The Epidemiology and Genomics Research Program (EGRP) has initiated a strategic planning effort to develop scientific priorities for cancer epidemiology research in the next decade in the midst of a period of great scientific opportunity but also of resource constraints. EGRP would like to engage the research community and other stakeholders in a planning effort that will include a workshop in December 2012 to help shape new foci for cancer epidemiology research.
EGRP Invites Your Feedback
To facilitate this process, we invite the research community to join in an ongoing Web-based conversation to develop priorities and influence the next generation of high-impact studies.
Our aim is to enhance the application of epidemiologic methods along the translational continuum from basic discoveries to population health impact. This week, we address the use of epidemiology in knowledge integration and meta research.
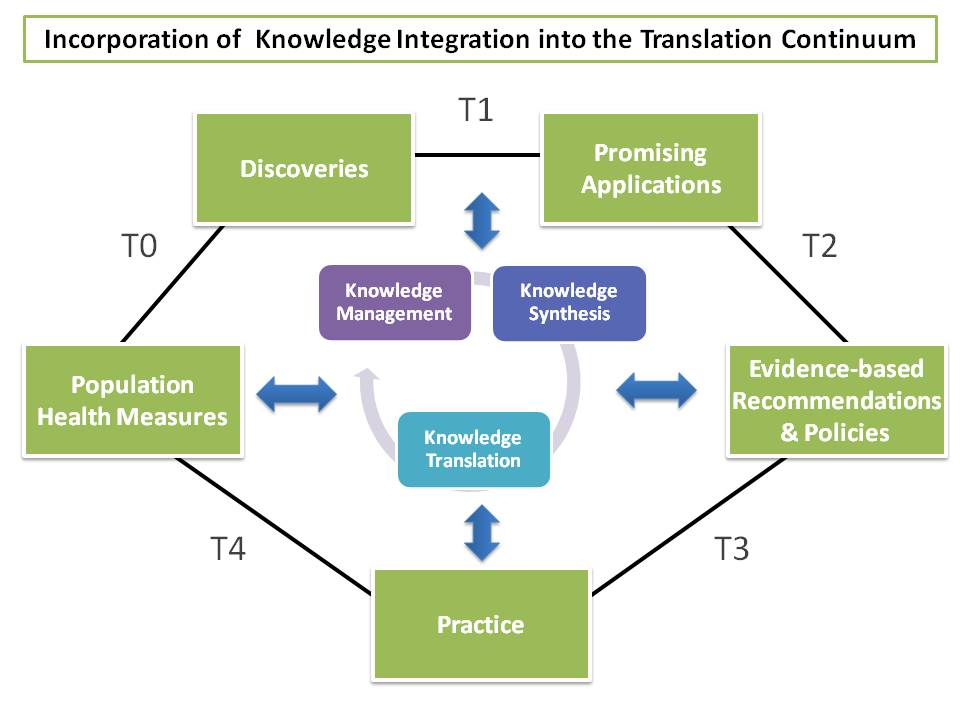
Figure 1. The continuum of translational research from discovery to reducing
the burden of disease in a population with the integration, incorporating the knowledge integration scheme.
Knowledge integration, as observed in Figure 1, serves as an engine to drive translational epidemiology by effectively using information that is generated, gathered, shared, and transformed into knowledge to improve health. Knowledge integration represents the methodological process of selecting, storing, collating, analyzing, integrating, and disseminating information within and across disciplines for the benefit of population health.
Knowledge integration consists of three processes, including knowledge management, knowledge synthesis, and knowledge translation.
Knowledge management is the process of scanning, selecting, storing, curating, and tracking information from multiple disciplines and phases of translation. An emerging field called infoveillance adopts a methodological approach to horizon scanning and surveillance by using technologies to scan databases, registries, publications for relevant information.
Knowledge synthesis is a systematic review of information across multiple disciplines to assess the validity and utility of information. Using direct and indirect evidence, knowledge synthesis models the value of information and employs meta-analysis and decision analysis.
Knowledge translation is the process of disseminating synthesized information to influence policy, develop guidelines, and close knowledge gaps, furthering the research agenda along the translation continuum. It largely involves engagement of stakeholders and knowledge brokering, a strategy which promotes interactions between researchers and end users and builds capacity for informed decision making based on sound evidence and integrated knowledge.
Knowledge integration is a multi-stakeholder enterprise that calls for adequate resources and support from both public and private sectors. The volume and rapid evolution of information requires knowledge integration to be robustly incorporated in all phases of translational epidemiology as a means of transforming knowledge that drives policy, practice, and further research.
We would like to get your feedback on the following fundamental question:
- How can epidemiology help integrate knowledge from basic, clinical, and population sciences to accelerate translation from research to practice?
Please use the comment section below to share your perspectives.
We encourage you to be as specific as possible. You can use or be inspired by the NCI Provocative Questions exercise. Your comments will be used to shape the workshop discussion in December, aspiring to transform the future of cancer epidemiology in the next decade.
