NIH State-of-the-Science Conference:
Role of Active Surveillance in the
Management of Men With
Localized Prostate Cancer
December 5–7, 2011
Bethesda, Maryland
Return to conference home
Program & Abstracts
Documents in PDF format require the Adobe Acrobat Reader®. If you
experience problems with PDF documents, please download the latest
version of the Reader®.
- Conference Sponsors
- About the Consensus Development Program
- Upcoming & Recent Conferences
- General Information
- Background
- About the Artwork
- Consensus Development Panel
- Speakers
- Planning Committee
- Educational Planners
- Agenda & Abstracts
- How have the patient population and the natural history of prostate cancer diagnosed in the United States changed in the last 30 years?
- How are active surveillance and other observational strategies defined?
- What factors affect the offer of, acceptance of, and adherence to active surveillance?
- What are the patient-experienced comparative short- and long-term health outcomes of active surveillance versus immediate treatment with curative intent for localized prostate cancer?
Presented by
National Cancer Institute,
NIH
Centers for Disease Control and Prevention
Office of Medical Applications of Research,
NIH
Partner
The Agency for Healthcare Research and Quality provided additional conference development support.
About the Consensus Development Program
The National Institutes of Health (NIH) Consensus Development Program has been organizing major conferences since 1977. The Program generates evidence-based consensus statements addressing controversial issues important to healthcare providers, policymakers, patients, researchers, and the general public. The Program is administered by the Office of Medical Applications of Research within the NIH Office of the Director. Typically, the conferences have one major NIH Institute or Center sponsor, with multiple cosponsoring agencies.
Topic Selection
NIH Consensus Development and State-of-the-Science Conference topics must satisfy the following criteria:
- Broad public health importance. The severity of the problem and the feasibility of interventions are key considerations.
- Controversy or unresolved issues that can be clarified, or a gap between current knowledge and practice that can be narrowed.
- An adequately defined base of scientific information from which to answer conference questions such that the outcome does not depend primarily on subjective judgments of panelists.
Conference Type
Two types of conferences fall under the purview of the NIH Consensus Development Program: State of-the-Science Conferences and Consensus Development Conferences. Both conference types utilize the same structure and methodology; they differ only in the strength of the evidence surrounding the topic under consideration. When it appears that there is very strong evidence about a particular medical topic, but that the information is not in widespread clinical practice, a Consensus Development Conference is typically chosen to consolidate, solidify, and broadly disseminate strong evidence-based recommendations for general practice. Conversely, when the available evidence is weak or contradictory, or when a common practice is not supported by high-quality evidence, the State-of-the Science label is chosen. This highlights what evidence about a topic is available and what directions future research should take, and alerts providers that certain practices are not supported by good data.
Conference Process
Before the conference, a systematic evidence review on the chosen topic is performed by one of the Agency for Healthcare Research and Quality’s Evidence-based Practice Centers. This report is provided to the panel members approximately 6 weeks prior to the conference, and posted to the Consensus Development Program website once the conference begins, to serve as a foundation of high-quality evidence upon which the conference will build.
The conferences are held over 2–1/2 days. The first day and a half of the conference consists of plenary sessions, in which invited expert speakers present information, followed by “town hall forums,” in which open discussion occurs among the speakers, panelists, and the general public in attendance. The panel then develops its draft statement on the afternoon and evening of the second day, and presents it on the morning of the third day for audience commentary. The panel considers these comments in executive session and may revise its draft accordingly. The conference ends with a press briefing, during which reporters are invited to question the panelists about their findings.
Panelists
Each conference panel comprises 12 to 16 members, who can give balanced, objective, and informed attention to the topic. Panel members:
- Must not be employees of the U.S. Department of Health and Human Services.
- Must not hold financial or career (research) interests in the conference topic.
- May be knowledgeable about the general topic under consideration, but must not have published or have a publicly stated opinion on the topic.
- Represent a variety of perspectives, to include:
- — Practicing and academic health professionals
- — Biostatisticians and epidemiologists
- — Clinical trialists and researchers
- — Nonhealth professionals with expertise in fields relevant to the specific topic (ethicists, economists, attorneys, etc.)
- — Individuals representing public-centered values and concerns
In addition, the panel as a whole should appropriately reflect racial and ethnic diversity. Panel members are not paid a fee or honorarium for their efforts. They are, however, reimbursed for travel expenses related to their service as panel members.
Speakers
The conferences typically feature approximately 21 presentations: 3 cover the information found in the Evidence-based Practice Center’s systematic review of the literature; the other 18 presentations feature experts in the topic at hand, who have likely published on the topic, and who may have strong opinions or beliefs on the topic. Where multiple viewpoints on a topic exist, every effort is made to include speakers who address all sides of the issue.
Conference Statements
The panel’s draft report is released online late in the conference’s third and final day. The final report is released approximately 6 weeks later. During the intervening period, the panel may edit its statement for clarity and to correct any factual errors that might be discovered. No substantive changes to the panel’s findings are made during this period.
Each Consensus Development or State-of-the-Science Conference Statement reflects an independent panel’s assessment of the medical knowledge available at the time the statement is written; as such, it provides a “snapshot in time” of the state of knowledge on the conference topic. It is not a policy statement of the NIH or the Federal Government.
Dissemination
Consensus Development and State-of-the-Science Conference Statements are broadly disseminated:
- A press briefing is held on the last day of the conference to assist journalists in preparing news stories on the conference findings.
- The statement is published online at consensus.nih.gov.
- The conference statement is published in at least one major peer-reviewed journal.
- Print copies are mailed to a wide variety of targeted audiences and are available at no charge through a clearinghouse.
Contact Us
For conference schedules, past statements, and evidence reports, please contact us:
NIH Consensus Development Program Information Center
P.O. Box 2577
Kensington, MD 20891
888–NIH–CONSENSUS (888–644–2667)
consensus.nih.gov
Upcoming Conferences
| NIH Consensus Development Conference: |
Diagnosing Gestational Diabetes Mellitus October 29–31, 2012 |
To receive registration notifications and updates about conferences and other program activities, please join the NIH Consensus Development Program Information Network at consensus.nih.gov/alerts.htm.
Recent Conferences
| NIH Consensus Development Conference: |
Inhaled Nitric Oxide Therapy for Premature Infants October 27–29, 2010 |
| NIH State-of-the-Science Conference: |
Preventing Alzheimer’s Disease and Cognitive Decline April 26–28, 2010 |
| NIH Consensus Development Conference: |
Vaginal Birth After Cesarean: New Insights March 8–10, 2010 |
| NIH Consensus Development Conference: |
Lactose Intolerance and Health February 22–24, 2010 |
| NIH State-of-the-Science Conference: |
Enhancing Use and Quality of Colorectal Cancer Screening February 2–4, 2010 |
| NIH State-of-the-Science Conference: |
Diagnosis and Management of Ductal Carcinoma In Situ (DCIS) September 22–24, 2009 |
| NIH State-of-the-Science Conference: |
Family History and Improving Health August 24–26, 2009 |
| NIH Consensus Development Conference: |
Management of Hepatitis B October 20–22, 2008 |
| NIH Consensus Development Conference: |
Hydroxyurea
Treatment for Sickle Cell Disease February 25–27, 2008 |
NIH State-of-the-Science Conference: |
Prevention of Fecal and Urinary Incontinence in Adults December 10–12, 2007 |
| NIH State-of-the-Science Conference: |
Tobacco Use: Prevention, Cessation, and Control June 12–14, 2006 |
| NIH State-of-the-Science Conference: |
Multivitamin/Mineral Supplements and Chronic Disease Prevention May 15–17, 2006 |
| NIH State-of-the-Science Conference: |
Cesarean Delivery on Maternal Request March 27–29, 2006 |
To access previous conference statements, webcasts, evidence reports, and other conference materials, please visit consensus.nih.gov.
General Information
Financial Disclosures
The National Institutes of Health, the Centers for Disease Control and Prevention, our planners, and our presenters wish to disclose that they have no financial interests or other relationships with the manufacturers of commercial products, suppliers of commercial services, or commercial supporters, with the exception of the following:
| Planning Committee Members | Company | Financial Relationship |
|---|---|---|
| Andriole, Gerald L., M.D. | Amgen |
Honorarium, Consultant |
Augmenix |
Stock, Consultant |
|
Bayer |
Honorarium, Consultant |
|
Cambridge Endo |
Stock, Consultant |
|
Caris |
Honorarium, Consultant |
|
Envisioneering Medical |
Stock, Investor/Medical Director |
|
Ferring Pharmaceuticals |
Honorarium, Consultant |
|
France Foundation |
Honorarium, Consultant |
|
GenProbe |
Honorarium, Consultant |
|
GlaxoSmithKline |
Honorarium, Consultant |
|
Myriad Genetics |
Honorarium, Consultant |
|
Ortho-Clinical Diagnostics |
Honorarium, Consultant |
|
Steba Biotech |
Honorarium, Consultant |
|
Viking Medical |
Stock, Medical Director |
|
| Lucia, M. Scott, M.D. | GenProbe |
Honorarium, Consultant |
GlaxoSmithKline |
Honorarium, Consultant |
|
| Newcomer, Lee, M.D. | United Healthcare |
Salary/Stock, Employee |
| Roach, Mack III, M.D., FACR |
American Cancer Society |
N/A, Board Member |
AstraZenca |
Honorarium, Lecturing |
|
CareCore National, LLC |
Consulting Fee, Board Member |
|
Ferring Pharmaceuticals |
Honorarium, Lecturing |
|
Handbook of Evidence-based Radiation Oncology |
Royalties, Educational Materials |
|
Molecular Insight Trofex |
Research Support |
|
National Comprehensive Cancer Network, Prostate Cancer Guidelines |
N/A, Member Guidelines Committee |
|
University of Pennsylvania |
Honorarium, Consultant |
|
UpToDate, Prostate Cancer |
Royalties, Educational Materials |
|
| All other planners and presenters signed statements that they have no financial or other conflicts of interest. | ||
There is no commercial support for this activity.
Policy on Panel Disclosure
Panel members signed a confirmation that they have no financial or other conflicts of interest pertaining to the topic under consideration.
Videocast
Live and archived videocasts may be accessed at videocast.nih.gov. Archived videocasts will be available approximately 1 week after the conference.
Dining
The dining center in the Natcher Conference Center is located on the main level, one floor above the auditorium. It is open from 6:30 a.m. to 2:30 p.m., serving hot breakfast and lunch, sandwiches and salads, and snack items. An additional cafeteria is available from 7:00 a.m. to 3:30 p.m., in Building 38A, Level B1, across the street from the main entrance to the Natcher Conference Center.
Online Content
All materials issuing from the NIH Consensus Development Program are available at consensus.nih.gov. In addition, remote participants will have the opportunity to provide comments on the panel statement by visiting consensus.nih.gov/comments.htm from 8:30 a.m. to 11:30 a.m. on Wednesday, December 7, 2011.
Background
Prostate cancer is the second leading cause of cancer-related deaths among men in the United States. It is estimated that in 2010, approximately 32,000 American men died of prostate cancer and 218,000 were newly diagnosed with the disease. Most prostate cancers are detected by a blood test that measures prostate-specific antigen (PSA), a tumor marker. More than half of cancers detected with PSA screening are localized (confined to the prostate), not aggressive at diagnosis, and unlikely to become life-threatening. However, 90 percent of patients receive immediate treatment for prostate cancer, such as surgery or radiation therapy. In many patients, these treatments have substantial short- and long-term side effects without any clinical benefit. Appropriate management of screen-detected, early-stage, low-risk prostate cancer is an important public health issue given the number of men affected and the risk for adverse outcomes, such as diminished sexual function and loss of urinary control.
Tools that can reliably predict which tumors are likely to progress and which are unlikely to cause problems are not available at present. Currently, clinicians rely on two observational strategies as alternatives to immediate treatment of early-stage prostate cancer: watchful waiting and active surveillance. Watchful waiting involves relatively passive patient follow-up, with palliative interventions if and when any symptoms develop. Active surveillance typically involves proactive patient follow-up in which PSA levels are closely monitored, prostate biopsies may be repeated, and eventual treatment is anticipated. Yet, it is unclear which men will most benefit from each approach and whether observational strategies will yield outcomes similar to immediate treatment when managing low-risk prostate cancer.
To better understand the benefits and risks of active surveillance and other observational management strategies for PSA-screening-detected, low-grade, localized prostate cancer, the National Institutes of Health has engaged in a rigorous assessment of the available scientific evidence. This process, sponsored by the National Cancer Institute, the Centers for Disease Control and Prevention, and the Office of Medical Applications of Research, will culminate in a State-of-the-Science Conference on December 5–7, 2011, that focuses on these key questions:
- How have the patient population and the natural history of prostate cancer diagnosed in the United States changed in the last 30 years?
- How are active surveillance and other observational strategies defined?
- What factors affect the offer of, acceptance of, and adherence to active surveillance?
- What are the patient-experienced comparative short- and long-term health outcomes of active surveillance versus immediate treatment with curative intent for localized prostate cancer?
- What are the research needs regarding active surveillance (or watchful waiting) in localized prostate cancer?
A multidisciplinary planning committee developed the questions, which will be addressed in an evidence report prepared through the Agency for Healthcare Research and Quality’s Evidence-based Practice Centers program. During the conference, invited experts, including the authors of the report, will present scientific evidence. Attendees will have opportunities to ask questions and provide comments during open discussion periods. After weighing the evidence, an unbiased, independent panel will prepare and present a statement addressing the key questions. The statement will be widely disseminated to practitioners, policymakers, patients, researchers, the general public, and the media.
About the Artwork
The illustration depicts the relationship between the healthcare provider and the patient, in this case a man diagnosed with localized prostate cancer. White lines symbolize active surveillance, and multicolored triangles depict the diversity of patients and the wide range of factors influencing a man’s access to, acceptance of, and adherence to alternatives to treatment. Active surveillance is an example of an alternative to immediate treatment and involves proactive patient follow-up and close monitoring.
The image was conceived and created by the National Institutes of Health’s Division of Medical Arts and is in the public domain. No permission is required to use the image. Please credit “Bryan Ewsichek/NIH Medical Arts.”
