FY 2008 Congressional Budget Justification
On this page:
- Organization Chart
- Budget Authority by Institute and Center
- Budget Authority by Mechanism
- Budget Authority by Program
- Justification of Budget Request
- Director’s Overview
- Justification by Activity Detail
Organization Chart
Budget Authority by Institute and Center
| Institute/Center | FY 2006 Actual | FY 2007 Continuing Resolution | FY 2008 Estimate | Change |
|---|---|---|---|---|
| NCI | $253,666,000 | $253,666,000 | $253,709,000 | $43,000 |
| NHLBI | 67,321,000 | 67,351,000 | 67,656,000 | 305,000 |
| NIDCR | 19,688,000 | 19,688,000 | 19,804,000 | 116,000 |
| NIDDK | 30,898,000 | 30,898,000 | 30,933,000 | 35,000 |
| NINDS | 45,937,000 | 45,799,000 | 43,878,000 | -1,921,000 |
| NIAID | 1,475,079,000 | 1,471,559,000 | 1,477,022,000 | 5,463,000 |
| NIGMS | 53,007,000 | 53,007,000 | 53,297,000 | 290,000 |
| NICHD | 133,555,000 | 133,555,000 | 133,850,000 | 295,000 |
| NEI | 10,585,000 | 10,585,000 | 10,585,000 | — |
| NIEHS | 7,513,000 | 7,513,000 | 5,310,000 | -2,203,000 |
| NIA | 5,389,000 | 5,389,000 | 5,555,000 | 166,000 |
| NIAMS | 4,866,000 | 4,866,000 | 4,136,000 | -730,000 |
| NIDCD | 1,412,000 | 1,412,000 | — | — |
| NIMH | 176,839,000 | 176,413,000 | 177,691,000 | 1,278,000 |
| NIDA | 297,201,000 | 296,470,000 | 296,506,000 | 36,000 |
| NIAAA | 26,681,000 | 26,617,000 | 27,033,000 | 416,000 |
| NINR | 12,114,000 | 12,114,000 | 12,264,000 | 150,000 |
| NHGRI | 6,835,000 | 6,835,000 | 6,228,000 | -607,000 |
| NIBIB | 1,038,000 | 1,038,000 | 738,000 | -300,000 |
| NCRR | 160,992,000 | 160,992,000 | 161,049,000 | 57,000 |
| NCCAM | 2,285,000 | 2,285,000 | 2,281,000 | -4,000 |
| NCMHD | — | — | — | — |
| FIC | 22,765,000 | 22,706,000 | 22,783,000 | 77,000 |
| NLM | 7,376,000 | 7,376,000 | 7,314,000 | -62,000 |
| OD | 60,235,000 | 60,290,000 | 58,290,000 | -2,000,000 |
| B&F | — | — | — | — |
| TOTAL, NIH | 2,883,277,000 | 2,878,424,000 | 2,877,912,000 | -512,000 |
| Roadmap | 18,582,000 | 24,859,000 | 27,307,000 | 2,448,000 |
| Total, including Roadmap | 2,901,859,000 | 2,903,283,000 | 2,905,219,000 | 1,936,000 |
Budget Authority by Mechanism
(Dollars in thousands)
| Mechanism | FY 2006 Actual | FY 2007 Continuing Resolution | FY 2008 Estimate | Change | ||||
|---|---|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | No. | Amount | |
| Research Projects | ||||||||
| Noncompeting | 1,991 | $1,029,689 | 1,907 | $1,092,525 | 2,013 | $1,202,283 | 106 | $109,758 |
| Administrative supplements | (164) | 123,003 | (81) | 15,944 | (81) | 14,889 | 0 | -1,055 |
| Competing | 564 | 405,981 | 816 | 431,183 | 839 | 337,077 | 23 | -94,106 |
| Subtotal, competing | 564 | 405,981 | 816 | 431,183 | 839 | 337,077 | 23 | -94,106 |
| Subtotal, RPGs | 2,555 | 1,558,673 | 2,723 | 1,539,652 | 2,852 | 1,554,249 | 129 | 14,597 |
| SBIR/STTR | 56 | 22,694 | 51 | 20,507 | 51 | 20,481 | 0 | 14,597 |
| Subtotal, RPGs | 2,611 | 1,581,367 | 2,774 | 1,560,159 | 2,903 | 1,574,730 | 129 | 29,194 |
| Research Centers | ||||||||
| Specialized/ comprehensive | 52 | 114,079 | 55 | 121,060 | 53 | 122,327 | -2 | 1,267 |
| Clinical research | 7 | 44,570 | 8 | 44,514 | 8 | 44,362 | 0 | -152 |
| Biotechnology | 2 | 3,777 | 3 | 3,951 | 3 | 3,951 | 0 | 0 |
| Comparative medicine | 16 | 58,100 | 15 | 59,907 | 15 | 59,935 | 0 | 28 |