Comments are also still welcome in response to first four questions of the strategic planning series:
- June 4, 2012: What Scientific Questions Should Cancer Epidemiology Address in the Next Decade to Impact Public Health?
- July 20, 2012: How Should New Technologies Be Integrated into Cancer Epidemiology?
- August 27, 2012: What Have We Learned from Epidemiology Cohorts and Where Should We Go Next?
- September 26, 2012: How Can We Use Epidemiology to Bridge Evidence Gaps and Translate Research Discoveries into Clinical and Public Health Practice?
EGRP’s Workshop Science Advisory Group





4 comments
Skip to comment form ↓
Katrina Goddard
October 16, 2012 at 2:39 PM (UTC -5) Link to this comment
There are many opportunities for epidemiology to promote knowledge synthesis to accelerate the translation of genomic applications from research to practice. What we know and understand about the human genome will continue to evolve rapidly with the advent of new technologies, such as for sequencing, and the capability to apply these technologies at a population level. The knowledge base is expanding to include novel gene-phenotype associations and greater characterization of the mutational spectrum within genes. As these technologies are applied to populations, we will refine our understanding of the magnitude of effect at a population level, which is not well represented by early cases that tend to be more extreme. These opportunities come with the major challenge of distinguishing the true signals from the background noise that is present in the flood of data from billions of observations in the genome. At the same time, for rare conditions or consideration of nuances in genetic subgroups we may never have sufficient sample size for what are considered rigorous and robust study designs in other settings. We cannot simply do nothing for these patients, but must employ more creative solutions such as modeling or observational designs using electronic medical record data to move the field forward. ‘Natural experiments’ in the delivery of genomic medicine through different public health and healthcare systems across nations will promote international collaboration as a tool for fostering a better understanding of what works. Harnessing this knowledge will require an efficient and flexible approach to integration, but we must also be careful to maintain rigor, reproducibility, and transparency for the system to be trustworthy. This balancing act can be supported by developments such as new electronic tools to improve automation and search algorithms, greater standardization in nomenclature, coding, and reporting, and clear delineation of evidentiary standards and frameworking. Additional tools including value of information, decision analysis, and modeling can pinpoint the most critical issues for knowledge synthesis, reserving the most resource intensive methods for use only when absolutely essential. Epidemiological principles are the foundation underlying these developments that will promote evidence-based practice in genomic medicine.
Robert Hiatt
October 22, 2012 at 9:20 AM (UTC -5) Link to this comment
Knowledge integration is “the effective incorporation of knowledge into the decisions, practices and policies of organizations and systems” (1). The discipline of epidemiology is unique in the biomedical sciences in its ability to integrate knowledge across multiple levels of organization from genes to social determinants. The imperatives of the 21st century require faster and more effective translation of scientific knowledge into applications; epidemiologists are well placed to be strong team members and leaders in this process. Rigorous etiologic and intervention research remains the core set of skills, but the role of the epidemiology will also be key to effective dissemination and implementation of that research into “decisions, practices and policies of organizations and systems”.
How can epidemiologists most effectively play this role?
Big Data. We live in an age of increasing amounts of data derived from multiple levels of organization from gene arrays and electronic medical records to mega-cohorts and social networks (2). Epidemiology has always worked to discern patterns in large aggregations of data, but the discipline must be directed with new energy to methods and approaches of detecting these patterns in ever larger datasets (e.g., data mining, complex systems analysis and social network analysis). Professional societies and funders need to support efforts to advance new methods for the analysis of large amounts of data. Academic institutions need to reorganize curricula and support cross-disciplinary groups to provide relevant education and research opportunities.
Team, transdisciplinary and translational science. Translational science is best carried out in collaborating teams taking a transdiscipinary approach to a common problem (3). Modern day epidemiologists work in teams, but they are often turned to for methodologic expertise only. Epidemiologists also have a unique perspective on the upstream origins of disease because of their understanding of population patterns and disease determinants. Because of this perspective they can make a larger contribution to translational science and knowledge integration. The dissemination and implementation of knowledge will require interactions with other non-biomedical sectors of society such as business, the law, engineering and urban planning. However, among barriers to effective team science are concerns about career advancement when researchers cross disciplinary boundaries. These barriers must be overcome if epidemiologists are to make their fullest contribution to advancing knowledge and action in population health.
Education and Training. Health professional training in the next century will be charged with incorporating a global perspective and an appreciation of population health into the curriculum. The Commission on Education of Health Professionals for the 21st Century has outlined a comprehensive approach to this broader orientation, now being explored by health science campuses around the world (4). Epidemiologists should seek opportunities to contribute to this exploration since our discipline is ideally situated to instilling an understanding of multilevel origins of disease and ill health as well as the methodologic tools to study them.
Thus, in at least these three areas of big data; translational, team and transdiscipliary science; and a transformation in health professional education epidemiology has a major role to play in knowledge integration in population health in the 21st century.
1. Best A, Hiatt RA, Norman CD. Knowledge integration: Conceptualizing communications in cancer control systems. Patient Educ Counseling 2008;71:319-327.