Consensus Development Panel
|
Patricia A. Ganz, M.D. Panel and Conference Chairperson Professor University of California, Los Angeles Schools of Medicine and Public Health Division of Cancer Prevention and Control Research Jonsson Comprehensive Cancer Center Los Angeles, California |
|
|
John M. Barry, M.D. Emeritus Professor of Surgery Divisions of Urology and Abdominal Organ Transplantation Oregon Health & Science University Portland, Oregon Wylie Burke, M.D., Ph.D. Professor and Chair Department of Bioethics and Humanities University of Washington Seattle, Washington Nananda F. Col, M.D., M.P.P., M.P.H., FACP Professor of Medicine Center for Excellence in the Neurosciences and Departments of Family Medicine and Geriatric Medicine University of New England Biddeford, Maine President Shared Decision Making Resources Georgetown, Maine Phaedra S. Corso, Ph.D., M.P.A. Professor and Head Department of Health Policy and Management College of Public Health University of Georgia Athens, Georgia Everett Dodson Community Health Educator Lombardi Comprehensive Cancer Center Georgetown University Hospital Washington, District of Columbia M. Elizabeth Hammond, M.D. Pathologist Intermountain Healthcare Professor of Pathology University of Utah School of Medicine Executive Editor for Pathology Products Amirsys, Inc. Salt Lake City, Utah Barry A. Kogan, M.D., FAAP, FACS Professor of Urology and Pediatrics Chief Division of Urology Albany Medical College Urological Institute of Northeastern New York Community Care Physicians Albany, New York |
Charles F. Lynch, M.D., Ph.D., M.S. Professor and Associate Head of Research Department of Epidemiology College of Public Health The University of Iowa Iowa City, Iowa Lee Newcomer, M.D. Senior Vice President of Oncology United Healthcare Minneapolis, Minnesota Eric J. Seifter, M.D., FACP Associate Professor of Medicine and Oncology The Johns Hopkins University School of Medicine The Sidney Kimmel Comprehensive Cancer Center Johns Hopkins at Greenspring Station Lutherville, Maryland Janet A. Tooze, Ph.D., M.P.H. Associate Professor Department of Biostatistical Sciences Division of Public Health Sciences Wake Forest School of Medicine Winston Salem, North Carolina Kasisomayajula “Vish” Viswanath, Ph.D. Associate Professor Department of Society, Human Development, and Health Harvard School of Public Health Associate Professor Department of Medical Oncology Dana Farber Cancer Institute Boston, Massachusetts Hunter Wessells, M.D., FACS Professor and Chair Department of Urology Nelson Chair in Urology University of Washington School of Medicine Seattle, Washington |
Speakers
|
Peter Albertsen, M.D. Medical Director UConn Medical Group Associate Dean Clinical Research Planning and Administration Associate Dean Clinical Affairs Division of Urology University of Connecticut Health Center Farmington, Connecticut Gerald L. Andriole, M.D. Robert K. Royce Distinguished Professor Chief of Urologic Surgery Washington University School of Medicine Barnes-Jewish Hospital Siteman Cancer Center St. Louis, Missouri Otis W. Brawley, M.D. Chief Medical Officer American Cancer Society Atlanta, Georgia Peter R. Carroll, M.D., M.P.H. Ken and Donna Derr – Chevron Distinguished Professor Department of Urology University of California, San Francisco (UCSF) Associate Dean UCSF School of Medicine Director of Clinical Services and Strategic Planning UCSF Helen Diller Family Comprehensive Cancer Center San Francisco, California H. Ballentine Carter, M.D. Professor Urology and Oncology Johns Hopkins Medicine Director Division of Adult Urology Bradley Urological Institute The Johns Hopkins Hospital Baltimore, Maryland Mei Chung, Ph.D., M.P.H. Assistant Director Tufts Evidence-based Practice Center Tufts Medical Center Boston, Massachusetts Issa Dahabreh, M.D., M.S. Research Associate Tufts Evidence-based Practice Center Tufts Medical Center Boston, Massachusetts Jenny Donovan, Ph.D. Head of School School of Social and Community Medicine University of Bristol UNITED KINGDOM Ann S. Hamilton, Ph.D. Associate Professor of Clinical Epidemiology Department of Preventive Medicine Division of Epidemiology Keck School of Medicine University of Southern California Los Angeles, California Richard M. Hoffman, M.D., M.P.H. Professor of Medicine University of New Mexico School of Medicine Staff Physician New Mexico Veterans Affairs Health Care System Albuquerque, New Mexico Lars Holmberg, M.D., Ph.D. Professor of Cancer Epidemiology Division of Cancer Studies King’s College London School of Medicine Guy’s Hospital UNITED KINGDOM |
Stanley Ip, M.D. Associate Director Tufts Evidence-based Practice Center Tufts Medical Center Boston, Massachusetts Laurence Klotz, M.D. Chief Division of Urology Sunnybrook Health Sciences Centre Professor of Surgery University of Toronto CANADA David A. Lipton, J.D. Director Securities Law Program Catholic University of America School of Law Washington, District of Columbia Mark S. Litwin, M.D., M.P.H. Professor of Urology and Health Services Chair Department of Urology David Geffen School of Medicine at the University of California, Los Angeles (UCLA) UCLA School of Public Health Los Angeles, California M. Scott Lucia, M.D. Associate Professor and Director Prostate Diagnostic Laboratory Co-Director Prostate Cancer Research Laboratories Co-Director Colorado Molecular Correlates Laboratory University of Colorado Denver School of Medicine Aurora, Colorado David F. Penson, M.D., M.P.H. Professor of Urologic Surgery Director Center for Surgical Quality and Outcomes Research Institute for Medicine and Public Health Vanderbilt University Nashville, Tennessee Daniella J. Perlroth, M.D. Research Associate Center for Health Policy Center for Primary Care and Outcomes Research Stanford University Stanford, California Mack Roach III, M.D., FACR Professor Departments of Radiation Oncology and Urology Chairman Department of Radiation Oncology University of California, San Francisco Helen Diller Family Comprehensive Cancer Center San Francisco, California Paul F. Schellhammer, M.D., FACS Professor East Virginia Medical School Medical Director Virginia Prostate Center Norfolk, Virginia Ian M. Thompson, Jr., M.D. Professor and Chair Department of Urology Executive Director Cancer Therapy and Research Center University of Texas Health Science Center at San Antonio San Antonio, Texas Timothy J. Wilt, M.D., M.P.H. Professor of Medicine and Core Investigator Minneapolis Veterans Affairs Center for Chronic Disease Outcomes Research and the University of Minnesota School of Medicine Minneapolis, Minnesota |
Planning Commitee
|
Bhupinder Mann, MBBS Planning Chairperson Head Genitourinary and Brain Cancer Therapeutics Cancer Therapy Evaluation Program National Cancer Institute National Institutes of Health Bethesda, Maryland |
|
|
Peter Albertsen, M.D. Medical Director UConn Medical Group Associate Dean Clinical Research Planning and Administration Associate Dean Clinical Affairs Division of Urology University of Connecticut Health Center Farmington, Connecticut Otis W. Brawley, M.D. Chief Medical Officer American Cancer Society Atlanta, Georgia Patricia A. Ganz, M.D. Panel and Conference Chairperson Director Division of Cancer Prevention and Control Research University of California, Los Angeles Schools of Medicine and Public Health Jonsson Comprehensive Cancer Center Los Angeles, California Ingrid Hall, Ph.D., M.P.H. Lead Epidemiologist/Team Lead Health Services Research Team Epidemiology and Applied Research Branch Division of Cancer Prevention and Control Centers for Disease Control and Prevention Atlanta, Georgia Barnett S. Kramer, M.D., M.P.H. Associate Director for Disease Prevention Office of the Director National Institutes of Health Bethesda, Maryland William Lawrence, M.D., M.S. Medical Officer Center for Outcomes and Evidence Agency for Healthcare Research and Quality Rockville, Maryland Kelli K. Marciel, M.A. Communications Director Office of Medical Applications of Research Office of the Director National Institutes of Health Bethesda, Maryland Jeffrey Metter, M.D. Medical Officer Longitudinal Studies Section National Institute on Aging National Institutes of Health Baltimore, Maryland Elizabeth Neilson, M.S.N., M.P.H. Senior Advisor Office of Medical Applications of Research Office of the Director National Institutes of Health Bethesda, Maryland |
Susanne Olkkola, M.Ed., M.P.A. Senior Advisor Consensus Development Program Office of Medical Applications of Research Office of the Director National Institutes of Health Bethesda, Maryland Peter A. Pinto, M.D. Director Fellowship Program Urologic Oncology Branch National Cancer Institute National Institutes of Health Bethesda, Maryland Scott Ramsey, M.D., Ph.D. Associate Professor of Medicine and Health Services Associate Member Cancer Prevention Research Program Division of General Internal Medicine Fred Hutchinson Cancer Research Center Seattle, Washington Lisa Richardson, M.D., M.P.H. Medical Officer Injury and Environmental Health Office of Noncommunicable Diseases National Center for Chronic Disease Prevention and Health Promotion Centers for Disease Control and Prevention Atlanta, Georgia Mack Roach III, M.D., FACR Professor Departments of Radiation Oncology and Urology Chairman Department of Radiation Oncology University of California, San Francisco Helen Diller Family Comprehensive Cancer Center San Francisco, California Paul F. Schellhammer, M.D., FACS Professor East Virginia Medical School Medical Director Virginia Prostate Center Norfolk, Virginia Paris A. Watson Senior Advisor Consensus Development Program Office of Medical Applications of Research Office of the Director National Institutes of Health Bethesda, Maryland Timothy J. Wilt, M.D., M.P.H. Professor of Medicine and Core Investigator Minneapolis Veterans Affairs Center for Chronic Disease Outcomes Research and the University of Minnesota School of Medicine Minneapolis, Minnesota |
|
Planning Committee members provided their input at a meeting held on August 11–13, 2010. The information provided here was accurate at the time of that meeting. |
|
Educational Planners
|
Sara E. Critchley, M.S., R.N. Continuing Education Learner Support Team Education Design and Accreditation Branch Division of Leadership and Practice Scientific Education and Professional Development Office Centers for Disease Control and Prevention Atlanta, Georgia Jennifer M. Croswell, M.D., M.P.H. Acting Director Office of Medical Applications of Research Office of the Director National Institutes of Health Bethesda, Maryland Ingrid Hall, Ph.D., M.P.H. Lead Epidemiologist/Team Lead Health Services Research Team Epidemiology and Applied Research Branch Division of Cancer Prevention and Control Centers for Disease Control and Prevention Atlanta, Georgia |
Elizabeth Neilson, M.S.N., M.P.H. Senior Advisor Office of Medical Applications of Research Office of the Director National Institutes of Health Bethesda, Maryland Trang N. Nguyen, M.P.H., CHES Health Education Specialist I Chicksaw Nation Industries Contractor Centers for Disease Control and Prevention Atlanta, Georgia Lisa Richardson, M.D., M.P.H. Medical Officer Injury and Environmental Health Office of Noncommunicable Diseases National Center for Chronic Disease Prevention and Health Promotion Centers for Disease Control and Prevention Atlanta, Georgia |
Abstracts
The abstracts are designed to inform the panel and conference participants, as well as to serve as a reference document for any other interested parties. We would like to thank the speakers for preparing and presenting their findings on this important topic.
The organizers would like to thank the planning committee, the panel, the Tufts Evidence-based Practice Center, and the Agency for Healthcare Research and Quality. We also would like to thank the National Cancer Institute and the Centers for Disease Control and Prevention. We appreciate your continued interest in both the National Institutes of Health Consensus Development Program and the area of active surveillance of men with localized prostate cancer.
Please note that where multiple authors are listed in an abstract, the underline denotes the presenting author.
