The Epidemiology and Genomics Research Program (EGRP) has initiated a strategic planning effort to develop scientific priorities for cancer epidemiology research in the next decade in the midst of a period of great scientific opportunity but also of resource constraints. EGRP would like to engage the research community and other stakeholders in a planning effort that will include a workshop in December 2012 to help shape new foci for cancer epidemiology research.
EGRP Invites Your Feedback
To facilitate this process, we invite the research community to join in an ongoing Web-based conversation to develop priorities and influence the next generation of high-impact studies.
Our aim is to enhance the application of epidemiologic methods along the translational continuum from basic discoveries to population health impact.
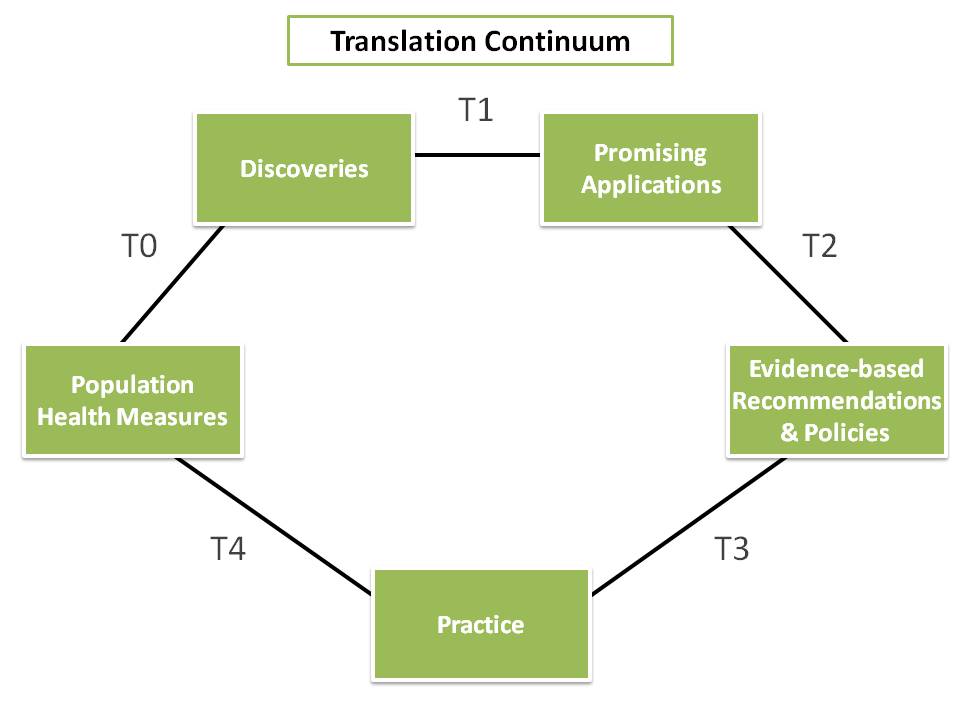
Figure 1. The continuum of translational research from discovery to reducing the burden of disease in a population.
This week, we address the use of epidemiologic research to bridge evidence gaps between scientific discoveries and population health impact, in particular, how we can use observational epidemiologic studies to supplement randomized clinical trials in advancing clinical and public health practice.
Translational research is a continuum from discovery to population health impact. Although this continuum is often considered a linear or unilateral process for laboratory scientists, the form shown in Figure 1 involves many iterations and feedback loops.
Within the translational research enterprise, epidemiology serves as a fundamental building block. The application of epidemiologic methods across all phases of translational research, T0 – T4, is known as translational epidemiology (Figure 1) and has been described in a 2010 publication in the American Journal of Epidemiology.
- T0, from population health measures to discoveries, consists of describing patterns of health outcomes by place, time, and person and finding determinants of health outcomes with observational studies.
- T1, from discoveries to promising applications, characterizes discovery and assesses potential health applications by using clinical and population studies.
- T2, from promising applications to evidence-based recommendations and policies, assesses the efficacy of interventions to improve health and prevent disease by using observational and experimental studies.
- T3, from evidence-based recommendations and policies to practice, assesses the implementation and dissemination of guidelines into practice.
- T4, from practice to population health measures, assesses the effectiveness of interventions on health outcomes.