| Research Centers in Minority Institutions | 3 | 11,649 | 3 | 12,518 | 3 | 12,518 | 0 | 0 |
| Subtotal, Centers | 80 | 232,175 | 84 | 241,950 | 82 | 243,093 | -2 | 1,143 |
| Other Research | ||||||||
| Research careers | 286 | 38,027 | 287 | 37,869 | 285 | 37,632 | -2 | -237 |
| Cancer education | 0 | 38 | 0 | 38 | 0 | 38 | 0 | 0 |
| Cooperative clinical research | 16 | 34,007 | 11 | 26,761 | 11 | 26,663 | 0 | -98 |
| Biomedical research support | 1 | 1,330 | 0 | 1,460 | 0 | 1,460 | 0 | 0 |
| Minority biomedical research support | 2 | 615 | 2 | 615 | 2 | 615 | 0 | 0 |
| Other | 117 | 60,484 | 109 | 58,219 | 109 | 58,569 | 0 | 350 |
| Subtotal, Other Research | 422 | 134,501 | 409 | 124,962 | 407 | 124,977 | -2 | 15 |
| Total Research Grants | 3,113 | 1,948,043 | 3,267 | 1,927,071 | 3,392 | 1,942,800 | 125 | 30,352 |
| Ruth L. Kirschstein Training Awards: | FTTPs | FTTPs | FTTPs | FTTPs | ||||
| Individual awards | 82 | 3,408 | 81 | 3,384 | 81 | 3,384 | 0 | 0 |
| Institutional awards | 709 | 31,318 | 700 | 31,284 | 693 | 31,116 | -7 | -168 |
| Total, Training | 791 | 34,726 | 781 | 34,668 | 774 | 34,500 | -7 | -168 |
| Research & development contracts | 289 | 447,760 | 262 | 463,128 | 270 | 450,846 | 8 | -12,282 |
| (SBIR/STTR) | (3) | (182) | (3) | (182) | (3) | (182) | (0) | (0) |
| Intramural research | 289,325 | 288,781 | 286,212 | -2,569 | ||||
| Research management and support | 95,812 | 97,110 | 97,950 | 840 | ||||
| Construction | 0 | 0 | 0 | 0 | ||||
| Library of Medicine | 7,376 | 7,376 | 7,314 | -62 | ||||
| Office of the Director | 60,235 | 60,290 | 58,290 | -2,000 | ||||
| Total BA without Roadmap | 2,883,277 | 2,878,424 | 2,877,912 | -512 | ||||
| RoadMap Support | 18,582 | 24,859 | 27,307 | 2,448 | ||||
| Total BA including Roadmap | 2,901,859 | 2,903,283 | 2,905,219 | 1,936 | ||||
Budget Authority by Program
(Dollars in thousands)
| Area of Emphasis | FY 2004 Actual | FY 2005 Actual | FY 2006 Actual | FY 2007 Continuing Resolution | FY 2008 Estimate | Change |
|---|---|---|---|---|---|---|
| HIV Microbicides1 | — | — | $85,693 | $85,453 | $96,400 | $10,947 |
| Vaccines | $452,269 | $508,974 | 581,450 | 591,679 | 596,194 | 4,515 |
| Behavioral and Social Science | 413,946 | 418,106 | 406,217 | 398,761 | 398,761 | — |
| Therapeutics | 728,492 | 732,159 | 635,434 | 633,100 | 622,427 | -10,673 |
| Etiology and Pathogenesis | 731,526 | 741,662 | 716,239 | 709,107 | 709,107 | — |
| Natural History and Epidemiology | 320,010 | 297,070 | 269,835 | 264,311 | 261,463 | -2,848 |
| Training, Infrastructure, and Capacity Building | 154,164 | 168,645 | 160,686 | 169,591 | 168,211 | -1,380 |
| Information Dissemination | 39,601 | 42,765 | 27,723 | 26,422 | 25,349 | -1,073 |
| Subtotal | 2,840,008 | 2,909,381 | 2,883,277 | 2,878,424 | 2,877,912 | -512 |
| Roadmap | 10,572 | 11,130 | 18,582 | 24,859 | 27,307 | 2,448 |
| Total | $2,850,580 | $2,920,511 | $2,901,859 | $2,903,283 | $2,905,219 | $1,936 |
1 Beginning in FY 2008, HIV Microbicides will be a separate activity. Dollars for HIV Microbicides were previously included within other science areas, such as Therapeutics, Etiology and Pathogenesis, and Behavioral and Social Science. The FY 2006 and FY 2007 amounts are comparable budget figures.