Agenda
Monday, December 5, 2011
| 8:30 a.m. |
Opening Remarks James H. Doroshow, M.D., FACP Director Division of Cancer Treatment and Diagnosis National Cancer Institute National Institutes of Health |
| 8:40 a.m. |
Charge to the Panel Paul M. Coates, Ph.D. Director Office of Dietary Supplements and Acting Director Office of Disease Prevention Office of the Director National Institutes of Health |
| 8:50 a.m. |
Conference Overview and Panel Activities Patricia A. Ganz, M.D. Panel and Conference Chairperson Professor University of California, Los Angeles Schools of Medicine and Public Health Division of Cancer Prevention and Control Research Jonsson Comprehensive Cancer Center |
| 9:00 a.m. |
Director Securities Law Program Catholic University of America School of Law |
| 9:20 a.m. |
A Urologist’s Personal Experience With Prostate Cancer Paul F. Schellhammer, M.D., FACS Professor Eastern Virginia Medical School Medical Director Virginia Prostate Center |
| 9:40 a.m. |
Cancer Diagnosis and Overdiagnosis Robert K. Royce Distinguished Professor Chief of Urologic Surgery Washington University School of Medicine Barnes-Jewish Hospital Siteman Cancer Center |
| 10:00 a.m. | Discussion Participants with questions or comments for the speakers should proceed to the designated microphones and wait to be recognized by the panel chairperson. Please state your name and affiliation. Questions and comments not heard before the close of the discussion period may be submitted on the computers in the registration area. Please be aware that all statements made at the microphone or submitted later are in the public domain. |
|
|
| 10:30 a.m. |
Temporal Trends in the Epidemiology of Prostate Cancer Otis W. Brawley, M.D. Chief Medical Officer American Cancer Society |
| 10:50 a.m. |
Research Associate Tufts Evidence-based Practice Center Tufts Medical Center |
| 11:10 a.m. |
Temporal Changes in the Pathologic Assessment of Prostate Cancer Associate Professor and Director Prostate Diagnostic Laboratory Co-Director Prostate Cancer Research Laboratories Co-Director Colorado Molecular Correlates Laboratory University of Colorado Denver School of Medicine |
| 11:30 a.m. |
Professor and Chair Department of Urology Executive Director Cancer Therapy and Research Center University of Texas Health Science Center at San Antonio |
| 11:50 a.m. | Discussion |
| 12:30 p.m. | Lunch—Panel Executive Session |
|
|
| 1:30 p.m. |
What Is the Risk Posed by Prostate Cancer? Medical Director UConn Medical Group Associate Dean Clinical Research Planning and Administration Associate Dean Clinical Affairs Division of Urology University of Connecticut Health Center |
| 1:50 p.m. |
Tumor and Patient Metrics, Eligibility, and Inclusion for Active Surveillance for Prostate Cancer Professor Urology and Oncology Johns Hopkins Medicine Director Division of Adult Urology Bradley Urological Institute The Johns Hopkins Hospital |
| 2:10 p.m. |
Active Surveillance: Inclusive Approach Chief Division of Urology Sunnybrook Health Sciences Centre Professor of Surgery University of Toronto |
| 2:30 p.m. | Discussion |
|
|
| 3:00 p.m. |
Associate Director Tufts Evidence-based Practice Center Tufts Medical Center |
| 3:20 p.m. |
Presenting Treatment Options to Patients With Localized Prostate Cancer Head of School School of Social and Community Medicine University of Bristol |
| 3:40 p.m. |
Improving the Communication of the Benefits and Harms of Treatment Strategies Richard M. Hoffman, M.D., M.P.H. Professor of Medicine University of New Mexico School of Medicine Staff Physician New Mexico Veterans Affairs Health Care System |
| 4:00 p.m. |
Peter R. Carroll, M.D., M.P.H. Ken and Donna Derr – Chevron Distinguished Professor Department of Urology University of California, San Francisco (UCSF) Associate Dean UCSF School of Medicine Director of Clinical Services and Strategic Planning UCSF Helen Diller Family Comprehensive Cancer Center |
| 4:20 p.m. | Discussion |
| 5:00 p.m. | Adjournment |
Tuesday, December 6, 2011
| 8:30 a.m. |
Factors Influencing Patients’ Acceptance of and Adherence to Active Surveillance Professor of Urologic Surgery Director Center for Surgical Quality and Outcomes Research Institute for Medicine and Public Health Vanderbilt University |
| 8:50 a.m. |
Associate Professor of Clinical Epidemiology Department of Preventive Medicine Division of Epidemiology Keck School of Medicine University of Southern California |
| 9:10 a.m. | Discussion |
|
|
| 9:30 a.m. |
Overview of Randomized Controlled Trials for Localized Prostate Cancer Professor Departments of Radiation Oncology and Urology Chairman Department of Radiation Oncology University of California, San Francisco Helen Diller Family Comprehensive Cancer Center |
| 9:50 a.m. |
Results From the Scandinavian Prostate Cancer Group 4 Trial (SPCG-4) Professor of Cancer Epidemiology Division of Cancer Studies King’s College London School of Medicine Guy’s Hospital |
| 10:10 a.m. |
Results From the Prostate Cancer Intervention Versus Observation Trial Professor of Medicine and Core Investigator Minneapolis Veterans Affairs Center for Chronic Disease Outcomes Research and the University of Minnesota School of Medicine |
| 10:30 a.m. |
Impact of Different Management Strategies on Quality of Life in Localized Prostate Cancer Professor of Urology and Health Services Chair Department of Urology David Geffen School of Medicine at the University of California, Los Angeles (UCLA) UCLA School of Public Health |
| 10:50 a.m. |
Economic Analysis of Different Management Strategies for Localized Prostate Cancer Research Associate Center for Health Policy Center for Primary Care and Outcomes Research Stanford University |
| 11:10 a.m. |
Assistant Director Tufts Evidence-based Practice Center Tufts Medical Center |
| 11:30 a.m. | Discussion |
| 12:30 p.m. | Adjournment |
Wednesday, December 7, 2011
| 9:00 a.m. | Presentation of the Draft Consensus Statement The panel chairperson will read the draft statement to the assembled audience. |
| 9:30 a.m. | Discussion The panel chairperson will call for questions and comments from the audience on the draft statement, beginning with the introduction and continuing through each subsequent section, in turn. Please confine your comments to the section under discussion. The chairperson will use discretion in proceeding to subsequent sections so that comments on the entire statement may be heard during the time allotted. Participants with comments should proceed to the designated microphones and wait to be recognized by the panel chairperson. Please state your name and affiliation. Questions and comments not heard before the close of the discussion period may be submitted on the computers in the registration area. For participants viewing the remote webcast, comments may be submitted online at consensus.nih.gov/comments.htm. Comments will not be accepted after 11:30 a.m. Please be aware that all statements made at the microphone or submitted later are in the public domain. |
| 11:00 a.m. | Adjournment Panel Meets in Executive Session The public portion of the conference ends at 11:00 a.m. The panel meets in its last executive session to review public comments on the draft statement. |
| 2:00 p.m. | Press Telebriefing The panel will provide a summary of its findings to the press and will answer questions from reporters via telebriefing. Only members of the press are permitted to ask questions of the panel during this time. Interested conference participants who are not members of the press may call in (from a remote location) to listen to the live telebriefing. Please go to consensus.nih.gov for instructions on joining the call. The panel’s draft statement will be posted to consensus.nih.gov as soon as possible after the close of proceedings, and the final statement will be posted 4 to 6 weeks later. |
You’re Kidding....I Have Cancer? A Patient’s Perspective on Coping With Prostate Cancer and Why “Active Surveillance” Was Not Chosen
David A. Lipton, J.D.
Introduction and Scope of Talk
I teach. In fact, I teach law. One might guess that my training has led me to approach life’s challenges with a somewhat defined methodology.
As happens to others, the news that I had prostate cancer pretty much took my breath away. After I became resigned to the disappointing news, I hunkered down to a familiar pattern of investigation and evaluation. Perhaps I was a tad compulsive. Perhaps I was excessive. But, as the saying goes, “this was MY cancer.” Whether formally or informally, I suspect I spoke with probably 10 physicians in the field of urology, oncology, and radiology. In retrospect, my goals appeared to be to educate myself on the pluses and minuses of the variety of choices with which I was confronted and then to evaluate which physician I thought I could work with best. Obviously, there were issues that I overlooked and with which I became concerned only after I had undergone my treatment. Three days from now will be the third anniversary of my robotic radical prostatectomy.
The process of selecting the physicians with whom I sought to consult was perhaps as mysterious and tangled as the information that I culled. I remain in contact with several of the doctors from this convoluted web of consultations. Possibly, I gained a friend or two. As events unfolded, sometime after my procedure, two of the doctors who were willing to share with me both time and information came to play a role in the preparation of this program that we are attending today. Apparently, after consultation with one another, they decided to ask me to speak and give some sense of what it felt like to be the patient in the process that I think of as a decision tree for deciding on both a treatment and a treatment provider for prostate cancer. When asked to speak here, I naively assumed that the ideal talk I could present would be to focus on the selection process I had engaged. I had hoped that in discussing this uncertain and anxiety-producing process, which indeed was not without its flaws, thoughts might be generated regarding possible missing tools in the universe of workshops for educating prostate cancer patients.
As I focused more on the significance of the conference’s theme of active surveillance, it became increasingly apparent that an “ideal talk” would have to include a discussion of why I did not choose active surveillance. The release of additional studies in the past several years suggesting that there is marginal or no difference in the rate of mortality between active surveillance and intervention made more pressing the need to include in my talk significant discussion of why active surveillance was not my treatment of choice. As fate, or more likely, reason would have it, the two discussion topics proved to be integrally tied. The discussion of my treatment selection inexorably led to a companion analysis of why active surveillance was not a compelling choice.
What Was Learned From Engaging in a Treatment Selection Process
What is it that might be gleaned from an analysis of a decisionmaking process for responding to a diagnosis of prostate cancer, which was conducted by a relatively rational, educated, academic, cancer patient?
For starters, at least this patient felt confused by the literature, the Internet sites, and the information that existed.1 This patient found it necessary to create his own “decision tree” to discern how to proceed. If this anecdotal account is typical of other similarly situated patients, then there is something lacking in the decisionmaking materials that are provided or are available for the patient. The missing “something” might be as simple as an informed physician assistant or nurse practitioner who is willing to sit down with the patient and help the patient determine what is important to him and which approach might best preserve his lifestyle. That informed medical extender would be helping the patient to frame the decisions that would have to be made. I remember feeling like I was shooting craps over the issue of did I want to take the risk of immediate erectile dysfunction that might be the outcome of surgery, or was I more willing to wait the 2 to 5 years after radiation therapy to discover that I had ended up in the same place? Ultimately, we all have to make that decision. But it turned out that it also was my task to construct the inquiry.
In retrospect, a fascinating discovery for me was that not all intervention treatment providers were created equal. Needless to say, I expected skill differentials, many of which would be difficult to assess. What I had not anticipated was the wide spectrum of personalities, ranging from “warm and fuzzy” to “intolerable.” Among those on the less positive end of the scale was the brachytherapist who did not explain to me, until a second visit, that he would be working in tandem with a urologic surgeon and that I would alternate between the two in posttherapy treatment. Should I have figured that out on my own? I do not believe so. For different reasons, I felt disappointed with a robotic surgeon who waited for a second visit to tell me that I had to remember, regardless of my preferences, that he was “in charge.” I walked away from both of those physicians. Could a physician assistant or nurse practitioner have alerted me to these issues ahead of time? Probably not. But certainly, a thoughtful adviser might have said, “Figure out what matters to you. Do you want someone who shares with you exactly how a procedure will work or are you willing to say ‘let the doctor decide’?”
Not surprisingly, my exploration process failed to explore all issues that would prove vital. Most significantly, I did not inquire about the chosen doctor’s availability were something to go wrong after the operation. In hindsight, perhaps I should have recognized that a top surgeon with a warm patient manner and user-friendly personality was going to be terribly popular, busy, and perhaps difficult to get hold of if it was determined, as indeed it was, that I was experiencing postoperation internal bleeding. Were I to do it over, I would have inquired whether the surgeon had good back-up that would be available to assist me at the hospital emergency room during my two late night visits. Again, a guide, helping me to formulate my selection methodology, might have urged me to make such inquiries. After my bleeding was attended to through follow-up surgery, I did share with my surgeon my concerns about dealing with emergency room personnel who were unfamiliar with my surgery. Hopefully, my experience has benefited others who came after me.
Why the Selection Process Did Not Lead This Patient To Choose “Active Surveillance”
Let us assume that this patient fashioned a vaguely competent approach for deciding on his prostate cancer treatment and the selection of his physician. Let us also assume that the perceived need itself, to engage in such a process, reflects deficiencies in the available resources to assist patients in making these decisions. We understand that there might not be agreement with either of these assumptions. For the purpose of this conference, however, the question is, “How is it that the process of searching for the proper treatment did not lead me to the selection of active surveillance?” More importantly, why was active surveillance not a serious consideration? And finally, and this is very much a matter of an individual preference, how is it that if I had it to do over again, I still would chose intervention rather than active surveillance?
These are not difficult questions from my perspective. When I learned of my prostate cancer, I was advised of my choices by the urologist who had first suggested that I get a biopsy. He briefly outlined the different approaches and some of the advantages and drawbacks of each approach. Active surveillance was not given prominence. Perhaps I indicated that I would probably seek intervention. But one way or the other, active surveillance, which was then called “watchful waiting,” was not given the emphasis given to intervention. Within my investigations, I saw one other urologist/surgeon for general advice. Again, perhaps it appeared to him that my mind was set, but there was no urging of active surveillance. Indeed, had I chosen active surveillance, I would not have known of a urologist who would have provided the surveillance. Then as now, I did not and do not have the sense that there are a large number of active surveillance physicians, although there are certainly some and they certainly must recommend active surveillance. On the other hand, there clearly are substantial numbers of radical prostatectomy surgeons, robotic surgeons, radiologists, and brachytherapists. It is quite possible that the ratio of active surveillance practitioners to “interventionists” has increased in the past 3 years. But, in 2008, it was difficult to find a practitioner who was a strong proponent of the active surveillance strategy.2
The apparent absence of promoters of active surveillance should not be surprising. Physicians train to be surgeons, to be radiologists, or perhaps to learn new robotic skills. Having learned those skills, it would be counterintuitive to expect the physician not to promote the chosen studied treatment. In addition, hospitals promote intervention treatment. Financial investments in robotic surgery equipment and new radiation devices stimulate hospitals to advertise these procedures. In July of this year, The Washington Post reviewed a study by Martin Markary about hospital advertisement of new technology. Markary, a pancreatic surgeon at Johns Hopkins School of Medicine, found that “among 164 U.S. hospital websites featuring robotic technology, 18 percent made use of what the study termed ‘emotionally appealing phrases,’ 73 percent used manufacturer-provided images or text, and 33 percent linked directly to a manufacturer’s website.”3
In some regards, the information about what active surveillance entails seems surprisingly limited. Although it was a clever linguistic move to reconfigure and expand the terminology from “watchful waiting” to “active surveillance,” it is not always clear to the patient what makes it “active.” When informed that one has cancer, there is a very human inclination to do “something.” Active surveillance, in fact, does not feel active, except for those who perceive undergoing frequent prostate-specific antigen (PSA) tests, rectal exams, and periodic biopsies as “doing something.” There is also very little discussion of what the survival rates are when active surveillance reveals a spike in a patient’s PSA (although I have been advised that these informational gaps will be filled by ongoing studies). The fact that several studies show no or limited impact on mortality rates as a result of intervention4 might indicate that mortality rates after a spike in PSA of a patient pursuing active surveillance should be no different than mortality rates were a rise in PSA found after intervention.5 In addition, the studies might indicate that the likelihood of PSA spiking during surveillance should not differ from the likelihood of it spiking after intervention. Those are difficult concepts to swallow, and all the more difficult to digest when it is “your cancer” in the balance. But they are important points to bring home if active surveillance is going to be viewed as an attractive option.6
Why This Patient Would Still Not Choose Active Surveillance
To be totally honest, were I to make my treatment decision today, I do not believe I would choose active surveillance. Indeed, I would have been a fairly good candidate for active surveillance (PSA less than 6, Gleason of 6 before the operation and revised to 7 after the operation, biopsy showing cancer in 1 of 12 needles, tumor size of 1 cm). Ultimately, however, the decision was very visceral and personal. The longevity of my parents and their siblings suggests that I have a fair chance of living another 30 years. I frankly plan to teach another 23 or 24 years…depending upon how much I am enjoying myself. There is one gland in my body that could have produced prostate cancer. That gland has been removed. I did not want to wake up each morning wondering if the previous night was the night that a cancer cell “flew” from my prostate and invaded other reaches of my body. I understand full well, and the studies cited confirm, that the cancer cell might already have flown. The fact that I continue to have my PSA tested every 6 months brings that point home to me twice a year. And statistically speaking, my decision lacks a certain amount of logic. But that is “statistically speaking.” If indeed that cancer cell did not travel from my prostate into my body before 3 years ago, there no longer is a prostate gland, invaded by cancer, from which a life-threatening cell may depart to cause me concern. That gives me some comfort, even though a number of studies suggest it should not.