The framework described above combines basic, clinical, and public health approaches to disease treatment, prevention, and control. It provides the necessary data to influence further research, policy, and practice by documenting what we know and do not know and what works and does not work. Translational epidemiology is an essential ingredient to accelerate the movement of discoveries from research into practice using evidence-based applications.
We would like to get your feedback on the following fundamental questions:
- What are new ways in which epidemiology can be used to fill evidence gaps between discoveries and population health impact in the cancer care continuum?
- How can observational epidemiology make the greatest scientific contributions in understanding cancer-related risk factors that cannot be studied through randomized clinical trials?
Please use the comment section below to share your perspectives.
We encourage you to be as specific as possible. You can use or be inspired by the NCI Provocative Questions exercise. Your comments will be used to shape the workshop discussion in December, aspiring to transform the future of cancer epidemiology in the next decade.
Comments are also still welcome in response to the first three questions posed as part of EGRP’s strategic planning series:
- June 4, 2012: What Scientific Questions Should Cancer Epidemiology Address in the Next Decade to Impact Public Health?
- July 20, 2012: How Should New Technologies Be Integrated into Cancer Epidemiology?
- August 27, 2012: What Have We Learned from Epidemiology Cohorts and Where Should We Go Next?
EGRP’s Workshop Science Advisory Group





5 comments
Skip to comment form ↓
Jeffrey Meyerhardt
October 16, 2012 at 7:57 AM (UTC -5) Link to this comment
Energy balance host factors have been shown to be a risk factor for a variety of cancers, including breast, colorectal, prostate, gynecological, and others.[1,2] Energy balance is impacted by both energy in (amount of calories eaten modified by absorption and/or ingestion) and energy out (influenced by level of physical activity, resting metabolic rate and thermic effect of food).[3] In addition to studies on the risk of cancer development from energy imbalance, there is increasing interest in how these factors impact patients with established disease.[4] Patient often ask what else can they do in addition to standard therapies to help their outcomes – what should they eat, should they exercise, should I lose weight?[5] These questions are both biologically relevant and very pressing to patients and until recently, little research was available for the oncologist or patient for guidance.
As oncologists, we recommend adjuvant chemotherapy choices based on data from randomized controlled trials. In the example of colon cancer, initial trials enrolled patients who underwent surgery for early stage colon cancer to either no therapy or chemotherapy. Subsequent trials have compared one regimen to another to determine best choice in chemotherapy. However, randomized trials on role of exercise, weight reduction, and dietary changes are considerably more challenging. Further, it is not known which energy balance factors are more or less relevant to alter to improve outcomes for patients.
An opportunity to understand the role of energy balance factors (and other host factors) in patients with colorectal cancer is to leverage the resources of a clinical trial. Clinical trial databases have the advantage of built in collection of tumor related factors and other demographics. Treatment is standardized for patients, reducing variability of care that may impact outcomes. Follow-up for survival endpoints is specifically delineated within the protocol, often with minimum loss to follow-up. The nature and timing of recurrences or disease progression is documented. Over the past decade, the Gastrointestinal Cancer Committee in the NCI-sponsored Cancer and Leukemia Group B (CALGB, now part of the ALLIANCE) has incorporated the use of patient completed questionnaires to provide an opportunity to maximize the benefits of their randomized clinical trials in colorectal cancer. In an adjuvant chemotherapy trial for stage III colon cancer, CALGB 89803, a companion study led by Dr. Charles Fuchs required patients participating in the treatment trial to complete comprehensive questionnaires that includes a semi-quantitative food frequency questionnaire, physical activity questionnaire, smoking history, multivitamin and other supplement usage, and anthropometric measurements. Questionnaires were completed during adjuvant therapy (approximately 2-3 months from surgical resection of their primary tumor) and 6-8 months after completion of adjuvant therapy (approximately 14-16 months after surgery). Compliance was very high – 95% of eligible patient completed the first questionnaire and 85% of eligible patients completed the second questionnaires.[6] To date, seven manuscripts have been published on physical activity, adiposity, diet, smoking, statin usage, multivitamin usage, and family history in stage III colon cancer leveraging the resources of CALGB 89803.[6-12] While studies are observational in nature, the use of clinical trial database provides an opportunity to study various host factors using statistical analyses that can test for reverse causality and adjust for other host factor confounders. Further, since the trial also collected tumor blocks for correlative science studies, studies to test for interactions with biological relevant markers can further understanding the biological effects of energy balance and other host factors.[13] These efforts have continued into a metastatic trial for colorectal cancer that recently completed accrual (CALGB 80405) and a currently enrolling adjuvant therapy trial (CALGB 80702).