Trans-NIH AIDS Research Budget Justification
| FY 2006 Actual | FY 2007 Continuing Resolution | FY 2008 Estimate | Increase or Decrease | |
|---|---|---|---|---|
| Budget Authority | $2,901,859,000 | $2,903,283,000 | $2,905,219,000 | +$1,936,000 |
Director’s Overview
The FY 2008 budget request for NIH AIDS research is $2,905,219,000, which represents an increase of $1,936,000 above the FY 2007 Continuing Resolution. This amount includes the total trans-NIH funding for intramural and extramural research; research management support; research centers; and basic and clinical research on HIV/AIDS, as well as the wide spectrum of its associated malignancies, opportunistic infections, co-infections, and clinical complications.
Global AIDS Pandemic
As of the End of 2006
- Approximately 40 million people worldwide are living with HIV/AIDS;
- Approximately 2.3 million are children under the age of 15 years;
- About half of the infected adults are women;
- An estimated 4.3 million people (adults and children) acquired HIV in 2006;
- The global HIV/AIDS epidemic killed approximately 3 million people in 2006; and
- More than 25 million people have died since the beginning of the epidemic.
Source: UNAIDS
The AIDS pandemic will continue to wreak devastating consequences around the world for decades to come for virtually every sector of society. The pandemic affects the future of families, communities, military preparedness, national security, political stability, national economic growth, agriculture, business, healthcare, child development, and education in countries around the globe. AIDS is the deadliest epidemic of our generation. The United Nations General Assembly’s Declaration of Commitment on HIV/AIDS states, “...the global HIV/AIDS epidemic, through its devastating scale and impact, constitutes a global emergency and one of the most formidable challenges to human life and dignity, as well as to the effective enjoyment of human rights, which undermines social and economic development throughout the world and affects all levels of society…” In the United States, HIV infection rates are continuing to climb among women, racial and ethnic minorities, young men who have sex with men, individuals with addictive disorders, and people over 50 years of age.1 The use of antiretroviral therapy is now associated with a series of side effects and long-term complications that may have a negative impact on mortality rates. The appearance of multi-drug resistant strains of HIV presents an additional serious public health concern.2 In addition, CDC has reported increased cases of HIV-tuberculosis (TB) coinfection and an increase in cases of drug-resistant TB. This is a major public health concern because of the highly contagious nature of TB. According to CDC reports, approximately one quarter of the HIV-infected population in the United States also is infected with hepatitis C virus (HCV). HCV progresses more rapidly to liver damage in HIV-infected persons and may also impact the course and management of HIV infection; and HIV may change the natural history and treatment of HCV.3 These data forebode an epidemic of even greater magnitude in the coming years.
NIH represents the largest and most significant public investment in AIDS research in the world. Our response to the pandemic requires a unique and complex multi-institute, multi-disciplinary, global research program. Perhaps no other disease so thoroughly transcends every area of clinical medicine and basic scientific investigation, crossing the boundaries of nearly every NIH Institute and Center (IC). This diverse research portfolio demands an unprecedented level of scientific coordination and management of research funds. The Office of AIDS Research (OAR), located within the Office of the Director, coordinates the scientific, budgetary, legislative, and policy elements of NIH AIDS research. Through its unique, comprehensive trans-NIH planning, budgeting, and portfolio assessment processes, OAR is enhancing collaboration, minimizing duplication, and ensuring that research dollars are invested in the highest priority areas of scientific opportunity that will lead to new tools in the global fight against AIDS.
Trans-NIH Strategic Plan: OAR develops the annual Trans-NIH Plan for HIV-Related Research, in collaboration with the ICs; non-government experts from academia, foundations, and industry; and community representatives. The Plan and the unique processes instituted by OAR to ensure its implementation allow NIH to pursue a united research front against the global AIDS epidemic. OAR has established trans-NIH Coordinating Committees for each of the major scientific areas of the Plan. These committees, comprised of representatives of the ICs with major research portfolios in that area, provide an ongoing mechanism for collaboration, coordination, and information exchange. The planning process serves to monitor and assess scientific progress on an annual basis, eliminating strategies where research is no longer necessary; adding new strategies where research has uncovered new questions; and reprioritizing objectives when the science has moved or changed. The Plan is used to: frame the development of the NIH AIDS research budget; determine the use of NIH AIDS-designated dollars; define those research areas for which AIDS-designated funds may be allocated; track and monitor AIDS research expenditures; and inform the scientific community, the public, Congress, and AIDS-affected communities about the priorities of the NIH AIDS research agenda.