Statistics do not necessarily control treatment decisions for prostate cancer. Gut perceptions also play an important role. If active surveillance is to become a more prevalently adopted treatment, it well may be that the medical profession will have to discover how to influence that gut perception.
Notes
- The reference book that I found most helpful was Walsh P, Farrar J. Dr. Patrick Walsh’s Guide to Surviving Prostate Cancer, 2nd ed. New York: Warner Wellness; 2007. Since my treatment, a new book has been published that focuses more on creating a decision tree: McHugh JC. The Decision: Your Prostate Biopsy Shows Cancer. Now What? Medical Insight, Personal Stories, and Humor by a Urologist Who Has Been Where You Are Now. Gainesville, GA: Jennie Cooper Press USA; 2010. The essay that provided me with the greatest comfort was Lange PH, Schellhammer PF. Reflections on prostate cancer: personal experiences of two urologic oncologists. In: Kirby RS, Partin AW, Feneley M, et al., eds. Prostate Cancer: Principles and Practice. London: Taylor and Francis; 2006. This essay allowed me to understand that the decision I faced was difficult, even for medical providers in the field.
- It is of interest to note that the National Institutes of Health/National Cancer Institute online patient version of the pamphlet Prostate Cancer Treatment (PDQ®), last modified in June 2011, provides, in the treatment option overview section, two lines to describe the active surveillance treatment option (still referred to as watchful waiting). See cancer.gov/cancertopics/pdq/treatment/prostate/Patient/page4#Keypoint14. Surgery, radiation, and hormone treatment have considerably greater coverage. In the health professional version of the pamphlet, although there is a discussion of studies suggesting limited or no advantages from intervention over active surveillance, the actual list of treatment options for prostate cancer does not include a mention of active surveillance. See cancer.gov/cancertopics/pdq/treatment/prostate/HealthProfessional/page4. Accessed August 11, 2011.
- Torres C. Robotic surgery extends its reach in health care, hospital marketing. The Washington Post. July 18, 2011. Available at: washingtonpost.com/national/robotic-surgery-extends-its-reach-in-health-care-hospital-marketing/2011/06/15/gIQAnw6HMI_story.html. Accessed August 11, 2011.
- Stattin P, Holmberg E, Johansson JE, et al. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst. 2010;102(13):950–958. Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11):1202–1209. Bill-Axelson A, Holmberg L, Filén F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian prostate cancer group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144–1154. Charnow JA. Active surveillance offers PCa survival similar to treatment. Renal and Urology News. May 17, 2011 (discussion of a report by Dr. Kiranpreet Khurana, presented at the May 2011 meeting of the American Urological Study in Washington, DC).
- The Stattin, Holmberg, Johansson study cited above and discussed online in the health providers’ version of the National Institutes of Health/National Cancer Institute Prostate Cancer Treatment (PDQ®) pamphlet does indeed suggest that there is no difference in mortality between those who choose immediate prostatectomy as opposed to those choosing a prostatectomy only after surveillance indicates the need. See the treatment overview section in the online patient version of the pamphlet available at: cancer.gov/cancertopics/pdq/treatment/prostate/HealthProfessional/page4. Accessed August 11, 2011.
- As close as the health providers’ version of the National Institutes of Health/National Cancer Institute online pamphlet on Prostate Cancer Treatment (PDQ®) cited above comes to suggesting the wisdom of active surveillance is the following sentence which precedes a discussion of one of the studies cited above: “Many men with screen-detected prostate cancer are candidates for active surveillance, with definitive therapy reserved for signs of tumor progression.” From cancer.gov/cancertopics/pdq/treatment/prostate/HealthProfessional/page4#Reference4.38. Accessed August 11, 2011.
A Urologist’s Personal Experience With Prostate Cancer
Paul F. Schellhammer, M.D.
Words matter, and words voiced by a physician during a patient interaction have a profound effect on the individual patient’s attitude and well-being.
The word “cure,” derived from the Latin “to care,” has evolved to imply that a procedure or medication will address the disease process such that it will be successfully resolved. When a patient hears that a disease is cured, in his mind it becomes past history. It no longer occupies attention or is a cause for anxiety. However, if the disease recurs, as is too often the case in the world of cancer, the caring that is required may not materialize—the cure and its expectations have failed, but the caring has been lost in translation. This conference will discuss the concept of “active surveillance.” Surveillance implies a proactive process to monitor rather than to initially render treatment for a selected cohort of men with newly diagnosed prostate cancer. It describes a strategy where the term “cure” largely loses relevance. Caring, with appropriate guidance and reassurance, on the other hand, rises front and center.
Other words that have found their way into common and frequent usage for the patient with cancer are “war” and “survivor.” War against cancer was declared legislatively by the President of the United States, Richard M. Nixon, in 1971, when he signed the National Cancer Act. Convention identifies those left standing during and after a war as “the survivors.” Prostate cancer is a disease that runs a long-time course. Virtually 100% of patients diagnosed with this disease survive 5 years and greater, and more than 90% survive 10 years. War requires a hypervigilant state of readiness; it is resource depleting and often exhausting. For the prostate cancer patient, a war mentality may very well deprive him of the opportunity to learn to live well and to live long with his cancer, both very realistic possibilities. Again, in the context of a surveillance strategy, war and survivorship do not find traction. Patience, partnership, and participant are more appropriate.
At the time of diagnosis, the word “cancer” is often too toxic to allow a “living well with cancer” discussion or a discussion of a surveillance strategy. I can personally attest to this based on a personal experience.1,2 Like so many men I have encountered, I too have experienced the two most common illnesses of aging, namely a cardiac event and the diagnosis of prostate cancer. I find it helpful to compare the emotions generated by each. At age 58, without any prior symptomatology, I experienced crushing chest pain. Prompt angioplasty and stents minimized cardiac damage. After my coronary occlusion, my mindset was one of establishing a program to heal my heart. Through diet modifications, exercise, and careful surveillance, I was committed to a partnership for mutual recovery with the injured organ. Implementing this lifestyle change was both satisfying and comforting. Reactions generated by my prostate cancer diagnosis were totally different. The sense of betrayal and hostility toward the offending organ was overpowering and was followed immediately by a commitment to destroy it by whatever means. Ironically, even though I intellectually recognized that the immediate and short-term threat to life was much greater secondary to coronary artery disease, this was overcome by my visceral reaction; my attention and anxiety were focused on the cancer diagnosis. My impression is that the majority of patients with both of these common diseases experience a similar emotional stance. The word “cancer” becomes the driving force in a patient’s view of his diagnosis and future. The astute diagnostician William Ostler recognized this emotionally driven mindset years ago when he advised that it is as important to know about the patient who has a disease as it is to know about the disease the patient has.
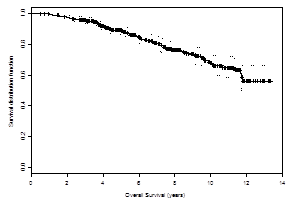
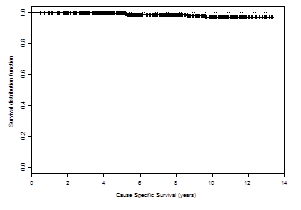
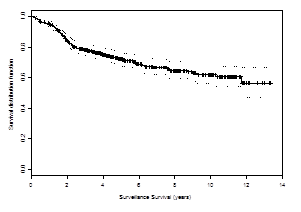
It has been 11 years since my diagnosis of prostate cancer. The prostate-specific antigen (PSA) clock has continued to tick up with regularity (Table 1). So the disease has not been cured. However, I am thankful that I have not been at war over this past decade and have attempted to adopt the same partnership stance with regard to prostate cancer that was my initial reaction in response to my cardiac event. And again, returning to the theme of “words matter,” I now like to consider myself a partner and participant rather than a war-weary survivor. I am a partner with my physicians in addressing the disease process; I am a participant in the strategies developed to address the disease whose presence has been identified by a series of PSA rises.3
Table 1. Tale of a Prostate Cancer Participant
|
Radical prostatectomy Rising PSA |
2000 |
| Salvage radiation and androgen deprivation Rising PSA |
2002 |
| Clinical trial (Phase 2) Rising PSA |
2006 |
| Intermittent to continuous combined androgen blockade Rising PSA |
2007 |
| Secondary hormone therapy—ketoconizole/GMCSF | 2008 |
| Transdermal estradial | 2009 |
| Stable PSA | 2010 |
| ? ? ? | 2011 |
PSA = prostate-specific antigen; GMCSF = granulocyte macrophage colony-stimulating factor.
Active surveillance is not an intuitive pathway for a patient to accept on receiving a diagnosis of cancer. Active surveillance does not describe a battle but rather a partnership with and participation in living well with a cancer game plan. To convincingly discuss the active surveillance strategy/game plan will take all the counseling skills of a physician, both at diagnosis and at the follow-up intervals. The vocabulary of cancer includes a term that physicians and scientists use to identify gaps in treatment protocols or pathways. The gap is termed an “unmet need.” This conference will present the option of active surveillance to address this unmet need. It will address the education of the patient and family so that they are comfortable with a discussion and decision that some prostate cancers—yes, even though the word “cancer” is spoken—may best be managed initially with a surveillance strategy rather than surgical or radiation or medicinal intervention.
References
- Lange PH, Schellhammer PF. Personal experiences of two urologic oncologists. In: Kirby RS, Partin AW, Feneley M, et al., eds. Prostate Cancer: Principles and Practice. London: Taylor and Francis; 2006:617–624.
- Schellhammer PF. Views from the “other side”: personal reflections about prostate cancer. In: Patient Information. Foundation for Urological Research; 2006. Available at: foundationforurologicalresearch.com. Accessed August 1, 2011.
- Schellhammer PF. Treater to target: experiences of a prostate cancer participant. In: Hemal AK, Menon M, eds. Robotics in Genitourinary Surgery. London: Springer-Verlag; 2011:627–634.
Cancer Diagnosis and Overdiagnosis
Gerald L. Andriole, M.D.
There is an enormous pool of prostate cancer prevalent in middle-aged and older men as shown in autopsy studies by Sakr et al.1 and Powell et al.2 (see Table 1) and in a series of studies evaluating cystoprostatectomy specimens (reported rates of incidental prostate cancer from 27% to 60% and clinically significant cancers from 18% to 53%).3–7 It is becoming clear that prostate-specific antigen (PSA) testing as commonly performed in the United States (annual PSA and digital rectal examination) results in overdetection of some prostate cancers that are not apt to ever become clinically relevant.
| Percentage With Prostate Cancer at Autopsy | ||
|---|---|---|
| Age Group | Black | White |
| 20–29 | 8 | 11 |
| 30–39 | 31 | 31 |
| 40–49 | 43 | 38 |
| 50–59 | 46 | 44 |
| 60–69 | 72 | 68 |
| 70–79 | 77 | 68 |
| From Powell IJ, Bock CH, Ruterbusch JJ, et al. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183(5):1792–1796. Reprinted with permission. Copyright © 2010 American Urological Association. All rights reserved. | ||
The magnitude of overdetection can be estimated by comparing a man’s current lifetime risk of prostate cancer (approximately 17%) with his approximate 3% risk of prostate cancer mortality. Others have estimated the magnitude of overdiagnosis by examining various cohorts of men. Welch and Black estimated that 60% of screen-detected cancers in the European Randomized Study of Screening for Prostate Cancer (which evaluated PSA testing every 4 years) met the definition of overdiagnosis based on the finding of 58 prostate cancers per 1,000 men in the screened arm versus 34 prostate cancers per 1,000 men not undergoing screening.8 Draisma et al. used a simulation analysis and reported that age of diagnosis heavily influenced the estimate of overdiagnosis, which ranged from 27% for young men to 56% for men age 75.9 These data are especially relevant for elderly men in light of the observations of Drazer et al. where, as seen in Figure 1, substantial PSA testing occurs among men age 65 and older.10 Etzioni et al. evaluated racial differences, and estimated overdiagnosis was more common in black men than white men (44% vs. 29%).11 They also estimated that the use of PSA would identify up to 15% of autopsy cancers in white men and 37% in black men. McGregor et al. reported an overdiagnosis rate of 84% when only lethal prostate cancer was defined as a clinically significant cancer.12 Moreover, overdiagnosis may be even higher when one considers the impact of comorbidity on a man’s chance of dying of prostate cancer as is illustrated by Albertsen et al. (see Table 2).13 Any diagnosis of prostate cancer substantially reduces men’s quality of life.14
Figure 1. Population-Based Prevalence of Prostate-Specific Antigen Screening in the United States
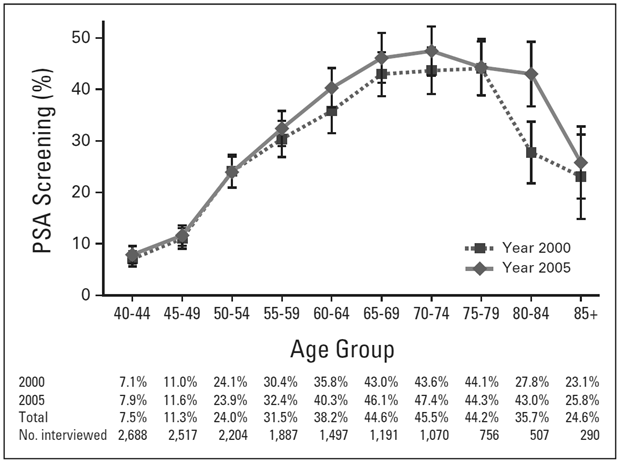
From Drazer MW, Huo D, Schonberg MA, et al. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J Clin Oncol. 2011;29(13):1736–1743. Reprinted with permission. Copyright © 2011 American Society of Clinical Oncology. All rights reserved.
