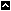Office of Human Research Compliance
Clinical Research
The overall mission of the Office of Human Research Compliance (OHRC) is to manage a comprehensive Human Research Protection Program at the National Institute of Environmental Health Sciences (NIEHS).
The OHRC accomplishes its mission by providing professional advice and leadership to NIEHS in the protection of human subjects participating in research through:
- Maintaining compliance by providing clarification and guidance in interpreting the Federal Regulations for the protection of human subjects, 45CFR46 and 21CFR50 & 56; NIH and NIEHS policies and guidance; and state regulations and guidance.
- Providing guidance to PIs during protocol development.
- Managing the administration of the Pre-IRB Process.
- Managing the daily administration of the NIEHS Institutional Review Board (IRB).
- Establishing educational and training programs with the goal of creating a culture of respect for and awareness of the rights and welfare of human research participants at NIEHS and its affiliated sites.
- Maintaining a Quality Assurance and Improvement Program to audit, monitor and continually improve the quality of its human research protections.
- Implementing and maintaining electronic resources and management systems for the NIEHS OHRC.
- Giving guidance and serving as the NIEHS central point of contact during the AAHRPP accreditation process. After accreditation has been achieved, the OHRC will develop guidelines and internal processes to maintain accreditation.
For information contact the Office of Human Research Compliance at:
Telephone: (919) 541-3852
FAX: (919) 541-3845
E-mail: NIEHS-OfficeofHRC@niehs.nih.gov
OHRC Staff
-
Joan P. Packenham, Ph.D. (http://www.niehs.nih.gov/research/clinical/join/ohrc/index.cfm)
NIEHS IRB Vice-Chair
Director, OHRC
DIR CRP OCR CD -
Tel (919) 541-0766
Fax (919) 541-9854
packenhm@niehs.nih.gov
-
Jane M. Lambert, CIP (http://www.niehs.nih.govhttp://www.niehs.nih.gov/research/clinical/join/ohrc/index.cfm)
IRB Administrator
DIR CRP OCR CD -
Tel (919) 541-5047
Fax (919) 541-9854
lambert@niehs.nih.gov
-
Craig Wladyka, MPA, RHIA, CIP (http://www.niehs.nih.govhttp://www.niehs.nih.gov/research/clinical/join/ohrc/index.cfm)
IRB Protocol Coordinator
DIR CRP OCR CD -
Tel (919) 541-3822
Fax (919) 541-9854
cwladyka@niehs.nih.gov
-
Edith M. Lee, MPA (http://www.niehs.nih.govhttp://www.niehs.nih.gov/research/clinical/join/ohrc/index.cfm)
IRB Protocol Specialist
DIR CRP OCR CD -
Tel (919) 541-3852
Fax (919) 541-9854
leeem@niehs.nih.gov
-
Lynnelle Fowler-McDonald
Technical IRTA -
Tel (919) 541-9844
lynnelle.fowlermcdonald@niehs.nih.gov
-
Terry Lewis, MPA
Program Specialist -
Tel (919) 541-7817
lewis8@niehs.nih.gov
-
Christine Philiput, Ph.D.
AAHRPP Accreditation & Quality Assurance Specialist