Trans-NIH Portfolio Analysis: OAR continues to reassess and refine the planning process to better capture the broadest range of scientific expertise and to identify the highest scientific priorities. In FY 2006, a critical new element was added to the annual process—a multi-tiered comprehensive trans-NIH review of all grants and contracts supported with AIDS-designated funds. This review established a new model to ensure that AIDS research dollars support the highest priority science taking into account the ever-changing domestic and international AIDS epidemic as well as the evolving scientific opportunities. This process allows OAR to redirect funds to better manage the AIDS research portfolio and assists OAR in developing the trans-NIH AIDS research budget.
Trans-NIH Budget: The trans-NIH AIDS research budget, developed by OAR, is explicitly tied to the objectives of the strategic Plan. The ICs submit their AIDS-related research budget requests to OAR for each scientific area, presenting their proposals for new or expanded program initiatives over the amounts committed for existing multi-year awards, coded to specific Plan objective(s). OAR reviews the IC initiatives in relation to the Plan, its priorities, and to other IC submissions to eliminate redundancy and/or to assure cross-Institute collaboration. The NIH Director and the OAR Director together determine the total amount to be allocated for AIDS-related research within the overall NIH budget. Within that total, OAR then develops each IC’s allocation for AIDS-related research based on the scientific priority of each proposed initiative. This process continues at each step of the budget development process up to the time of the final appropriation. The careful determination of the balance of the research budget—among Institutes, among areas of science, between AIDS and non-AIDS, between intramural and extramural, between basic and clinical, and between investigator-initiated and targeted—requires a comprehensive knowledge of the science and of the Institute portfolios. Dollars are allocated to the ICs based not on a formula, but on the priorities of the Plan, scientific opportunities, and the IC’s capacity to absorb and expend resources for the most meritorious science. At the time of the appropriation, OAR informs each IC of its AIDS-related budget allocation level, specifying amounts for each approved initiative. As each IC awards AIDS-related research grants, those dollars are coded to the appropriate objective(s) of the Plan and reported to the OAR’s AIDS Research Information System, a trans-NIH database of all AIDS-related expenditures, including extramural, intramural, and research management and support.
AIDS Research Conducted in International Settings: NIH maintains a strong portfolio of research conducted in international settings, now encompassing more than 90 countries around the world. Such research crosses all the scientific areas, including efforts to develop: HIV vaccine and microbicide candidates to prevent sexual transmission; behavioral strategies targeted to the individual, family, and community to alter risk behaviors associated with sexual activity and drug and alcohol use; drug and non-drug strategies to prevent mother-to-child transmission; therapeutics for HIV-related coinfections and other conditions; and approaches to using antiretroviral therapy in resource-poor settings. Before prevention and treatment interventions can be implemented in different geographic settings, their safety must be confirmed and efficacy demonstrated in such settings through clinical trials and other intervention research. To develop vaccines and other prevention strategies that will be effective globally, Phase I safety studies are first conducted in small populations in the U.S. To establish efficacy, large numbers of at-risk study participants are necessary. Because of the large populations at high risk of infection, prevention studies can be more efficiently conducted in international settings. Development of a research infrastructure is essential to these research programs. All expenditures for research conducted internationally are tracked by country through the AIDS Research Information System after the funds are awarded. Most of these funds are awarded to U.S.-based investigators for research in collaboration with scientists in the host country. Some funds are awarded directly to investigators in international research institutions.
AIDS Research Conducted in International Settings
(Dollars in millions)
| FY 2006 Actual | FY 2007 Continuing Resolution | FY 2008 Estimate |
|---|---|---|
| $373 | $372 | $373 |
Cross-Over Benefits: The NIH research investment is reaping even greater dividends as AIDS research is unraveling the mysteries surrounding many other infectious, malignant, neurologic, autoimmune, and metabolic diseases. AIDS research has provided an entirely new paradigm for drug design, development, and clinical trials to treat viral infections. For example, the drug known as 3TC, developed to treat HIV/AIDS, is now the most effective therapy for chronic hepatitis B infection. Drugs developed to prevent and treat AIDS-associated opportunistic infections also provide benefit to patients undergoing cancer chemotherapy or receiving anti-transplant rejection therapy. AIDS research also is providing a new understanding of the relationship between viruses and cancer.
Total NIH AIDS Research Funding by Fiscal Year
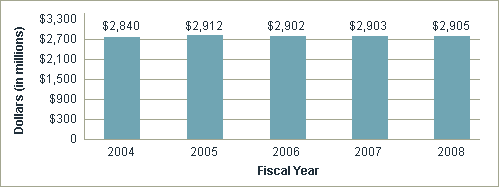
Justification of the FY 2008 Budget by Activity Detail
Through its trans-NIH planning, portfolio analysis, and budget processes, OAR shifted funds across ICs and across activities to ensure that the highest scientific priorities are supported in FY 2008. These priorities are primarily in the area of HIV prevention research, particularly the development of microbicides and vaccines. The AIDS pandemic continues to expand worldwide, and will only be slowed or halted through these critical prevention strategies. Within the total budget request, funding has been increased above the FY 2007 Continuing Resolution (CR) level only for the areas of microbicides and vaccine research. In order to provide those increases, funding for the activity areas of behavioral and social science, and for etiology and pathogenesis are unchanged from the FY 2007 CR; and funds have been reduced below the estimated FY 2007 level in all other activity areas.