A cancer diagnosis frequently results in aggressive treatment, and for many men this may be overtreatment. In the United States, approximately 90% of men with newly diagnosed prostate cancer, including those with low-risk factors, undergo aggressive treatment.15,16 In a typical radical prostatectomy series, about 20% of patients with a PSA <4 and 16% of those with a PSA >4 have trivial small-volume tumors.17
PSA is not prostate cancer specific; since it inevitably rises as men age, many men undergo biopsies of the prostate and are discovered to have incidental cancers merely because their benign prostatic hyperplasia is progressing. One way of potentially avoiding overdiagnosis in men on the basis of PSA is to consider using PSA in a different way. An analysis by Lilja et al. suggests that it is possible to identify a population of men at high risk of prostate cancer on the basis of their serum PSA level during their 40s and 50s, a time when PSA levels are not apt to be significantly confounded by benign prostatic hyperplasia.18 Other strategies might include the use of PSA-related kallikreins19 and nomograms to predict an individual man’s risk of prostate cancer and to intensively screen those at the highest risk for prostate cancer mortality while not screening those at low risk. Another option might be to improve the ability of PSA to identify aggressive cancer by use of 5-alpha reductase inhibitors.20–22 Since 5-alpha reductase inhibitors “stabilize” the amount of PSA produced by benign prostatic elements, PSA rises in men receiving 5-alpha reductase inhibitors are especially worrisome for high-risk prostate cancer.
| Gleason Grade | Medical Comorbidity | 10-Year Prostate Cancer Mortality (%) | 10-Year Overall Mortality (%) |
|---|---|---|---|
| 5–7 | 0 | 4.8 | 28.8 |
| 1 | 2.0 | 50.5 | |
| >1 | 5.3 | 83.1 | |
| 8–10 | 0 | 25.7 | 55.0 |
| 1 | 20.2 | 52.0 | |
| >1 | 13.7 | 64.3 |
Five-alpha reductase inhibitors, in addition to improving PSA, reduce a man’s chance of being diagnosed with a low-grade cancer by 25% to 30%.23,24 However, the exact role of 5-alpha reductase inhibitors remains uncertain given some concerns that they may predispose men to developing high-grade prostate cancer.25
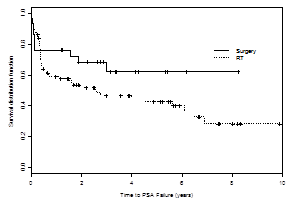
Potential strategies to eliminate overtreatment include more widespread implementation of active surveillance protocols,26 better identification of aggressive tumors based on improved biopsy,27,28 use of molecular markers,29 and potentially focal ablation of small tumors.30 Using conventional biopsy and considering men for active surveillance on the basis of relatively small-volume Gleason 6 tumors, active surveillance seems to be a safe option, although many men on active surveillance defect to aggressive treatment out of anxiety. Focal ablation therapy may be a valid adjunct to active surveillance as it may prevent biopsy progression. The use of 5-alpha reductase inhibitors also may be plausible agents to reduce biopsy progression of men on active surveillance.31
References
- Sakr WA, Haas GP, Cassin BF, et al. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150(2 Pt 1):379–385.
- Powell IJ, Bock CH, Ruterbusch JJ, et al. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol. 2010;183(5):1792–1796.
- Pettus JA, Al-Ahmadie H, Barocas DA, et al. Risk assessment of prostatic pathology in patients undergoing radical cystoprostatectomy. Eur Urol. 2008;53(2):370–375.
- Winkler MH, Livni N, Mannion EM, et al. Characteristics of incidental prostatic adenocarcinoma in contemporary radical cystoprostatectomy specimens. BJU Int. 2007;99(3):554–558.
- Gakis G, Schilling D, Bedke J, et al. Incidental prostate cancer at radical cystoprostatectomy: implications for apex-sparing surgery. BJU Int. 2010;105(4):468–471.
- Abdelhady M, Abusamra A, Pautler SE, et al. Clinically significant prostate cancer found incidentally in radical cystoprostatectomy specimens. BJU Int. 2007;99(2):326–329.
- Kouriefs C, Fazili T, Masood S, et al. Incidentally detected prostate cancer in cystoprostatectomy specimens. Urol Int. 2005;75(3):213–216.
- Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–613.
- Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101(6):374–383.
- Drazer MW, Huo D, Schonberg MA, et al. Population-based patterns and predictors of prostate-specific antigen screening among older men in the United States. J Clin Oncol. 2011;29(13):1736–1743.
- Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981–990.
- McGregor M, Hanley JA, Boivin JF, et al. Screening for prostate cancer: estimating the magnitude of overdetection. CMAJ. 1998;159(11):1368–1372.
- Albertsen PC, Moore DF, Shih W, et al. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29(10):1335–1341.
- Fall K, Fang F, Mucci LA. Immediate risk for cardiovascular events and suicide following a prostate cancer diagnosis: a prospective cohort study. PLOS Med. 2009;6(12):37–38.
- Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–1123.
- Andriole GL, Crawford ED, Grubb RL 3rd, et al.; PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. N Engl J Med. 2009;360(13):1310–1319.
- Pelzer AE, Bektic J, Akkad T, et al. Under diagnosis and over diagnosis of prostate cancer in a screening population with serum PSA 2 to 10 ng/ml. J Urol. 2007;178(1):93–97.
- Lilja H, Cronin AM, Dahlin A, et al. Prediction of significant prostate cancer diagnosed 20 to 30 years later with a single measure of prostate-specific antigen at or before age 50. Cancer. 2011;117(6):1210–1219.
- Vickers AJ, Cronin AM, Roobol MJ, et al. A four-kallikrein panel predicts prostate cancer in men with recent screening: data from the European Randomized Study of Screening for Prostate Cancer, Rotterdam. Clin Cancer Res. 2010;16(12):3232–3239.
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst. 2006;98(8):529–534.
- Andriole GL, Bostwick D, Brawley OW, et al. The effect of dutasteride on the usefulness of prostate specific antigen for the diagnosis of high grade and clinically relevant prostate cancer in men with a previous negative biopsy: results from the REDUCE study. J Urol. 2011;185(1):126–131.
- Marberger M, Freedland SJ, Andriole GL, et al. Usefulness of prostate-specific antigen (PSA) rise as a marker of prostate cancer in men treated with dutasteride: lessons from the REDUCE study. BJU Int. 2011 Jun 23. Epub ahead of print.
- Thompson IM, Goodman PJ, Tangen CM, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003;349(3):215–224.
- Andriole GL, Bostwick DG, Brawley OW, et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med. 2010;362(13):1192–1202.
- Theoret MR, Ning YM, Zhang JJ, et al. The risks and benefits of 5α-reductase inhibitors for prostate-cancer prevention. New Engl J Med. 2011;365(2):97–99.
- Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126–131.
- Onik G, Miessau M, Bostwick DG. Three-dimensional prostate mapping biopsy has a potentially significant impact on prostate cancer management. J Clin Oncol. 2009;27(26):4321–4326.
- Megwalu II, Ferguson GG, Wei JT, et al. Evaluation of a novel precision template-guided biopsy system for detecting prostate cancer. BJU Int. 2008;102(5):546–550.
- Cuzick J, Swanson GP, Fisher G, et al.; Transatlantic Prostate Group. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):244–255.
- Ahmed HU. The index lesion and the origin of prostate cancer. N Engl J Med. 2009;361(17):1704–1706.
- Fleshner N, Lucia MS, Melich K, et al. Effect of Dutasteride on Prostate Cancer Progression and Cancer Diagnosis on Rebiopsy in the REDEEM Active Surveillance Study. Presented at the American Society of Clnical Oncology, Genitourinary Cancers Symposium, Orlando, Florida, February 17–19, 2011.
Evidence-based Practice Center Presentation I: Systematic Review Methods and the Natural History of Prostate Cancer Diagnosed in the Last 30 Years
Issa Dahabreh, M.D., M.S.; Stanley Ip, M.D.;
Mei Chung, Ph.D., M.P.H.; Winifred W. Yu, Ph.D., M.S.;
Ethan M. Balk, M.D., M.P.H.; Joseph Lau, M.D.
Introduction
Radical prostatectomy and radiation therapy for prostate cancer have adverse effects, and their relative survival benefit over no treatment is unclear for men with localized and low-risk disease. Prostate cancer often has an indolent natural history, making observational management strategies, including active surveillance, potentially appealing. Throughout this report, we use the term “active surveillance” to describe management strategies of deferred treatment with monitoring of triggers to begin active treatment with curative intent.
Objectives
The objectives are to summarize the systematic review methods we used to address all sections of our report and to summarize changes in the natural history of prostate cancer in the United States over the last 30 years (Key Question 1).
Review Methods
The key questions were developed prior to the systematic review by a conference planning committee, which included experts in the field from the Federal Government, academia, and the clinical practice community. Guidance to the Tufts Evidence-based Practice Center was provided by an external Technical Expert Panel, separate from the conference planning committee. We searched MEDLINE® and the Cochrane Database of Systematic Reviews for relevant English-language publications, from inception through August 2011, using search terms related to prostate cancer, active surveillance, watchful waiting, expectant management, and other related strategies. We also searched for studies based on specific databases sourced from the U.S. population, such as the Surveillance, Epidemiology and End Results (SEER) and Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) databases. Additional studies were identified from reference lists of eligible articles and technical experts. We selected studies for inclusion by first reviewing titles and abstracts of all citations identified through our searches and then by reading the full text of potentially relevant citations. We also relied on two completed evidence reports on treatments for localized prostate cancer conducted for the Agency for Healthcare Research and Quality (AHRQ).1,2
For the question on the natural history of prostate cancer (Key Question 1), we included studies using large databases sourced from the U.S. population and reporting information on trends over time during the period between 1980 and 2011. For the question on definitions of observational management strategies (Key Question 2), we included primary studies of any design or study protocols that reported on observational management strategies (i.e., no immediate curative treatment) for patients with prostate cancer. For the question on the implementation (offer, acceptance, and adherence) of active surveillance or other observational management strategies (Key Question 3), three types of studies were considered eligible: (1) studies that used quantitative methods to identify predictors of the offer of, acceptance of, or adherence to observational management strategies; (2) studies that used qualitative research methods (e.g., focus groups or surveys) to obtain information on factors that affect the implementation of observational management strategies; and (3) experimental studies evaluating the effect of tools such as decision aids on the implementation of observational management strategies. For the question of the effectiveness of active surveillance compared with radical prostatectomy or radiation therapy (Key Question 4), we included all longitudinal comparative studies, whether randomized or nonrandomized, prospective or retrospective, that were performed in a multicenter setting, together with selected existing systematic reviews. To be included, nonrandomized studies of treatment efficacy had to use multivariable methods to adjust for possible confounding (including specifically adjustments for age and tumor stage). The population of interest was men with clinically localized prostate cancer (T1 or T2), without known lymph node (N0-X) or metastatic (M0-X) cancer spread. Outcomes of interest included prostate cancer mortality, all-cause mortality, morbidity of primary treatment, metastatic disease, quality of life, satisfaction with treatment, and costs.
From all eligible studies, we extracted data on study design, population demographics, the interventions or predictive factors assessed, and the outcomes of interest. For studies of treatment effectiveness, we assessed methodological study quality and rated the overall strength of evidence regarding active surveillance versus active treatment using established AHRQ Evidence-based Practice Center methods.3 We did not assess the quality of studies considered eligible for other key questions due to their largely descriptive (noninferential) nature.
Results: 30-Year Trends in the Natural History of Prostate Cancer in the United States
We identified 64 primary observational studies and one systematic review reporting data relevant to temporal trends in prostate cancer incidence; mortality/survival; or patient-, tumor-, and system-level characteristics at diagnosis. The majority of observational studies analyzed the SEER database of the National Cancer Institute or a subset of its component registries; other commonly used data sources were the linked SEER-Medicare database, the CaPSURE database, and the National Cancer Database. Given the extensive overlap in included populations across studies, the number of publications included in this review is not directly indicative of the amount of available evidence.
Prostate cancer incidence rose between 1975 and 1992 and then fell until around 1995. After a period of nonsignificant increase from 1995 to 2000, rates declined again from 2000 to 2007. These trends were observed both overall and among men age 65 or older (i.e., the age group in which the majority of prostate cancers are diagnosed). Prostate cancer incidence also increased in all racial/ethnic groups since the mid-1980s and peaked in the early 1990s. Studies consistently demonstrated that localized and regional prostate cancer cases were mainly responsible for the observed increase in prostate cancer incidence from the mid-1980s up to the mid-1990s. Studies also consistently demonstrated a decrease in incidence rates for all disease stages from the mid-1990s to 2000.
Studies generally reported a trend toward younger age at diagnosis. Studies also reported a decrease in the number of patients with low-grade and high-grade tumors, and a concomitant increase in patients with intermediate-grade tumors at diagnosis. Prostate-specific antigen (PSA) values at diagnosis have decreased over time such that an increasing number of patients diagnosed have PSA concentrations below 10 ng/ml. The proportion of prostate cancer patients diagnosed through biopsy (compared with those diagnosed through other procedures, such as transurethral resection of the prostate) also has increased over time.
For the overall U.S. population, prostate cancer mortality rates increased from 1975 to 1991 and decreased from 1994 to 2007. Deaths due to prostate cancer as a proportion of all deaths among patients diagnosed with the disease have decreased over time (i.e., prostate cancer patients are increasingly likely to die of non-prostate cancer causes), particularly for patients with early-stage disease at diagnosis or patients who were diagnosed at an older age. Blacks have been at higher risk for prostate cancer death compared with non-Hispanic whites, although the difference between the two groups appears to have decreased over time.
