Access to CADP Resources
Application Deadlines
The Clinical Assay Development Program (CADP) is designed to assist translation to the clinic of novel clinical assays. CADP is not a grant program. Approved proposals gain access to assay development services and resources through the CADP. A panel of experts will review the applications based on scientific merit, feasibility, clinical need or impact and path to clinical implementation. For more information, see Instructions to applicants.
CADP proposals are accepted following the schedule below:
| Cycle Open for Submission | Submission Deadline |
|---|---|
| January 10, 2013 | February 15, 2013 |
| April 29, 2013 | June 15, 2013 |
| August 26, 2013 | October 15, 2013 |
Note: At this time our access to blood samples is limited. If your project requires blood, please send an inquiry to NCI_CADPinfo@mail.nih.gov before submitting an application.
Applications are due by 11:59 PM ET on the deadline dates. Applications will not be accepted after the deadline. Applicants are advised to finalize submission of their proposals prior to the deadline date.
All proposals must be submitted online via the proposalCENTRAL site. Please note that the Principal Investigator (the Applicant) is responsible for final submission.
If you have used proposalCENTRAL in the past, you can use the same username and password.
Eligibility
Definitions:
Analytical performance (analytical validity): how accurately the test detects the analyte(s) of interest.
Clinical validity: how well the test relates to the clinical outcome of interest, e.g., response to therapy, survival, etc.
Clinical utility: whether the results of the test provide information that can contribute to and improve current optimal management of the patient’s disease.
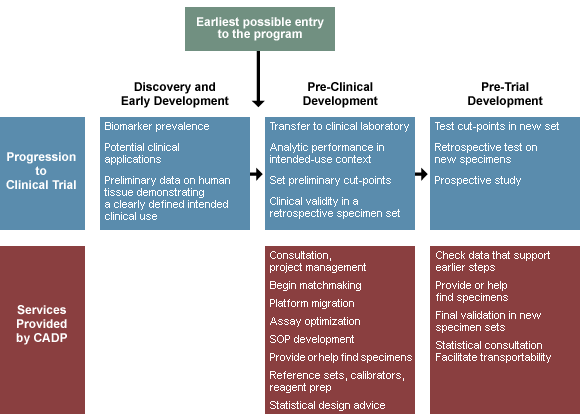
In order to be eligible for services of the CADP, a working prototype assay must exist and its intended clinical use must be clearly defined. As depicted in the figure, assays may be at various stages of technical and clinical development when submitted, and those assays that have progressed further in the analytical and clinical validation process often still need services provided by the CADP prior to use in a clinical study.
At a minimum the submitter must provide information about the following (detailed information requirements are noted in the Instructions section):
Clinical Use: basic description of the intended clinical use, including the disease type, stage, and treatment decision resulting from use of the assay. What is the expected clinical benefit resulting from the use of this assay? What is the potential harm resulting from a false assay result?
Technical Feasibility: description of the prototype assay including an assay protocol, a list of reagents and equipment used, and associated assay data obtained using human specimens. The specimens used also need to be described.
Current Status of Assay: Analytical and Clinical Validity: description of minimal clinical sensitivity, specificity, repeatability, precision, positive and negative predictive values required for successful application of this assay and data already generated. Specification of the intended clinical sample (e.g. blood, fresh tissue, formalin-fixed paraffin-embedded tissue section).
Plans for the Assay following CADP Development: description of planned clinical study of the assay (e.g. Will it be used for retrospective analysis of samples in a specific clinical trial? Will it be used as an integral assay in a prospective clinical trial?). What are the timelines for assay delivery?
Instructions
General Instructions
The type of information and data that will have to be submitted as part of an application for the services offered by the CADP is described below. The applicant should begin with the background and rationale, a brief description of the current state of the assay and a list of the services being requested from the CADP (no more than one page). For pre-trial assays further along in development, details covering the analytical validation steps and data should be included.
Required Documents for CADP Applications
1. CADP Application
Application to the Clinical Assay Development Program can only be made through proposalCENTRAL. Applicants will be asked to outline the scientific nature and rationale of the proposed project including the reason for development of the assay, a brief description of the current state of the assay and a list of the services being requested from the CADP. Data generated to date, related literature or a sound hypothesis that correlates the assay result (biomarker) with clinical outcome or a clinical action to support the further development are also requested. Detailed data and/or literature review can be supplied as appendices. If available, data that demonstrate the technical feasibility and analytic and clinical performance of the assay should be provided. The specimen requirements for the services requested should be described. The expected path to clinical implementation should be stated. If other assays exist for this clinical use, an explanation should be provided addressing why this assay is needed. Finally, the status of any real or potential intellectual property rights must be disclosed. The Principal Investigator will be expected to provide a biosketch in the NIH standard format. Additional documentation may be requested as appropriate. Complete instructions for filling out the application are at the proposalCENTRAL website. You can preview them here.
Applications should be submitted according to the published Application Deadlines.
Proposal Resubmission Instructions
A resubmission is submission of an application that has previously been reviewed. The NCI will accept only a single resubmission to the CADP. If the resubmission also fails to receive access to NCI resources, the applicant should submit a new application. A new application should be a completely new project or a substantive redesign of the project, and should not simply incorporate minor changes in response to the previous review.
The resubmission can only be submitted through proposalCENTRAL.
Resubmission applications should be resubmitted according to the published Application Deadlines.
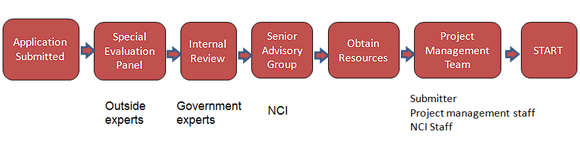
Prioritization Criteria and Selection Process
All applications will be evaluated by a special emphasis panel (SEP) of outside experts. The evaluation criteria and rating system are shown below.
| Scientific Merit (30%) | There should be a sound biologic hypothesis and preliminary data for the intended use that associate marker(s) for drug effects or state of disease with a known clinical outcome supported by the current state of the field. The marker should be expected to have clinical utility. |
|---|---|
| Impact/ Clinical Need (30%) | If successful, the proposed diagnostic should be likely to change clinical practice and have a major impact on the outcome of treatment. |
| Feasibility (30%) | The approach should be technically feasible. The assay platform and specimen requirements should allow straightforward implementation for clinical use. |
| Path to Clinical Implementation (10%) | The path to clinical implementation should be clear. |
The results of the SEP’s evaluations will be forwarded to an internal committee of NCI and other government agency representatives. The internal committee will make recommendations based on available resources and prioritization criteria including:
- The predicted clinical impact
- Clinical specimen needs/availability
- Scientific rigor, including of the translational science to move the assay into a clinical study
- Overall feasibility
- Relationship with a trial partner for a specific trial
- Cost of services (which could include assessment of cost-sharing plans)
The final decisions regarding which applications will be supported will be made by the NCI Senior Advisory Committee, which will also examine the full portfolio of projects, timelines related to clinical trials, and other factors related to potential impact.