2. National Research Council. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease, 2011, National Academies Press, Wash D.C.
3. Hiatt RA. Epidemiology: key to translational, team and transdisciplinary science. Ann Epidemiol 2008;18:859-861. PMID: 18823793.
4. Frenk J, Chen L, Bhutta ZA, et al. Health professionals for a new century: transforming education to strengthen health systems in an interdependent world. Lancet 2010;376:1923-1958.
John Ioannidis
October 31, 2012 at 2:24 PM (UTC -5) Link to this comment
Knowledge integration includes knowledge management, synthesis, and translation processes. It aims to maximize the utility of collected scientific information and accelerate translation of discoveries into individual and population health benefits. Accumulated evidence in cancer epidemiology constitutes a large share of the 2.7 million papers on cancer in PubMed. We examine the landscape of knowledge integration in cancer epidemiology. Past approaches have mostly used retrospective efforts of knowledge management and traditional systematic reviews and meta-analyses. Systematic searches identify 2,332 meta-analyses, about half of which are on genetics and epigenetics. Meta-analyses represent 1:86-1:1162 of published articles in various cancer sub-fields. Recently there are more collaborative meta-analyses with individual-level data, including those with prospective collection of measurements (e.g. genotypes in genome-wide association studies); this may help increase the reliability of inferences in the field. However, most meta-analyses are still done retrospectively with published information. There is also a flurry of candidate gene meta-analyses from China with spuriously prevalent “positive” results. Prospective design of large research agendas, registration of datasets, and public availability of data and analyses may improve our ability to identify knowledge gaps, maximize and accelerate translational progress or – at a minimum – recognize dead ends in a more timely fashion.
Ann Graham Zauber
November 15, 2012 at 3:44 PM (UTC -5) Link to this comment
How Can Epidemiology Help Integrate Knowledge from Basic, Clinical and Population Sciences to Accelerate Translation from Research to Practice?
Population based microsimulation modeling is now a readily usable tool to integrate knowledge from basic, clinical and population sciences. Furthermore such a tool has the facility to evaluate how best to translate actions from research to practice. In particular the Cancer Intervention and Surveillance Modeling Network (CISNET), sponsored by the National Cancer Institute, provides for population based answers to interventions of risk factors, screening, and treatment for 5 cancer types: breast, colon, esophageal, lung, and prostate. These models include a natural history of disease from initiation, pre-cancer, pre-clinical cancer, clinical cancer, and cancer death. The models are informed by epidemiological studies on natural history, risk factor impact, and screening, as well as clinical studies of treatment. A summary of these models and a model profiler for each can be obtained at http://cisnet.cancer.gov/.
As summarized by the recent publication of the ISPOR-SMDM modeling good research practices1, “models are essentially communication tools that allow the complexity of a given system to be reduced to its essential elements.” In this sense the CISNET models describe the natural history of disease and the impact of intervening on the natural history of disease for a population over an extended time period.
The natural history of disease is informed from epidemiological and clinical studies. For colorectal cancer (CRC) the natural history model is based on the prevalence of adenomas, which are the pre-cursor lesions for colorectal cancer, as primarily ascertained in autopsy studies, and as linked to the resulting incidence rates in the pre-screening era of 1975-1979 as detailed in the SEER9 registries. What is relatively unknown is which combination of transition factors trigger one of more adenomas to advance to cancer. In addition what is the shape and range of the sojourn time from an adenoma to transition to cancer? For example the transition from adenoma to carcinoma is expected to be shorter for those affected with Lynch syndrome than those in the general population.
The impact of interventional strategies of risk factor modulation and screening scenarios on colorectal cancer mortality projections is presented at http://cisnet.cancer.gov/projections/colorectal/. The impact of these interventions is heavily provisional on whether members of the population are willing to undertake behavioral changes of risky behavior and also to take up CRC screening on a regular basis. Furthermore the pathway of effect of an intervention is not clearly known. These modeling exercises also delineate where knowledge is still needed. Does a smoking cessation program affect the initiation of adenomas or the transition of adenomas to cancer at a faster rate or for a larger number of adenomas? A benefit of modeling to inform health policy is illustrated by a report to the United States Preventive Services Task Force in 20082 by the CRC CISNET group to assess screening recommendations for ages to begin screening (40, 50, 60), to end screening, (75 or 85), types of screening tests (fecal occult blood tests, flexible sigmoidoscopy, and colonoscopy), and intervals of repeat screening (1,2, 3 for FOBT, and 5, 10, or 20 for endoscopy).
Examples of the use of these models to inform health policy and where there are gaps in knowledge will be presented.
1. Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices – overview: a report of ISPOR-SMDM modeling good research practices task force -1. Http://mdm.sagepub.com/content/32/5/667
2. Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008 Nov 4; 149(9):659-69. Epub 2008 Oct 6. Summary for patients in: Ann Intern Med. 2008 Nov 4; 149(9):I-44.