Most studies demonstrated decreasing trends in the proportion of patients being managed with observational management strategies of no active treatment (active surveillance, watchful waiting, or expectant management, with or without androgen deprivation therapy). The proportion of patients receiving active surveillance or watchful waiting has remained low, even among patients with low-risk disease.
Conclusions
Over the past 30 years in the United States, patients with prostate cancer have been increasingly diagnosed with early-stage, low-risk disease. Only a small proportion of men with prostate cancer are managed with observational strategies. Studies indicate that patients diagnosed with prostate cancer in recent years are more likely to die of non-prostate-cancer-related causes.
References
- Wilt TJ, Shamliyan T, Taylor B, et al. Comparative effectiveness of therapies for clinically localized prostate cancer. AHRQ Comparative Effectiveness Reviews. Report No. 08-EHC010-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- Ip S, Dvorak T, Yu WW, et al. Comparative Evaluation of Radiation Treatments for Clinically Localized Prostate Cancer: An Update. Rockville, MD: Agency for Healthcare Research and Quality, Technology Assessment Program; August 13, 2010. Available at: cms.gov/mcd/viewtechassess.asp?where=index&tid=69. Accessed August 31, 2011.
- Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions—Agency for Healthcare Research and Quality and the effective health-care program. J Clin Epidemiol. 2010;63(5):513–523.
Temporal Changes in the Pathologic Assessment of Prostate Cancer
M. Scott Lucia, M.D.
In 30 years, there have been dramatic changes in the manner in which we diagnose and manage prostate cancer. The advent of the prostate-specific antigen (PSA) screening in the late 1980s, coupled with increased public awareness of the disease, has resulted in a marked shift in stage toward clinically localized disease.1–3 Pathologically, pre-PSA-era tumors tended to be large, occupying the majority of the glandular volume, and often had extensive extraprostatic extension. In contrast, tumors of today are typically smaller in volume, organ confined, and associated with improved therapeutic outcomes.2,3 Concern arises that we are now detecting more clinically insignificant cancers, cancers that might be better managed expectantly.
Prostate cancer is a multifocal disease: large tumors often result from the assimilation of multiple smaller tumors as they grow to confluence.4 Individual tumors may display marked differences in grade, molecular markers, and DNA ploidy from region to region even within a single tumor.5 The most important pathologic prognostic factor for prostate cancer on biopsy is the Gleason grade. The Gleason system is a five-tiered classification that categorizes tumors by their architectural pattern of growth rather than cytologic features. Since its first description by Donald Gleason in 1966,6 the grading system has undergone a number of modifications over time by Gleason and others.7,8 Tumors often display more than one pattern of growth. This was originally addressed by the Gleason system by adding the two most prominent patterns, a primary pattern (majority of tumor) and a secondary pattern (second most extensive pattern), together to obtain a Gleason “score.” If only one pattern was present, then the grade was doubled with the resulting sums between 2 (grade 1 + grade 1) and 10 (grade 5 + grade 5).
Years of experience with Gleason grading in relation to disease outcomes, along with changes in the diagnostic tools available, have produced shifts in grading practices among academic genitourinary pathologists. In 2005, 80 genitourinary pathologists of the International Society of Urological Pathology participated in a practice survey and consensus conference to document and address trends in and refine the guidelines for Gleason grading.8 Most notable of the changes to the classic Gleason grading system included (1) restrictions on the assignment of very low grades (patterns 1 and 2) to biopsy specimens,(2) refinement of the separation of pattern 3 from pattern 4, (3) guidelines for assigning grade to cribriform patterns, (4) Gleason grading of variant carcinomas, and (5) Gleason scoring of biopsies when minor amounts of high-grade tumor or tertiary-grade patterns are present.
In the classic Gleason system, a grade pattern had to represent at least 5% of the tumor to be included in the Gleason score as a secondary pattern. In the 2005 modified system, higher grade patterns, regardless of quantity, were included in the score (98% pattern 3 and 2% pattern 4 is scored as 3+4 = 7). If more than two grades existed for a tumor on biopsy, then the most extensive pattern (the primary pattern) and the highest pattern of those remaining regardless of relative amount were included in the score (e.g., a tumor with 70% pattern 3, 25% pattern 4, and 5% pattern 5 is scored as 3+5 = 8). Theoretically, this could result in a trend toward higher Gleason scores on biopsy when the modified Gleason grading system is used. Conversely, these modifications could ultimately result in fewer prostate cancers being upgraded upon prostatectomy, a situation that occurs frequently when comparing biopsy grade with final tumor grade on prostatectomy.9 The refinements documented in the 2005 International Society of Urological Pathology Consensus Conference represent an attempt to standardize grading trends already practiced by leading genitourinary pathologists around the world rather than a new approach to Gleason grading.
Ultimately, the goal of pathologic examination of prostate biopsies is to (1) establish a diagnosis of cancer, and (2) help determine the aggressiveness (grade) and extent of the tumor to guide management decisions. Since most prostate tumors of today are not clinically palpable, prostate biopsies are taken systematically but randomly from the right and left sides of the prostate from base to apex. What began as sextant biopsies in the 1980s has been extended to 10, 12, or more biopsies concentrating on lateral portions of the gland in attempts to improve tumor detection. Nevertheless, even extended biopsy schemes sample a limited portion of the prostate and frequently miss tumors.10 Consequently, many men undergo repeat biopsies that may or may not be necessary. Moreover, the Gleason score as determined on biopsy may not be the same as, and is often lower than, that determined on subsequent prostatectomy when the entire gland is examined.9 Furthermore, although the number of cores positive for cancer in a given set of biopsies correlates with tumor volume, the finding of small amounts of tumor on a single biopsy does not always indicate a clinically inconsequential tumor.11
The most commonly used definition of “insignificant” tumor is a tumor confined to the prostate with a volume of <0.5 cm3 and a Gleason score of 6 or less (no pattern 4 or 5) at prostatectomy.12 Epstein et al. define a tumor at biopsy as being “potentially insignificant” if the following criteria are met: (1) stage T1c, (2) PSA density <0.15 ng/ml/gm, (3) Gleason score ≤6 (no pattern 4 or 5), and (4) tumor involving less than three cores with no core having more than 50% tumor involvement.13 However, attempts to predict clinical significance using biopsy criteria are imperfect with sensitivities ranging from 35% to 83% and specificities ranging from 68% to 98%.14
In an era when more conservative management options exist for prostate cancer, including targeted focal therapy and expectant management, it becomes more crucial to be able to determine the aggressiveness and extent of tumors accurately. The pathologist’s ability to do this is hampered by limitations in the amount of information obtainable from routine prostate biopsies. The tissue obtained on biopsy is a static view of a tumor at a particular point in the course of a dynamic process in which the tumor continues to evolve over time. Predicting the behavior of a tumor from a single biopsy is much like trying to define the slope of a curve from a single data point. It is therefore critical that improved diagnostic techniques be developed to optimize management decisions.
References
- Stephenson RA. Population-based prostate cancer trends in the PSA era: data from the Surveillance, Epidemiology and End Results (SEER) Program. Monogr Urol. 1998;19:3–19.
- Falzarano SM, Magi-Galluzzi C. Prostate cancer staging and grading at radical prostatectomy over time. Adv Anat Pathol. 2011;18(2):159–164.
- Moul JW, Wu H, Sun L, et al. Epidemiology of radical prostatectomy for localized prostate cancer in the era of prostate-specific antigen: an overview of the Department of Defense Center for Prostate Disease Research national database. Surgery. 2002;132(2):213–219.
- Miller GJ, Cygan JM. Morphology of prostate cancer: the effects of multifocality on histological grade, tumor volume and capsule penetration. J Urol. 1994;152(5 Pt 2):1709–1713.
- Karavitakis M, Ahmed HU, Abel PD, et al. Tumor focality in prostate cancer: implications for focal therapy. Nat Rev Clin Oncol. 2011;8(1):48–55.
- Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50(3):125–128.
- Gleason DF. Histological grading and clinical staging of prostatic carcinoma. In: Tannenbaum M, ed. Urologic Pathology: The Prostate. Philadelphia, PA: Lea & Feibiger; 1977:171–198.
- Epstein JI, Allsbrook WC Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29(9):1228–1242.
- Sved PD, Gomez P, Manoharan M, et al. Limitations of biopsy Gleason grade: implications for counseling patients with biopsy Gleason score 6 prostate cancer. J Urol. 2004;172(1):98–102.
- Raja J, Ramachandran N, Munneke G, et al. Current status of transrectal ultrasound-guided prostate biopsy in the diagnosis of prostate cancer. Clin Radiol. 2006;61(2):142–153.
- Quann P, Jarrard DF, Huang W. Current prostate biopsy protocols cannot reliably identify patients for focal therapy: correlation of low-risk prostate cancer on biopsy with radical prostatectomy findings. Int J Clin Exp Pathol. 2010;3(4):401–407.
- Stamey TA, Freiha FS, McNeal JE, et al. Localized prostate cancer: relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer. 1993;71(3 Suppl):933–938.
- Epstein JI, Sanderson H, Carter HB, et al. Utility of saturation biopsy to predict insignificant cancer at radical prostatectomy. Urology. 2005;66(2):356–360.
- Noguchi M, Stamey TA, McNeal JE, et al. Relationship between systematic biopsies and histological features of 222 radical prostatectomy specimens: lack of prediction of tumor significance for men with nonpalpable prostate cancer. J Urol. 2001;166(1):104–109; discussion 109–110.
Temporal Changes in the Clinical Approach to Diagnosing Prostate Cancer: How the Cancer of Today Differs From the Cancer of Yesterday
Ian M. Thompson, Jr., M.D.
Prior to the mid-1980s, prostate cancer was largely a disease that presented with symptoms. A small series of screenings using digital rectal examination were performed, but these demonstrated that as many as two-thirds of cases diagnosed were extraprostatic at the time of diagnosis and thus potentially incurable.1 With the advent of prostate-specific antigen (PSA), a dramatic series of initial changes in diagnosis occurred.1 An increasing fraction of men participated in early detection activities, reaching about 50% of men at the present time.2 A significantly greater fraction of men underwent prostate biopsy as approximately 8% of the general population has a PSA >4.0 ng/ml, the initial upper limit of normal for prostate biopsy.
With the rapid adoption of PSA screening in the United States, an enormous increase in the incidence of prostate cancer occurred, from about an 8% lifetime risk to an estimated 16% to 17% lifetime risk at this time. Although a steady-state detection rate would have initially been predicted, including a “harvest” of early cases leading to both a stage shift and an “age shift” toward a lower and lower age at diagnosis, several other events occurred that changed patterns of care.
The first of these events was in the mid-1990s as it became evident that a four-core prostate biopsy missed many prostate cancers. With the initial report on the “sextant” biopsy, further increases in detection of cancers occurred.2 Thereafter, a 10-core and then a 12-core biopsy were recommended by various subject matter experts. It is not uncommon in some settings to now see as many as 20 cores and, with “saturation” biopsies, as many as 30 to 40 cores. Obviously, with a high background rate of small, low-grade prostate cancers in the general population, what would be expected is an increase in the detection of these small tumors and thus a further increase in disease incidence.
The next event that affected rates of detection was in 2004 when the results of the Prostate Cancer Prevention Trial were presented.3 In this study, all men, regardless of PSA, underwent prostate biopsy. An overall rate of prostate cancer in the population of men with a PSA <4.0 ng/ml was found to be 15% with as high as 30% rates in men with a PSA of 3.5–4.0 ng/ml. Additional investigation found that older men and African American men as well as men with a family history of prostate cancer had a further increase in risk, thus increasing the number of men undergoing biopsy with PSA values <4.0 ng/ml.
As a result of these changes in clinical activities, an increase in the number of small, low-grade tumors has been witnessed. With about 90% of these patients undergoing treatment, an increasing number of whom have radical prostatectomy, fewer older men “at risk” of prostate cancer are present in the population, leading to significant changes in age at diagnosis of the disease.
Changes in patterns of detection are to be expected in the years to come. With the increased understanding that the detection of a low-volume, low-grade tumor in many men may actually not be a benefit but a harm (risk of complications of biopsy, treatment, anxiety without a measurable benefit vis-a-vis cancer risk), increased focus is being placed on methods to reduce biopsies in men who have a low risk of aggressive prostate cancer. New biomarkers such as PCA3 or TMPRSS2:ERG fusion protein show considerable promise to help select those men who may benefit most from prostate biopsy.4,5
References
- Thompson IM, Ernst JJ, Gangai MP, et al. Adenocarcinoma of the prostate: results of routine urological screening. J Urol. 1984;132(4):690–692.
- Hodge KK, McNeal JE, Terris MK, et al. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142(1):71–74.
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level ≤4.0 ng per milliliter. N Engl J Med. 2004;350(22):2239–2246.
- Sokoll LJ, Sanda MG, Feng Z, et al. A prospective, multicenter, National Cancer Institute Early Detection Research Network study of [-2]proPSA: improving prostate cancer detection and correlating with cancer aggressiveness. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1193–1200.
- Schilling D, deReijke T, Tombal B, et al. The prostate cancer gene 3 assay: indications for use in clinical practice. BJU Int. 2010;105(4):452–455.
What Is the Risk Posed by Prostate Cancer?
Peter Albertsen, M.D.
For the past century and a half, prostate cancer has challenged clinicians and researchers. Prior to Thompson’s 1852 monograph, The Enlarged Prostate, prostate cancer was an unknown disease.1 Forty years later, Von Recklinghausen reported that prostate cancer could present as a small local lesion and that metastases had a preference for bone.2 Improvements in microscopy resulted in growing numbers of case reports of prostate cancer, so that by 1900 most clinicians recognized the presenting symptoms of this disease. Urologists of the early 20th century were often called to palliate symptoms of prostate cancer. Radiation seed implants were used to alleviate bladder outlet obstruction. Benjamin Barringer, a prominent urologist in New York City, recognized that most patients with symptoms had advanced disease that was uniformly lethal.3 Only 36 of his first 352 patients lived more than 5 years.