Microbicides
Purpose and Methods of Operation: The vulnerability of women to acquiring HIV infection requires the development of effective and acceptable female-controlled chemical and physical barrier methods, such as topical microbicides, to reduce HIV transmission. NIH supports a comprehensive microbicide research program that includes the screening, discovery, development, preclinical testing, and clinical evaluation of compounds with the potential to act as antimicrobial agents with both spermicidal and non-spermicidal activity. Animal model testing and toxicity studies of potential candidate compounds are conducted through NIH-sponsored contracts before these agents are considered for clinical trials. NIH also supports Phase I, II, and III clinical trials of various topical microbicides, as well as behavioral and social science research on the acceptability and use of microbicides among different populations. The Office of AIDS Research coordinates microbicide research across the NIH and in collaboration with other Federal agencies, providing administrative accountability and funding coordination for this priority research area.
Budget Policy: The FY 2008 budget request for this activity is $96,400,000, which represents an increase of $10,947,000 over the FY 2007 Continuing Resolution. Around the world, most HIV infections are spread through heterosexual transmission; and half of all infected adults are women. Women have no means to protect themselves from HIV if their partners do not use a condom or allow a female condom to be used. Prevention methods such as abstinence or being faithful are not likely to protect married women or those who are sexually abused. Therefore, the development of a safe and effective microbicide is a high priority for NIH research. The science of microbicides is moving rapidly forward. NIH has undertaken critical efforts over the years to attract investigators into this field. A large number of microbicide candidates have been developed and will soon enter Phase I clinical trials. OAR will use its authorities to improve NIH management and support for this crucial area of science. A separate division of OAR now will be dedicated to microbicide research and other issues relevant to women. OAR is convening a newly constituted NIH Microbicide Research Coordinating Committee with members from the ICs with significant microbicide portfolios, as well as CDC and USAID. The Committee will assist in the development of the microbicides component of the Plan, foster information-sharing and trans-NIH coordination, and help identify scientific opportunities and gaps for increased attention. A Microbicide Research Working Group is being established of non-government experts to advise OAR, NIAID, NIH, and other government and non-government entities in this priority area. In addition, the National Institute of Allergy and Infectious Diseases (NIAID) Division of AIDS (DAIDS) is developing a new Prevention Sciences Program, which will include a Microbicide Research Branch. OAR will support a number of conferences, workshops, and symposia to continue to enhance scientific interest in conducting these important studies. NIH will increase collaborations with academia, industry, and foundations to identify and explore new and existing compounds as potential topical microbicidal agents. Important areas of research include the establishment of a new microbicide clinical trials network and the necessary infrastructure to conduct microbicide trials, especially in developing countries; the development of criteria for selecting potential products to be evaluated in clinical trials and for advancing them through the different phases of clinical studies; and research on ethical and behavioral issues impacting these clinical trials. Funds will be provided to support the evaluation of lead candidates in non-human primate models; a new initiative for development of new innovative microbicide concepts; and an integrated pre-clinical/clinical program for development of microbicide candidates. OAR will also provide $5 million, to be matched by the Government of India, for an international initiative to support collaborative U.S.-India research on HIV/AIDS. High priority will be given to microbicide research projects.
Vaccines
Purpose and Methods of Operation: Safe and efficacious vaccines are essential for global control of the AIDS pandemic. As a result of increased NIH vaccine research funding, many new approaches are being pursued. Basic research in vaccine design, studies of immune responses in small animals and non-human primates, and vaccine product development are underway. New vaccine designs have been developed, and several will enter Phase I clinical trials within the next 2 years. Recent HIV vaccine research studies in animal models have provided strong scientific rationales to further explore and develop several vaccine concepts and to move additional candidate vaccines into clinical testing. More than 55 products or combinations have been tested to date in over 85 Phase I and II trials involving more than 24,000 volunteers. The NIH supports a new consortium, the Center for HIV/AIDS Vaccine Immunology, to identify protective immune responses and to test vaccine strategies that might induce protective immune responses. NIH-funded independent investigators are pursuing many different HIV vaccine approaches. Initial studies are leading to improved vaccine candidates that may provide better protection. NIH supports a broad program encompassing basic, preclinical, and clinical research on candidate vaccine products. As promising candidates move further in the vaccine pipeline, expanded trials with populations at increased risk for HIV infection will become increasingly important. HIV/AIDS vaccine research requires trained health care, medical research, and prevention specialists, as well as populations at risk who will be integrally involved in the development of vaccine candidates and clinical vaccine and prevention trials. International and domestic sites are being developed, including a cadre of trained personnel, to conduct vaccine trials.