1. Hursting SD, Lashinger LM, Colbert LH, et al. Energy balance and carcinogenesis: underlying pathways and targets for intervention. Curr Cancer Drug Targets 2007;7(5):484-91.
2. Kaaks R. Nutrition, insulin, IGF-1 metabolism and cancer risk: a summary of epidemiological evidence. Novartis Found Symp 2004;262:247-60; discussion 260-68.
3. Demark-Wahnefried W, Platz EA, Ligibel JA, et al. The role of obesity in cancer survival and recurrence. Cancer Epidemiol Biomarkers Prev 2012;21(8):1244-59.
4. Goodwin PJ, Meyerhardt JA, Hursting SD. Host factors and cancer outcome. J Clin Oncol 2010;28(26):4019-21.
5. Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62(4):243-74.
6. Meyerhardt JA, Heseltine D, Niedzwiecki D, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 2006;24(22):3535-41.
7. Meyerhardt JA, Fuchs C. Cancer Recurrence and Survival Associated With Dietary Patterns in Stage III Colon Cancer-Reply Jama 2007;298(19):2263.
8. Meyerhardt JA, Niedzwiecki D, Hollis D, et al. Impact of body mass index and weight change after treatment on cancer recurrence and survival in patients with stage III colon cancer: findings from Cancer and Leukemia Group B 89803. J Clin Oncol 2008;26(25):4109-15.
9. Chan JA, Meyerhardt JA, Niedzwiecki D, et al. Association of family history with cancer recurrence and survival among patients with stage III colon cancer. JAMA 2008;299(21):2515-23.
10. McCleary NJ, Niedzwiecki D, Hollis D, et al. Impact of smoking on patients with stage III colon cancer: results from Cancer and Leukemia Group B 89803. Cancer 2010;116(4):957-66.
11. Ng K, Meyerhardt JA, Chan JA, et al. Multivitamin use is not associated with cancer recurrence or survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol 2010;28(28):4354-63.
12. Ng K, Ogino S, Meyerhardt JA, et al. Relationship between statin use and colon cancer recurrence and survival: results from CALGB 89803. J Natl Cancer Inst 2011;103(20):1540-51.
13. Ogino S, Chan AT, Fuchs CS, et al. Molecular pathological epidemiology of colorectal neoplasia: an emerging transdisciplinary and interdisciplinary field. Gut 2011;60(3):397-411.
Michael S. Lauer
November 7, 2012 at 2:16 PM (UTC -5) Link to this comment
When trials can’t be done
When I call on clinicians and public health officials to subject their beliefs to clinical trials, I’m often told that trials can’t be done. Maybe they’re impracticable. Or unethical — the answer is clear. Or they’re too expensive, or too slow.
Esther Duflo is a French-born economist, a professor at MIT, who recently won acclaim as a recipient of the John Bates Clark medal, a medal given to promising economists under the age of 40. Professor Duflo has made her mark by questioning conventional solutions for poverty. She and her colleagues essentially introduced the randomized trial to the world of economic development.1 She found, that for improving learning, traditional education aids don’t work, but inexpensive anti-worm medications do. Before Professor Duflo, it was assumed that the only kind of economic development data available would be observational data – after all, how can one possibly do poverty experiments?
Professor Duflo’s work should remind us that randomized trials are possible in what may seem like the most unlikely situations. The Moving to Opportunity experiment randomized poor families to different kinds of housing vouchers.2 The investigators found that moving families into lower-poverty neighborhoods led to modest improvements in well-being, obesity, and metabolic health. This landmark experiment expanded on a wealth of epidemiological data linking neighborhood characteristics3 to health by demonstrating that public policy could be redirected to positive effect.