The dismal prognosis associated with prostate cancer changed following the report by Huggins and Hodges in 1941 that prostate cancer was an endocrine-dependent tumor.4 By the 1950s, orchiectomy and/or diethylstilbestrol had become the treatment of choice for men with clinically symptomatic disease. Although the average patient responded for only 3 years, many responded for much longer. The prognosis for this disease improved dramatically.
The Veterans Administration Cooperative Urologic Research Group was organized in the early 1960s to determine the appropriate treatment of prostate cancer. Researchers had no difficulty recruiting to trials involving men with metastatic disease, but they had difficulty identifying men with localized disease. Probably the most significant accomplishment of these trials was the Gleason scoring system used to evaluate tumor histology. Gleason’s scoring system provided significant prognostic information, and the diagram developed by Gleason (Figure 1) helped standardize the evaluation of prostate cancer. This system remains the most powerful predictor of clinical prognosis for this disease.
The 1987 manuscript by Stamey et al. concerning prostate-specific antigen (PSA) began the modern era of prostate cancer diagnosis and management.5 This publication along with Catalona’s 1991 report advocating PSA testing to screen for prostate cancer dramatically altered the incidence of this disease.6 Since 1987, the number of incident cases has doubled, although the death rate from this disease has declined about 20%.7 The PSA era has produced a dramatic change in how men present with this disease. Before PSA testing, most men complained of either back pain or difficulty voiding. These men often had metastatic disease and were treated with some type of androgen deprivation. Now more than 80% of men present with localized disease as a result of a biopsy recommended because of an elevated PSA.
Researchers have attempted to describe the natural history of prostate cancer. Albertsen et al. published a series of graphs (Figure 2) depicting the competing risks of prostate cancer and other causes stratified by patient age at presentation and Gleason score.8 Men with low-grade tumors rarely died from their disease compared with men with high-grade tumors who often died within 5 to 10 years of diagnosis.
Figure 1. The Gleason Scoring System
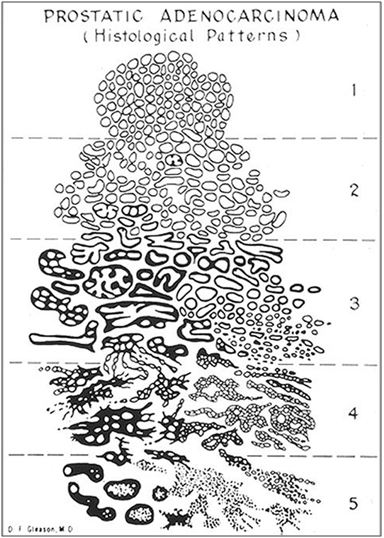
Reprinted from Gleason D. Classification of prostate carcinomas. Cancer Chemother Rep. 1966;50:125–128.
These studies, however, do reflect contemporary outcomes. Annual PSA testing has advanced the date of diagnosis for the majority of patients. Draisma et al. estimate that the lead time for men age 55 is approximately 12 years and for those age 75 approximately 6 years.9 Equally important is the recognition that PSA testing leads to the discovery of indolent disease never destined to become clinically significant. Draisma et al. estimate that 27% of cancers diagnosed at age 55 and 56% of cancers diagnosed at age 75 are clinically unimportant.9 These estimates support the findings of Sakr et al., who showed from autopsy studies that the incidence of small-volume, low-grade cancers increased by 10% per decade so that a man age 50 has about a 50% chance of harboring a small, indolent prostate cancer.10 The finasteride chemoprevention trial also demonstrated that small-volume, low-grade prostate cancers are very prevalent.11 More than 20% of men originally noted to have PSA values within the normal range at the time of enrollment were eventually found to harbor prostate cancer. Three-quarters of the cancers were low-volume, low-grade tumors.
Figure 2. 20-Year Survival Estimates of Men Diagnosed in the Pre-Prostate-Specific Antigen Era
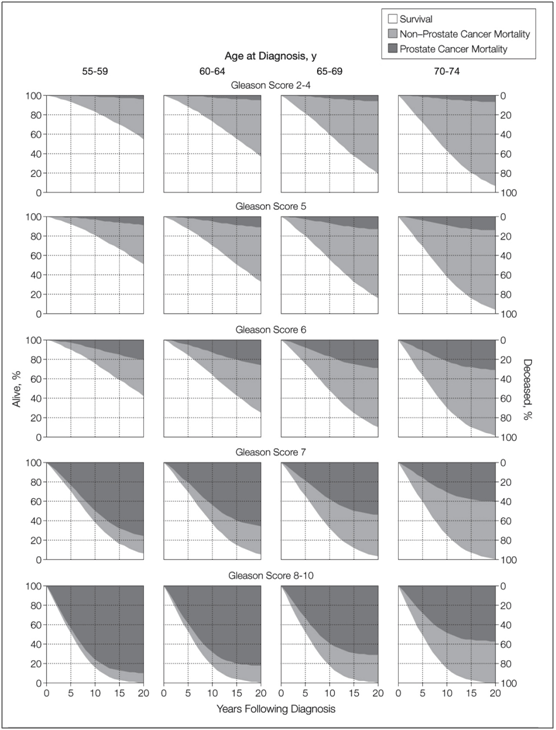
From Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095–2101. Reprinted with permission. Copyright © 2005 American Medical Association. All rights reserved.
Another factor influencing our understanding of the natural history of prostate cancer is the changing interpretation of the Gleason scoring system. Contemporary pathologists no longer utilize Gleason patterns 1 and 2, and many features that were originally part of pattern 3 are now considered pattern 4.12 These changes in the classification system have resulted in a significant upgrading of disease during the past two decades (Figure 3).
Figure 3. Change in the Interpretation of Gleason Score Patterns: Results Recorded in 1990 Versus Results Recorded in 2007
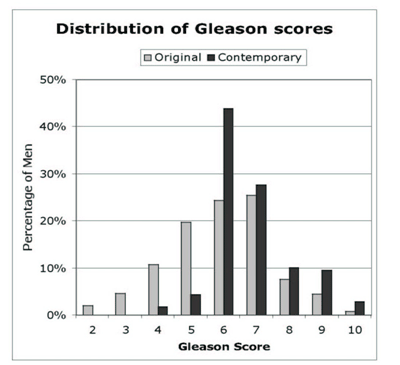
From Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248–1253. Reprinted with permission. Copyright © 2005 Oxford University Press. All rights reserved.
To adjust for the lead time introduced by PSA testing and the changes in the interpretation of the Gleason scoring system, Lu-Yao et al. analyzed the clinical outcomes of men diagnosed with localized prostate cancer who received no treatment for their disease at the time of diagnosis.13 These results are presented in Figure 4. She and her colleagues also explored the impact of patient comorbidity in another competing risk analysis.14 These 10-year survival curves reflect the current best estimates of the risk posed by prostate cancer diagnosed in contemporary practice in the United States.
Figure 4. Contemporary 10-Year Survival Estimates for Men With Gleason 5–7 Disease
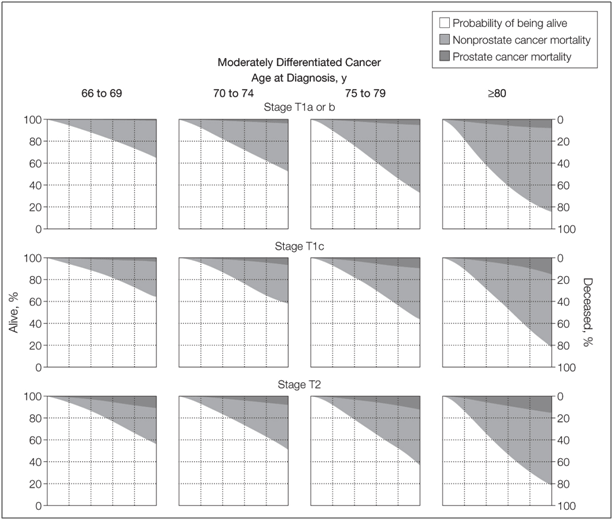
From Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA 2009;302:1202–1209. Reprinted with permission. Copyright © 2009 American Medical Association. All rights reserved.
The natural history of prostate cancer is extraordinarily variable. Before prostate cancer screening with PSA, most men presented with clinical evidence of prostate cancer and often succumbed to their disease several years later following treatment with hormonal manipulation. Since the advent of screening with PSA, most men are diagnosed with localized disease. The most powerful predictor of long-term outcome is the Gleason scoring system. Over the past two decades, however, pathologists have modified their use of this system such that men previously classified with Gleason 2–5 tumors are now classified as Gleason 6 disease and men with Gleason 6 disease are often now classified as Gleason 7 disease. Men with high-grade cancers (Gleason 8–10) often progress to metastatic disease and death despite treatment. Men with low-grade cancers have an excellent prognosis even in the absence of treatment. Unfortunately, we are still unable to predict accurately the risk posed by a specific prostate cancer.
References
- Thompson H. The Enlarged Prostrate. London: John Churchill; 1852.
- Von Recklinghausen F. Ueber die multiplen Fibrome der Haut und ihre Beziehung zu den multiplen Neuromen. Berlin: A. Hirschwald; 1882.
- Barringer BS. Treatment of prostatic carcinoma. Bull N Y Acad Med. 1943;19(6):417–422.
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;(1):293.
- Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med. 1987;317(15):909–916.
- Catalona WJ, Smith DS, Ratliff TL, et al. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324(17):1156–1161.
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300.
- Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095–2101.
- Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95(12):868–878.
- Sakr WA, Haas GP, Cassin BF, et al. The frequency of carcinoma and intraepithelial neoplasia of the prostate in young male patients. J Urol. 1993;150(2 Pt 1):379–385.
- Thompson IM, Ankerst DP, Chi C, et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA. 2005;294(1):66–70.
- Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97(17):1248–1253.
- Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302(11):1202–1209.
- Albertsen PC, Moore DF, Shih W, et al. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29(10):1335–1341.
Tumor and Patient Metrics, Eligibility, and Inclusion for Active Surveillance for Prostate Cancer
H. Ballentine Carter, M.D.
The management of favorable-risk prostate cancer is controversial and, in the absence of controlled trials to inform best practice, choices are driven by personal beliefs with resultant wide variation in practice patterns. Men with favorable-risk prostate cancer diagnosed today often undergo treatments that will not improve overall health outcomes. A shared decision approach for selecting optimal management of favorable-risk disease should account for (1) individual tumor metrics, (2) patient age and overall health, and (3) patient preferences that consider living with a cancer and the potential side effects of curative treatments.
Tumor Metrics
Gleason score (cancer grade) based on prostate biopsy, cancer stage, and prostate-specific antigen (PSA) have been used as tumor metrics to risk stratify men with newly diagnosed prostate cancer before recommending management. Based on these criteria, the National Comprehensive Cancer Network recognizes favorable-risk prostate cancer as a low-risk or very low-risk disease1 (Figure 1). Most published prostate cancer surveillance programs have preferentially included men with favorable-risk tumor metrics using the criteria below (Figure 1)—or similar criteria2—thought to identify men with a low risk of cancer progression in the absence of treatment.
Figure 1. Criteria for Identifying Favorable-Risk Prostate Cancer (Low Risk and Very Low Risk)
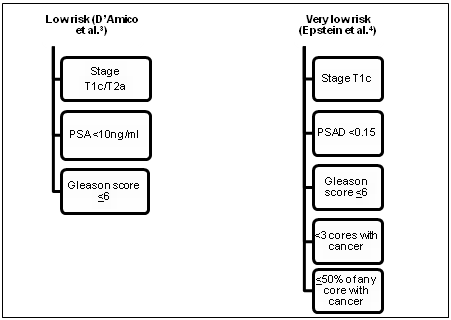
PSA = prostate-specific antigen; PSAD = PSA density (PSA/prostate volume).
Note: Core refers to prostate biopsy tissue.
The most recent update of the Johns Hopkins Active Surveillance Program begun in 1995 includes 769 men (median age 66), of whom 80% met the criteria for very low-risk disease5 (Figure 1). The median survival time free of intervention was 6.5 years after diagnosis. Overall, 33% underwent curative intervention at a median of 2.2 years after diagnosis triggered by biopsy reclassification in 74% of men. The estimated 15-year prostate-cancer-specific mortality adjusted for competing risks ranged from 1.1% to 1.8% and 1.7% to 2.7% for men enrolled in the program 5 and 10 years, respectively.
Patient Age and Overall Health
The recently published 15-year follow-up data from the Scandinavian Prostate Cancer Group Study 4 comparing surgery to watchful waiting for men without screen-detected prostate cancers demonstrated no cancer-specific or metastatic-free survival advantage for surgery among men over age 65.6 Thus, these data suggest that for favorable-risk prostate cancer diagnosed with screening, most men over age 65 to 70—especially those with comorbidities—should defer treatment based on life expectancy and the long natural history of prostate cancer. Yet, 71% of men age 75 years and older with favorable-risk prostate cancer undergo some form of active treatment.7
Patient Preferences
In decision analyses comparing active surveillance with curative intervention for management of prostate cancer using Monte Carlo simulations, individual patient preferences are critical in determining the optimal approach for favorable-risk prostate cancer.8,9 Two important considerations are the ability to live with untreated disease without significant anxiety and utilities for side effects of treatment such as urinary, bowel, and sexual problems. Thus, in addition to tumor metrics that help identify men for surveillance, a shared decision approach that explores a man’s preferences for living with cancer and the potential side effects of treatment should be included when a choice of surveillance or curative intervention is being considered.
References
- Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8(2):162–200.
- Eggener SE, Mueller A, Berglund RK, et al. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J Urol. 2009;181(4):1635–1641.
- D’Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280(11):969–974.
- Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271(5):368–374.
- Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29(16):2185–2190.
- Bill-Axelson A, Holmberg L, Ruutu M, et al.; SPCG-4 Investigators. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–1717.
- Hamilton AS, Albertsen PC, Johnson TK, et al. Trends in the treatment of localized prostate cancer using supplemented cancer registry data. BJU Int. 2011;107(4):576–584.
- Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304(21):2373–2380.
