caBIG Clinical Trials Suite
![]() The caBIG® Clinical Trials Suite is an integrated set of open-source standards-based tools that facilitate electronic management of clinical trials and associated data, and enable comprehensive sharing and integration of clinical research information. The Suite supports not only cancer clinical trials, but all clinical trials. The Suite is part of the National Cancer Institute's overarching goal to connect the people, institutions, and data in the research community through caBIG®. The open-source tools in the caBIG® Clinical Trials Suite are intended for use by clinical researchers.
The caBIG® Clinical Trials Suite is an integrated set of open-source standards-based tools that facilitate electronic management of clinical trials and associated data, and enable comprehensive sharing and integration of clinical research information. The Suite supports not only cancer clinical trials, but all clinical trials. The Suite is part of the National Cancer Institute's overarching goal to connect the people, institutions, and data in the research community through caBIG®. The open-source tools in the caBIG® Clinical Trials Suite are intended for use by clinical researchers.
|
Suite Tools
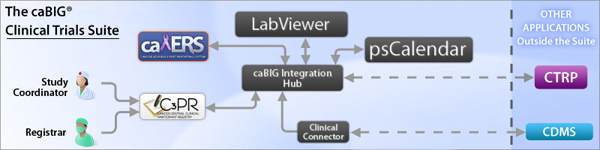
caBIG® Clinical Participant Registry (C3PR) – enables efficient and streamlined registration of participants into clinical trials. Improvements from previous versions include a user setup module and improved performance and usability.
caBIG® Patient Study Calendar (PSC) – allows organizations to centrally and consistently manage study participant schedules in clinical trials. Improvements from previous versions include a user-friendly dashboard interface and enhanced management of templates.
caBIG® Adverse Event Reporting System (caAERS) – used to manage the collection and reporting of adverse event data obtained during clinical trials. Improvements from previous versions include support for new versions of the NCI Enterprise Services, expanded support of FDA and NCI adverse event reporting requirements, and enhanced robustness of functionality.
caBIG® Lab Viewer – used to view laboratory data in transit between clinical source systems (typically in-house clinical chemistry laboratory systems) and clinical trials systems (typically Clinical Data Management Systems). Improvements from previous versions include complete integration with NCI Enterprise Services and enhanced search capability.
The caBIG® Clinical Connector - provides a semantically integrated service layer via caGrid that allows users of a Clinical Data Management System (CDMS) to expose functions within the CDMS. The exposed service layer uses a BRIDG-based model and defines service operations (including patient registration and loading of laboratory test data) that can be implemented by a CDMS.
caBIG® Integration Hub (formerly caXchange) – enables tools and applications in the caBIG® Clinical Trials Suite, and beyond, to interface seamlessly with one another. Improvements from previous versions include integration with secure NCI Enterprise Services and integration with Clinical Connector for subject registration and load lab messages.
Deploying the Clinical Trials Suite
All the tools include support for NCI's Build and Deployment Automation (BDA) framework, enabling quick and easy deployments of the tools in the Suite, and have enhanced support for NCI Enterprise Services to improve consistency and interoperability of information across the enterprise. The caBIG® program is actively researching and developing a comprehensive set of services that describe key functionality across the biomedical enterprise, and is updating all existing software applications to reuse these components. These efforts, in addition to more accurately describing the business processes within a biomedical research organization, will reduce software development time going forward while enabling greatly improved interoperability between applications, whether they are developed internally or externally.
Each tool provides an end-user-oriented graphical user interface as well as a Java-based Application Programming Interface (API) and a caGrid-compatible Grid Service for programmatic access to data. All of the applications are built on a common set of open-source technologies for ease of installation and maintenance.
The caBIG® Clinical Trials Suite can be downloaded, either as a single bundled distribution or as individual standalone components.