Budget Policy: The FY 2008 budget request for this activity is $596,194,000, which is an increase of $4,515,000 above the FY 2007 Continuing Resolution. AIDS vaccine research has been the highest priority for the past several years, and has received significant increases to ensure that new and innovative concepts continue to advance through the pipeline. In FY 2008, support will be provided for the design and development of new vaccine concepts and the pre-clinical/clinical development of vaccine candidates in the pipeline. Funds will support a planned large-scale clinical trial of the first multi-gene, multi-strain vaccine candidate developed by the NIAID Dale and Betty Bumpers Vaccine Research Center. This protocol will be conducted collaboratively in several clinical trial networks. This approach could be of benefit in many parts of the world with different viral strains. Funds also will be provided to initiate a clinical trial of another innovative vaccine approach to test a vaccine candidate developed for one viral strain in a population at risk for a different strain to determine if cross-strain protection is possible.
Behavioral and Social Science
Purpose and Methods of Operation: NIH supports research to further our understanding of how to change the behaviors that lead to HIV transmission—including preventing their initiation—and how to maintain protective behaviors once they are adopted in all populations at risk. NIH sponsors research related to: developing, implementing, and evaluating behavioral and social science interventions to reduce HIV transmission in various populations and settings; strengthening our understanding of the determinants, trends, and processes of HIV-related risk behaviors and the consequences of HIV infection; developing and evaluating behavioral strategies for preventing or ameliorating the negative physical, psychological, and social consequences of HIV infection; and improving the methodologies employed in behavioral and social science research. A better understanding of social and cultural factors associated with HIV risk or protection, particularly in minority communities, will contribute to the successful implementation of a broader range of preventive or therapeutic strategies.
Budget Policy: The FY 2008 budget request for this activity is $398,761,000, which is the same as the FY 2007 Continuing Resolution. NIH will support ongoing research to develop and test effective HIV-related interventions that build on studies of substance addiction and the complex interaction of alcohol use, drug use, and disinhibition. Behavioral issues associated with adherence to therapies are another area of ongoing investigation. Lack of complete adherence to drug regimens may result in the development of drug-resistant strains of HIV, which could have devastating public health implications. In addition, HIV-infected individuals taking antiretroviral therapies who experience improved health and a decline in detectable virus may believe that they are less infectious and may lapse into unsafe sexual and drug-using behaviors. This could have the effect of increasing HIV transmission, if the virus is still viable at undetectable levels. New initiatives will support global partnerships for social science research on AIDS and studies on the role of behavioral and social networks in HIV transmission. To support these priority areas, OAR has redirected funds from expiring grants that had supported the discovery, development, and clinical evaluation of drug abuse treatments in persons who are not HIV-infected. While these studies provided findings that had applicability to HIV-infected individuals, the trans-NIH portfolio review determined that they are now of a lower AIDS-related priority.
Therapeutics
Purpose and Methods of Operation: Many HIV-infected people are living with the benefits resulting from NIH-supported therapeutics research. The development of combination regimens has extended the length and quality of life for many HIV-infected individuals in the United States and Western Europe. The use of antiretroviral therapy continues to result in improved immune function in patients who are able to adhere to the treatment regimens and tolerate the toxicities associated with antiretroviral drugs. NIH supports a comprehensive AIDS therapeutics program, from drug discovery through to the conduct of large-scale clinical trials, particularly of multi-drug regimens. NIH plays a unique role in therapeutics research both in basic and clinical science. NIH etiology and pathogenesis research (described below) provides the basic science building blocks on which new drugs can be designed and developed. In addition, NIH supports clinical trials that test drugs from different drug companies against each other. Industry does not carry out these studies.