Sometimes randomized trials happen independent of the direct intervention of researchers. The State of Oregon used a lottery to determine who would receive expanded Medicaid coverage. Early observations derived from this “natural experiment” provide support for policies that enhance access to health care for economically disadvantaged people.4
I believe that we should stop thinking of epidemiology and clinical experimentation as separate disciplines. In an era of expanding digital connectivity, patient engagement, and health care integration, it should become possible to assemble mega-cohorts, cohorts of hundreds of thousands to millions of people, who then would serve as “platforms” for many practical randomized trials. The United Kingdom Biobank5 assembled a well-phenotyped cohort of over 500,000 people at a cost of $200 per person; these people are being followed through existing electronic data infrastructures at a cost of $15 per person per year. One can imagine enrolling subsets of these people in a wide array of randomized trials, trials that could, at exceedingly low marginal cost, answer high-impact questions.6
We are already seeing this. Scandinavian investigators have introduced the term “clinical registry trial” when describing a randomized study of thrombus aspiration in patients with acute myocardial infarction. The investigators are embedding their trial into existing observational registries.7 American investigators are embedding within an existing registry a trial of radial-artery-directed percutaneous coronary interventions in women.
Just as health care is becoming increasingly integrated, the time has come to integrate epidemiology into clinical care, clinical trials, and public health. Instead of begging off by arguing that randomized trials are not feasible, future epidemiologists will enable a whole new generation of large-scale, high-impact, low-cost trials that will discover and enable whole new paradigms of investigation and prevention.
References
1. Banerjee AV, Duflo E. Poor economics : a radical rethinking of the way to fight global poverty. 1st ed. New York: PublicAffairs; 2011.
2. Ludwig J, Sanbonmatsu L, Gennetian L, et al. Neighborhoods, obesity, and diabetes–a randomized social experiment. N Engl J Med. Oct 20 2011;365(16):1509-1519.
3. Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. Jul 12 2001;345(2):99-106.
4. Finkelstein A, Taubman S, Wright B, et al. NBER Working Paper 17190. The Oregon Health Insurance Experiment: Evidence from the First Year (available at http://www.nber.org/papers/w17190.pdf, accessed on November 7, 2012).
5. Manolio TA, Collins R. Enhancing the feasibility of large cohort studies. JAMA. Nov 24 2010;304(20):2290-2291.
6. Lauer MS. Time for a creative transformation of epidemiology in the United States. JAMA. Nov 7 2012;308(17):1804-1805.
7. Frobert O, Lagerqvist B, Gudnason T, et al. Thrombus Aspiration in ST-Elevation myocardial infarction in Scandinavia (TASTE trial). A multicenter, prospective, randomized, controlled clinical registry trial based on the Swedish angiography and angioplasty registry (SCAAR) platform. Study design and rationale. Am Heart J. Dec 2010;160(6):1042-1048.
David F. Ransohoff
November 7, 2012 at 2:22 PM (UTC -5) Link to this comment
Cultivating cohorts
How can epidemiological research advance clinical and public health practice by “bridging an evidence gap,” using observational (non experimental) studies to address T0-T4 questions? In the last 10-20 years much research has assessed T1 questions about diagnosis, prognosis, and prediction, producing measurements of sensitivity, specificity (for diagnosis), and predictive value (for prognosis or prediction). One problem has been that much early or “discovery” research results turn out to be not reproducible in subsequent studies. (1, 2) A major reason is lack of attention to “study design” (e.g., addressing issues of appropriateness of comparison and avoidance of bias). At one extreme, convenience samples may be used for discovery (or for validation), where little though has been given to “design” of the study that produced the samples that became available to analyze. At another extreme are examples of high-quality observational studies that have produced results that changed practice or provided a strong foundation for future work. (1, 2)
The purpose of the discussion today is, by considering lessons from the past, to develop the concept of “cultivating cohorts” that may be used in observational research about discovery or validation of markers, focusing mainly on T1 studies. The discussion will examine published successes and disappointments in order to draw larger lessons about how to take advantage of existing cohorts and infrastructure so that they may be used in strong observational research about markers for discovery and validation.
One example of success assessed whether an RNA expression profile can predict prognosis. The OncoTypeDx test study was conducted “retrospectively”, in that the research question was asked after the phenomena that would be analyzed had occurred. (3) I.e., The study used banked specimens and data from the already-completed NSABP B-14 study). Topics to be discussed include: What “new” effort was involved in conceptualization, design, and conduct of this study? In what ways was the study strong or not? What are lessons for cultivating other similar cohorts for use in clinical research about markers?
