| 2010 |
5,150,710,000 |
|
1 Reflects enacted supplementals, rescissions, and reappropriations.
2 Excludes funds for HIV/AIDS research activities consolidated in the NIH Office of AIDS Research.
Top
Detail of Full-Time Equivalent Employment (FTEs)
| OFFICE/DIVISION |
FY 2008 Actual |
FY 2009 Estimate |
FY 2010 Estimate |
| Office of the Director |
745 |
758 |
773 |
| Center for Cancer Research |
1,474 |
1,500 |
1,530 |
| Division of Cancer Biology |
38 |
39 |
40 |
| Division of Extramural Activities |
86 |
88 |
89 |
| Division of Cancer Treatment and Diagnosis |
186 |
189 |
193 |
| Division of Cancer Prevention |
79 |
80 |
82 |
| Division of Cancer Control and Population Sciences |
124 |
126 |
129 |
| Division of Cancer Epidemiology and Genetics |
150 |
153 |
156 |
| Total |
2,882 |
2,933 |
2,992 |
| Includes FTE's which are reimbursed from the NIH Roadmap for Medical Research |
| FTE's supported by funds from Cooperative Research and Development Agreements |
(10) |
(10) |
(10) |
| FISCAL YEAR |
Average GM/GS Grade |
| 2006 |
12.0 |
| 2007 |
12.0 |
| 2008 |
12.0 |
| 2009 |
12.0 |
| 2010 |
12.0 |
Top
Detail of Positions
| GRADE |
FY 2008
Actual |
FY 2009
Estimate |
FY 2010
Estimate |
| Total, ES Positions |
5 |
6 |
7 |
| Total, ES Salary |
$792,096 |
$995,950 |
$1,141,093 |
| GM/GS-15 |
236 |
240 |
245 |
| GM/GS-14 |
383 |
390 |
398 |
| GM/GS-13 |
368 |
375 |
382 |
| GS-12 |
470 |
479 |
489 |
| GS-11 |
192 |
195 |
199 |
| GS-10 |
11 |
11 |
11 |
| GS-9 |
137 |
139 |
142 |
| GS-8 |
92 |
94 |
96 |
| GS-7 |
83 |
84 |
86 |
| GS-6 |
20 |
20 |
21 |
| GS-5 |
15 |
16 |
16 |
| GS-4 |
11 |
11 |
11 |
| GS-3 |
3 |
3 |
3 |
| GS-2 |
2 |
2 |
2 |
| GS-1 |
0 |
0 |
0 |
| Subtotal |
2,023 |
2,059 |
2,101 |
| Grades established by Act ofJuly 1, 1944 (42 U.S.C. 207): |
|
| Assistant Surgeon General |
1 |
1 |
1 |
| Director Grade |
31 |
31 |
31 |
| Senior Grade |
13 |
13 |
13 |
| Full Grade |
16 |
16 |
16 |
| Senior Assistant Grade |
1 |
1 |
1 |
| Assistant Grade |
4 |
4 |
4 |
| Subtotal |
66 |
66 |
66 |
| Ungraded |
901 |
917 |
936 |
| Total permanent positions |
2,120 |
2,158 |
2,201 |
| Total positions, end of year |
2,995 |
3,049 |
3,110 |
| Total full-time equivalent (FTE) employment, end of year |
2,882 |
2,933 |
2,992 |
| Average ES salary |
158,419 |
165,991 |
170,805 |
| Average GM/GS grade |
12.0 |
12.0 |
12.0 |
| Average GM/GS salary |
81,373 |
85,263 |
87,736 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research.
Top
New Positions Requested
| |
FY 2010 |
| |
Grade |
Number |
Annual Salary |
| Biologist |
GS 11 |
10 |
$73,214 |
| Chemist |
GS 12 |
2 |
93,296 |
| Clinical Fellow |
AD/602/0 |
4 |
82,420 |
| Epidemiologist |
GS 14 |
1 |
125,759 |
| Health Scientist Administrator |
GS 14 |
8 |
125,156 |
| Medical Officer |
GS 15 |
1 |
208,129 |
| Microbiologist |
GS 12 |
2 |
93,843 |
| Nurse Specialist |
GS 14 |
2 |
109,223 |
| Research Chemist |
RS/1320/0 |
1 |
187,520 |
| Research Fellow |
AD/401/0 |
3 |
80,019 |
| Senior Investigator |
AD/602/0 |
6 |
221,705 |
| Investigator |
AD/401/0 |
3 |
118,485 |
| Staff Clinician |
AD/602/0 |
1 |
166,228 |
| Staff Scientist |
AD/401/0 |
7 |
111,349 |
| IT Specialist |
GS 12 |
1 |
89,007 |
| Contract Specialist |
GS 13 |
1 |
105,540 |
| Grant Management Specialist |
GS 13 |
2 |
98,713 |
| Administrative Officer |
GS 12 |
3 |
82,570 |
| Purchasing Agent |
GS 8 |
1 |
56,819 |
| Total Requested |
|
59 |
|
Top
|