Budget Policy: The FY 2008 budget request for this activity is $622,427,000, which represents a decrease of $10,673,000 below the FY 2007 Continuing Resolution. A high priority of NIH-sponsored AIDS therapeutics research continues to be the development of better drugs and therapeutic regimens that are less toxic and have fewer side effects, limit the development of drug resistance, enter viral reservoirs to inhibit viral replication, promote easier adherence, and are more readily accessible. The global impact and continued spread of the AIDS pandemic in both developed and developing nations underscore the importance of the development of therapeutic regimens that can be implemented in international settings. NIH will support ongoing research to address the metabolic complications, including insulin resistance, and body composition changes such as deforming fatty tissue deposits, that have emerged in individuals who have been on long-term antiretroviral regimens. More deaths occurring from liver failure, kidney disease, cardiovascular complications, and malignancies are being observed in this patient population. NIH will support important ongoing studies to address these complications. NIH-supported research demonstrated the effectiveness of antiretroviral therapy to reduce mother-to-child HIV transmission. As a result of the implementation of these regimens, less than 200 HIV-infected babies are born each year in the U.S. However, NIH is continuing to develop regimens that can be implemented in resource-constrained nations, including strategies to prevent transmission associated with breast-feeding. A restructured clinical trials network for the conduct of perinatal, pediatric, and maternal clinical studies will place a greater emphasis on sites in developing countries. To support these initiatives, funds will be redirected from expiring grants that supported basic research on coinfections and opportunistic infections. These studies provided important findings that contributed to the development of regimens for the prevention and treatment of these infections; however, the incidence of these infections in HIV-infected individuals has diminished as a result of the effectiveness of antiretroviral treatments.
Etiology and Pathogenesis
Purpose and Methods of Operation: Tremendous progress has been made in understanding the fundamental steps in the life-cycle of HIV, the host-virus relationship, and the clinical manifestations associated with HIV infection and AIDS. Groundbreaking research on basic HIV biology and AIDS pathogenesis has revolutionized the design of drugs, methodologies for diagnosis, and monitoring of the safety and effectiveness of antiviral therapies. The results of this research are the basic building blocks for the development of new drugs, vaccines, and microbicides. Continued support for basic research is the critical foundation of our fight against HIV/AIDS. This research is focused on gaining a better understanding in two areas: (1) how HIV infection is established and maintained; and (2) what causes the profound immune deficiency and severe clinical complications that accompany this infection. NIH researchers are studying the ways in which sex and gender confer vulnerability to, or protection from, HIV infection and AIDS among women and girls—in general, and relative to men—in diverse geographical settings and during different stages of the life course. There are many research questions that remain unanswered about specific anatomical and physiological characteristics of women and girls that might play a role in transmission, acquisition, or resistance to HIV infection. Studies are focused on factors in HIV acquisition, including the influence of hormonal modulation on viral replication and immune responses in the reproductive tract, and co-factors, such as coincident infections with other sexually transmitted pathogens.
Budget Policy: The FY 2008 budget request for this activity is $709,107,000, which is the same as the FY 2007 Continuing Resolution. NIH will continue to support ongoing investigator-initiated research in this area, including initiatives addressing the important pathogenic mechanisms more commonly observed in women, children, and adolescents infected with HIV. This basic knowledge is critical for our efforts to prevent and control HIV infection and disease progression. Ongoing research will be supported to understand the normal development and functioning of the human immune system. These studies are crucial to our understanding of the pathogenesis of AIDS and the development of new and better treatments and prevention strategies. Support will be provided to investigator-initiated studies to address critical questions that remain in this area, including the role of specific HIV proteins in the viral life cycle; the primary modes of HIV transmission between cells and between individuals; how the immune system controls the infection and disease progression; the mechanisms involved in cell injury and death in the immune, nervous, and other organ systems; host factors and cofactors that influence primary infection and disease course; and the relationship of HIV infection to its associated malignancies, opportunistic infections and coinfections, neurological impairments, and metabolic disturbances. To support these initiatives, funds will be redirected from expiring grants that supported basic research on opportunistic infections and coinfections. These studies provided important findings that contributed to the development of regimens for the prevention and treatment of these infections; however, the incidence of these infections in HIV-infected individuals has diminished as a result of the effectiveness of antiretroviral treatments.
Natural History and Epidemiology
Purpose and Methods of Operation: Natural history and epidemiologic research is needed to monitor epidemic trends, develop and evaluate prevention modalities, follow the changing clinical manifestations of HIV disease in different populations, and measure the effects of treatment regimens. NIH supports research in U.S. and international settings to examine HIV transmission, HIV/AIDS disease progression, (including the occurrence of coinfections and opportunistic infections, malignancies, metabolic complications, neurological and behavioral dysfunctions), the development of other HIV/AIDS-related conditions, and improved methodologies to support this research. In the United States, the population groups most affected by the AIDS epidemic are racial and ethnic minorities, women, drug users, and adolescents. Prevalence of HIV infection in racial and ethnic minority communities is disproportionately higher than in majority communities. NIH supports research to develop interventions that will have the greatest impact on these groups. These include interventions that address the co-occurrence of other sexually transmitted diseases, hepatitis, drug abuse, and mental illness; and interventions that consider the role of culture, family, and other social factors in the transmission and prevention of these disorders. The use of potent antiretroviral therapy has delayed the progression of HIV disease, extending the time between HIV infection and development of AIDS. A more complex pathology, however, is being uncovered as HIV-infected people live longer and develop age-related comorbidities. In addition, while effective in improving the health of many HIV-infected individuals, antiretroviral therapy has been associated with a wide variety of undesired effects in many organ systems. Epidemiologic research has been instrumental in identifying and describing such effects, disentangling effects related to treatment from those related to HIV disease itself.