Another example of a strong observational study result addressed whether a blood based proteomics test can diagnose early ovarian cancer. (4) Several promising panels of markers were assessed by using specimens that had been “already banked” in NCI’s PLCO (Prostate, Lung, Colon, Ovary RCT) biorepository. What features of that biorepository made specimens suitable (or not) for use? How much effort was involved? Why were study results strong or not? What are lessons for cultivating other similar cohorts for use in clinical research about markers?
A third example was a prospectively-planned (and expensive and time-consuming) study to assess a stool DNA test to detect colon cancer. (5) The study involved new collection of specimens, because stools had to be obtained before determination of the “true state” by colonoscopy. Could this kind of study have been done, and perhaps with less expense, in other settings?
Based on these examples and others, we will consider how to cultivate cohorts in a variety of settings, including on-going RCTs, on-going observational cohort studies, and in practice settings such as HMOs. (1, 2) What are long-term opportunities for addressing T1 kinds of questions? What planning, resources, and infrastructure might be useful to do that planning?
REFERENCES
1. Ransohoff DF, Gourlay ML. Sources of bias in specimens for research about molecular markers for cancer. J Clin Oncol 2010;28(4):698-704.
2. Ransohoff DF. How to improve reliability and efficiency of research about molecular markers: roles of phases, guidelines, and study design. J Clin Epidemiol 2007;60(12):1205-1219.
3. Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004;351(27):2817-2826.
4. Zhu CS, Pinsky PF, Cramer DW, Ransohoff DF, Hartge P, Pfeiffer RM, et al. A framework for evaluating biomarkers for early detection: validation of biomarker panels for ovarian cancer. Cancer Prev Res (Phila) 2011;4(3):375-383.
5. Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N Engl J Med 2004;351(26):2704-2714.
Barnett S. Kramer
November 19, 2012 at 9:45 AM (UTC -5) Link to this comment
As I have pointed out in an editorial years ago, the tools of traditional epidemiology play a well deserved, venerated role in public health and in evidence-based decision making. (1) The National Cancer Institute’s Physician Data Query (PDQ) Screening and Prevention Editorial Board, which I chair, incorporates traditional epidemiology studies into its formal consideration of levels of evidence for clinical and public health interventions. (2) That role has not been diminished with the advent of increasingly sophisticated mechanistic in vitro and animal models, or by molecular epidemiology approaches. Many important health questions simply cannot be addressed without the tools of traditional epidemiology. We often don’t even know the positive and negative predictive values of our in vitro and animal model systems for health outcomes in humans. We frequently rely on pure logic when extrapolating from such studies to humans. With rare exceptions, we cannot ethically test the effect of exposures postulated to have a net harm in randomized trials.
But if the tools of epidemiology are sharp, they are also double-edged. Epidemiology studies are often powered to detect small effects of exposures. This requires large datasets. For the sake of efficiency data on multiple variables and multiple exposures are collected, and the data are explored for multiple endpoints. This can lead to multiplicity and a large number of falsely positive results. Selective reporting of appealing findings is also tempting. Advocacy positions and intellectual conflicts may also get in the way. In addition, no matter how many variables are collected, it is difficult to collect sufficient information on, and adjust for, some important confounding factors. So false positive associations, as well as non-causal associations, are frequent. Both types of findings can lead to misplaced public health policy and to wasted resources as others try to replicate or follow up on incorrect findings. Sometimes epidemiology studies provide hypotheses that are so attractive and intuitively appealing that we simply forget to actually test the hypothesis using a more rigorous study design. Therefore, if the question is important enough, we should always ask if the answer from an epidemiology study can be ethically confirmed in a randomized trial. The future of epidemiology is strengthened by epistemological humility.
1. Weed DL and Kramer BS: Induced abortion, bias, and breast cancer: why epidemiology hasn’t reached its limit (editorial). J. Natl. Cancer Inst, 88(23):1698-1700, (1996).
2. National Cancer Institute PDQ (Physician Data Query): Levels of Evidence for Cancer Screening and Prevention Studies: http://www.cancer.gov/cancertopics/pdq/screening/levels-of-evidence/HealthProfessional/page1/AllPages (last accessed 17November2012)
Olufunmilayo I. Olopade
December 10, 2012 at 9:02 AM (UTC -5) Link to this comment
Epidemiology in the 21st Century: Going where the money is?
I have often wondered why there are not more cancer epidemiologists working in and out of hospitals and clinics to collect observational data or going to hotspots with the highest burden of cancer deaths. Afterall, every hospital has an “Infection Control” team that monitors patterns of infection in real time. One might be surprised to find out how much can be learned by going to where the money is. By having a broader understanding of human biology and the context of transition from heath to disease state, the next generation of cancer epidemiologists will be better equipped to work in interdisciplinary teams and create new knowledge with the highest potential to impact clinical and public health practice.
Despite significant gains in our basic understanding of the genetic basis of cancer, huge knowledge gaps remain in the translational continuum that hinder our ability to use epidemiology to drive change in the clinic or population at large. While clinicians in oncology have witnessed the “n of one” patient with dramatic response to expensive targeted therapy, and epidemiology has given us new ways to interrogate increasingly large cohorts and consortia using genome wide approaches, the impact in reducing cancer morbidity and mortality has been modest. Moreover, the public has become confused about screening for early detection of cancer and whether we can make our screening recommendations more precise with better risk prediction models that integrate biology. The challenge of the next decade is knowing how to make these scientific advances matter for the average cancer patient and the population at large. How do we implement “precision medicine”, “precision risk assessment”, lower cost of cancer care and reduce cost of large epidemiological studies. “Big Data” holds promise and this is where epidemiology can bridge evidence gaps if we do it right (1). Classical epidemiology has documented racial and ethnic differences in cancer survival during the past decade and it is now time to collect and integrate granular data that will advance the field (2).
In the US and throughout the developing world, cancer is projected to become the leading cause of death as the population ages (3). With health care reform and the Affordable Care Act, it is expected that millions of previously uninsured Americans will enter the health care system and we could begin to collect longitudinal data on these individuals. Large studies evaluating multi-level determinants of disease and outcomes when integrated with genomics, biologically relevant biomarkers and patient reported outcomes could be powerful drivers of innovations in clinical care and population health in the next decade. Cancer Registration could be much more democratic with widespread use of Electronic Health Records. Every newly diagnosed cancer patient becomes a subject for longitudinal studies and quality improvement. Substantial investments could be put into disruptive technologies capable of facilitating patient enrollment in studies even in the most remote parts of the world. Mobile technologies are going to be a big part of service delivery even as the Internet expands use of social media for epidemiological studies. Now, less than 1% of eligible cancer patients participate in randomized clinical trials, public-private partnership with pharmaceutical companies such as envisioned by the newly created TransCelerate BioPharma could collect and integrate the same granular data used for registration in all post marketing surveillance after a drug has been approved. These data could be shared across studies and disease sites and be mined to aid fundamental discovery research on mechanisms of disease, especially for populations that have been understudied and where there are huge knowledge gaps.
Lastly, as long as geographic variations in cancer mortality rates exist, epidemiology of the 21st century should take advantage of the tremendous interest and enthusiasm for global health to improve the scorecard on global cancer disparities (4). Epidemiologists can no longer sit on the sidelines when people are dying of cancer because of lack of awareness and poor access to care. Learning from AIDS activism of the past decade, the cancer community should galvanize towards collective action to control cancer. Genomics can be used to improve public health and these tools can be deployed at minimal costs for great gains. There are opportunities for innovative studies in implementation and communication sciences that could be vigorously pursued in resource poor settings at home and abroad. Let’s go where the money is.
References
1. Wang X, Liu L, Fackenthal J, Chang P, Newstead G, Chmura S, Foster I, Olopade OI. Towards an Oncology Database (ONCOD) Using a Warehousing Approach. AMIA Summits Transl Sci Proc. 2012;2012:105. Epub 2012 Mar 19
2. Blase N. Polite, Brooke E. Sylvester, and Olufunmilayo I. Olopade. Race and Subset Analyses in Clinical Trials: Time to Get Serious About Data Integration JNCI J Natl Cancer Inst (2011) 103(20): 1486-1488 first published online October 12, 2011 doi:10.1093/jnci/djr382
3. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Research Data, Nov 2010 Sub (1973-2008) —Linked To County Attributes—Total U.S., 1969-2009 Counties. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; http://www.seer.cancer.gov. Released April 2011, based on the November 2010 submission Accessed August 5, 2011.
4. Harold Varmus and Edward L. Trimble. Integrating Cancer Control into Global Health
Sci Transl Med 21 September 2011 3:101cm28. DOI:10.1126/scitranslmed.3002321