Budget Policy: The FY 2008 budget request for this activity is $261,463,000, which represents a decrease of $2,848,000 below the FY 2007 Continuing Resolution. NIH will support high-priority ongoing rigorous epidemiology studies on new groups and populations affected by HIV that are changing the face of the epidemic. In particular, a study of the unique natural history of the disease in women, including its complications and manifestations, will be recompeted. NIH will support ongoing translational research in international settings to define the optimal parameters of treatment and care to achieve the best outcomes. NIH will continue support for epidemiologic studies to investigate the mechanisms of disease progression, the impact of therapy in changing the spectrum of HIV disease, and the causes of death. To support this research, funds will be redirected from expiring grants of lower priority research, including some basic studies of oral and ocular manifestations that have been mediated by new therapeutic advances.
Training, Infrastructure, and Capacity Building
Purpose and Methods of Operation: NIH supports training of domestic and international biomedical and behavioral AIDS researchers, as well as the improvement of facilities and equipment for the conduct of AIDS-related research, including facilities for animal model research. Numerous NIH funded programs have increased the number of training positions for AIDS-related research, including programs specifically designed to recruit individuals from minority communities into research careers and to build research infrastructure in minority institutions. The NIH Loan Repayment Program (LRP) was mandated by Congress under Public Law 100-607 in 1988 and authorized under 42 USC 288-1 to encourage health professionals to engage in AIDS-related research at NIH. Specific international infrastructure needs include: (1) developing research sites through establishment of stable, targeted, study populations, development of recruitment strategies, and enhancement of laboratory, clinical, and data management capabilities; (2) increasing the number of scientists, clinicians, and health care workers trained in basic, clinical, and behavioral research, data management, and ethical considerations; (3) developing research collaborations; and (4) transferring appropriate clinical and laboratory technologies.
Budget Policy: The FY 2008 budget request for this activity is $168,211,000, which represents a decrease of $1,380,000 below the FY 2007 Continuing Resolution. NIH will continue to support ongoing commitments for efforts to increase the supply of non-human primates, particularly rhesus macaques, for AIDS research and other areas of biomedical research both in the U.S. and abroad. NIH also will continue to support training programs for U.S. and international researchers to build the critical capacity to conduct AIDS research both in minority communities in the U.S. and in developing countries.
Information Dissemination
Purpose and Methods of Operation: Effective information dissemination approaches will continue to be integral to HIV prevention and treatment efforts. Such programs are critical in light of the continuing advent of new and complex antiretroviral treatment regimens, the adherence issues related to HIV/AIDS treatment, the need for research communities to work and communicate globally, and the need to translate behavioral and social prevention approaches into practice. The changing pandemic and the increasing number of HIV infections in specific population groups, such as minorities and women, also underscore the need to disseminate HIV research findings and other related information to communities at risk. The flow of information among researchers, health care providers, and the affected communities represents new opportunities to rapidly translate research results into practice and to shape future research directions.
Budget Policy: The FY 2008 budget request for this activity is $25,349,000, which represents a decrease of $1,073,000 below the FY 2007 Continuing Resolution. NIH will continue to support initiatives to enhance dissemination of research findings, including scientific conferences, the development of state-of-the art treatment guidelines, and the distribution of those guidelines through AIDSInfo, a web-based service to provide information to caregivers and patients. NIH will support efforts to recruit and retain participants in clinical studies, including women and minorities. Funding will maintain ongoing commitments in this area, but new initiatives will not be undertaken.
Global Fund for HIV/AIDS, Tuberculosis and Malaria: In addition to the $2,905 million in the NIH AIDS research activities described in this section, the Administration requests a total of $300 million through the Non-AIDS program of the NIH for the Global Fund for HIV/AIDS, Tuberculosis and Malaria, as part of the President's Emergency Plan for AIDS Relief (PEPFAR). The contributions of multilateral institutions and international organizations to combating HIV/AIDS provide a vital opportunity for a comprehensive response to the disease. The diverse drivers and consequences of HIV/AIDS, as well as its complex interactions with a variety of other social, political and economic circumstances demand leadership from diverse international partners with varied expertise.
1A Glance at the AIDS Epidemic, CDC (2006).
2World Health Report on Infectious Diseases: Overcoming Antimicrobial Resistance,@ (WHO, 2000).
3NIH Consensus Conference Statement: Management of Hepatitis C: 2002, p 76–77.