- Liu D, Lehmann HP, Frick KD, et al. Which men with low-risk prostate cancer should be treated? Med Decis Making. 2011;31:E95–E118.
Active Surveillance: Inclusive Approach
Laurence Klotz, M.D.
Introduction
The world of prostate cancer has changed dramatically in the last 20 years. Prostate-specific antigen (PSA) first became available in the mid-1980s and was not introduced in Canada until about 1988. In both the United States and Canada, PSA was immediately embraced as a biomarker for the early identification of patients at risk for prostate cancer. The result was a sharp increase in the incidence of prostate cancer. This initial spike in incidence was due to the diagnosis of the many prevalent slow-growing, previously undiagnosed cases in the population. Many of these patients had substantial volume of disease; if serial PSA screening had been available, they would have been diagnosed years before.
With the passage of time, the prevalent cases were diagnosed and treated, and the median volume of prostate cancer in newly diagnosed patients began to fall. This process took about 5 years. By the mid-1990s, the “incident” cases began to predominate. A dramatic increase occurred in the number of patients with minimal low-grade disease on biopsy. Other than stage T1a prostate cancer seen following a transurethral resection of the prostate, such patients had previously been uncommon.
Discussion
My colleagues and I who worked together in a multidisciplinary genitourinary oncology group at Sunnybrook Health Sciences Centre were very cognizant of the high rate of prostate cancer found at autopsy and also were influenced by the excellent results of conservative management of T1a disease. Surprisingly, a widespread and relatively unremarked-on consensus existed that T1a prostate cancer (<5% of chips showing Gleason 6 or less prostate cancer on a transurethral resection of the prostate specimen) should not be treated. Yet this consensus was not applied to the diagnosis of T1c prostate cancer. Patients diagnosed with any prostate cancer at all based on an elevated PSA and transrectal ultrasound-guided prostate biopsy were offered radical therapy. In the United States, based on Cancer of the Prostate Strategic Urologic Research Endeavor data, 90% to 95% of such patients received radical treatment.1
It seemed obvious to us that there was an incongruity between the conservative approach taken successfully and without fanfare for T1a disease and the insistence on radical therapy for T1c disease. Many of the arguments by knowledgeable individuals seemed specious. For example, it was argued that American men diagnosed with cancer demanded treatment, yet this had not been the case for T1a disease. Another rationale was that some of these patients would progress and die of disease, even though their baseline parameters were favorable; this also had been true for T1a disease, which had a 15% progression rate at 10 years.2 It was argued that patients managed with watchful waiting were deprived of an opportunity for cure, but experience suggested curative therapy offered after several years of observation might still be effective. Thus, we reasoned, perhaps an initial conservative approach of expectant management using the natural history of the patient’s own disease, including PSA kinetics and serial biopsy to determine treatment, might go a long way toward reducing the overall morbidity of treatment. PSA kinetics was an appealing tool in managing these patients, based on emerging data at the time that PSA kinetics correlated with tumor aggressivity and volume. The concept of personalized therapy also was beginning to emerge and further drove support for the concept.
Active surveillance has evolved to become a standard of care for favorable-risk prostate cancer. It is advocated as the treatment of choice for favorable-risk disease in several national guidelines (National Comprehensive Cancer Network, National Institute for Health and Clinical Excellence). The published experience with surveillance now encompasses approximately 2,000 patients (Table 1). While the median follow-up of some of these studies is short, collectively at least 400 patients have been followed for more than 10 years. The prostate cancer mortality in these patients remains low.
| Author | Year | Number | Median Age | Median F/U Mo | pT3 in Radical Prosta- tectomy Patients |
OS | CSS | On Surveil- lance (%) |
|---|---|---|---|---|---|---|---|---|
| Van As3 Cancer J. |
2007 | 326 | 67 | 22 | 8/18 44% |
98 | 100 | 73 |
| Carter4 J Urol. |
2007 | 407 | 66 | 41 | 10/4 20% |
98 | 100 | 59 |
| van den Bergh5 Eur Urol. |
2008 | 533 | 70 | 48 | 4/24 17% |
90 | 99 | 50 |
| Soloway6 BJU Int. |
2008 | 99 | 66 | 45 | 0/2 0% |
100 | 100 | 92 |
| Roemeling7 Eur Urol. |
2007 | 278 | 70 | 41 | 89 | 100 | 71 | |
| Khatami8 Int J Cancer |
2007 | 270 | 64 | 63 | Not stated | 100 | 61 | |
| Klotz9 J Clin Oncol. |
2010 | 452 | 70 | 73 | 14/24 58% |
82 | 97 at 10 yrs |
53 |
| Total | 2,130 | 68 | 43 | 90 | 99.7 | 64 | ||
| F/U Mo = follow-up months; OS = overall survival; CSS = cause-specific survival. | ||||||||
Advocates for surveillance generally agree that patients with small-volume Gleason 6 prostate cancer (based on extended biopsies) with PSA <10 are candidates. In these patients, over time, approximately one-third are eventually reclassified as higher risk and treated radically. The likelihood of cancer death is, at 10 years, about 1/20th the risk of other cause mortality in patients managed this way.9 Although the risk of prostate cancer death will increase over time, so will that of other-cause mortality. This large excess of other-cause mortality suggests that more patients may be candidates than just those with a small volume of disease and PSA <10. It is very plausible that some patients with higher volume Gleason 6 disease, PSA >10, or small elements of Gleason 4 pattern also may be at low risk for disease mortality during their lifetime and therefore are candidates for surveillance.
Since November 1995, 453 patients at our center have been managed with active surveillance.9–13 Median follow-up is 8.0 years (range 1–16 years). Overall survival is 78.6% (Figure 1a), and 10-year prostate cancer actuarial survival is 97.2% (Figure 1b). Five of 450 patients (1.1%) have died of prostate cancer, and 30% of patients have been reclassified as higher risk and offered definitive therapy (Figure 2a). The most common indication for treatment was a PSA doubling time <3 years (44%) or Gleason upgrading (26%). Of 117 patients treated radically, the PSA failure rate was 50% (Figure 2b). This represents 13% of the total cohort. Most PSA failures occurred early; at 2 years, 44% of the treated patients had PSA failure. The hazard ratio for nonprostate cancer to prostate cancer mortality was 18.6 at 10 years (Figures 3a and 3b).
In the initial Toronto experience (1995–2000), we included, in patients older than age 70, men with Gleason 3+4 or PSA 10–15. Since 2000, we restricted the group to men with Gleason 6 and PSA <0. In fact, in multivariate analysis of predictive factors for progression, Gleason score was not an independent predictor. This likely reflected the fact that for most of these patients, the Gleason 4 pattern was <10% of the cancer present.
A detailed analysis of the five men who died of prostate cancer after being entered on surveillance revealed that (1) the PSA doubling time was <2 years in all five; (2) all had Gleason 7 or higher disease on repeat biopsy; (3) three of the five were treated within 1 year of diagnosis and had metastatic disease within 1 year of treatment; (4) one had an early trigger for diagnosis (upgrading at 1 year) and refused treatment; and (5) only one had radical treatment after a period of observation of 2 years and went on to a late prostate cancer death, possibly avoidable by earlier treatment.13
The recent large experience of radical prostatectomy patients reported by Eggener et al. supports the view that Gleason pattern 3 (i.e., Gleason score 6) has little or perhaps no metastatic potential.14 In 12,000 patients with pathologic Gleason 6 or less only (i.e., no pattern 4 disease in the radical prostatectomy specimen), the 20-year prostate cancer mortality rate was 0.2%. Indeed, according to the author, even these few patients dying of disease had some Gleason 4 pattern (personal communication). Although there is a major treatment effect, one would expect that a disease with some lethality would result in a few deaths from disease in spite of treatment. One would not expect surgery to be curative in 100% of patients with a lethal disease.
|
Figure 1a. Overall Survival
|
Figure 1b. Cause-Specific Survival
|
|
Figure 2a. Likelihood of Remaining Alive and on Surveillance
|
Figure 2b. PSA Failure in 117 Patients Treated With Surgery or Radiation After a Period of Surveillance
|
|
Figure 3a. Cumulative Hazard Ratio for Nonprostate Cancer to Prostate Cancer Mortality
|
Figure 3b. Cumulative Hazard Ratio for Mortality by Cause and Age, Stratified Around Age 70
|
Another recent analysis by Wolters et al. examined the European Randomized Study of Screening for Prostate Cancer data to derive a contemporary definition of “clinically insignificant” prostate cancer using the same approach taken by Stamey in 1979, who arrived at the figure of <0.5 cc of Gleason 6 or less disease.15 Their conclusion was that a more realistic threshold was <1.3 cc of cancer.15 In fact, their analysis suggests that there may be no minimum threshold for clinically insignificant Gleason 6 disease.
Table 2 summarizes the current approaches to surveillance taken by various groups, from very stringent to very inclusive. Biomarkers and better imaging offer the promise of more accurate identification of both indolent and aggressive disease. There are two fundamental decision points where biomarkers and predictive tools would enhance the current approach to surveillance. First is the accurate identification of candidates for active surveillance. More accurate predictive tools would allow an even more inclusive approach to surveillance, assuming that the minority with aggressive disease could be identified at the outset.
Table 2. Active Surveillance: Who Is a Candidate?
| 1. Very stringent approach: | Age >65, Gleason 6, <3 positive cores, no core >50% involved, PSA <0 (Epstein criteria) |
| 2. Stringent approach: | Any age, Epstein criteria |
| 3. Inclusive approach: | Any age, all Gleason 6, PSA <10 |
| 4. Very inclusive approach: | Inclusive approach plus, in men >70, PSA <15, and/or Gleason 3+4 = 7 |
| PSA = prostate-specific antigen. | |
The second challenge is to improve the early identification of those apparently favorable-risk patients who harbor more extensive or higher grade disease. Approximately 20% of patients are found to have Gleason 4 elements on first rebiopsy, and an additional 10% are identified on subsequent biopsies. In a smaller proportion, very aggressive disease was missed on initial biopsy. Earlier identification of these patients is a major priority. We used a PSA doubling time as a trigger for intervention between 1995 and 2008.13 The initial PSA doubling-time threshold was 2 years; in 2000, this was increased to 3 years, as the 2-year doubling-time threshold appeared overly stringent (encompassing <10% of patients). We used the generalized linear mixed model (GLMM) to adjust for baseline PSA. Other PSA triggers (velocity >2 ng/ml per year or PSA doubling time <3 years by linear regression) resulted in inappropriate triggers for intervention in up to 50% of stable patients.16 Although the GLMM approach seemed reasonably successful, recent analysis of PSA kinetics suggests that it is an unreliable guide in this setting.17 Thus we now use a short PSA doubling time as an indication for further evaluation, either multiparametric magnetic resonance imaging (MRI) or repeat biopsy with biomarker evaluation if necessary.
Multiparametric MRI has recently shown tremendous progress in identifying higher grade and higher volume cancer. Multiparametric MRI encompasses T2 weighted image, dynamic contrast-enhanced imaging, and diffusion-weighted imaging. This approach offers the appealing combination of high sensitivity for high-grade or high-volume disease, and low sensitivity for unwanted low-grade or low-volume disease.18 Recent reports suggest that the negative predictive value for high-grade prostate cancer in sectors of the prostate that show no abnormal signal is as high as 97%. If confirmed, this negative predictive value is sufficient to make this technology a critically important partner in the management of men on surveillance. If the “new” threshold for clinically insignificant disease is indeed 1.3 cc, one can expect MRI to have an extremely high accuracy for detecting lesions of this size.
Several biomarkers have been evaluated in patients on surveillance. The two challenges are to identify those few patients with only Gleason 6 disease whose disease has metastatic potential, and (more importantly), the 25% of those who harbor higher grade disease. PCA3, a urine-based analysis of RNA expression-associated prostate cancer, has shown definite correlation with increased grade and increased cancer volume in surveillance patients. The negative predictive value of this assay for high-grade cancer in the low-risk population has not yet been established. Some studies have linked the TMPRSS2-ERG translocation to a more aggressive disease phenotype, although no consistent associations have been identified between the presence of ETS alteration and clinical outcome, with the possible exception that duplication of the ETS-related gene, reflecting aneuploidy, is associated with poor outcome.19,20 In a recent radical prostatectomy series,21 the TMPRSS2 translocation was associated with lower grade and not with biochemical recurrence, metastases, or death. The Aureon test, a systems pathology approach to biopsy tissue, is able to predict more aggressive natural history. Utilizing prostate needle biopsy specimens from men with T1c-T3 stage prostate cancer, who had been treated by curative-intent radical prostatectomy and followed for 8 years, the risk of systemic metastasis was predicted with 74% accuracy and a hazard ratio of 5.12.22 Single-nucleotide polymorphism analysis, while able to identify a high-risk population for prostate cancer, has not yet been demonstrated to be able to predict more aggressive disease in men with low-risk prostate cancer.
Conclusions
Active surveillance for Gleason 6 prostate cancer for men of all ages, and Gleason 3 plus a small element of Gleason 4 disease in men over age 70, is feasible and appears safe in the 10- to 15-year timeframe. Common sense dictates that the uncertain increased risk of disease progression in those with high-volume Gleason 6 cancer should be weighed against patient age and comorbidity. This strategy provides the benefit of an individualized approach based on the demonstrated risk of clinical or biochemical progression with time. In this cohort, the likelihood of dying of other causes was 18.6 times greater than the likelihood of prostate cancer death. Uncertainty remains regarding the long-term impact of delayed treatment in men reclassified as higher risk after a period of observation and repeat biopsy.
References
- Cooperberg MR, Broering JM, Carroll PR, et al. Time trends and local variation in treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–1123.
- Matzkin H, Patel JP, Altwein JE, et al. Stage T1A carcinoma of prostate. Urology. 1994;43(1):11–21.
- van As NJ, Parker C. Active surveillance with selective radical treatment for localized prostate cancer. Cancer J. 2007;13(5):289–294.
- Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178(6):2359–2364.
- van den Bergh RC, Roemling S, Roobol MJ, et al. Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur Urol. 2008 Sep 8. Epub ahead of print.
- Soloway MS, Soloway CT, Williams S, et al. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int. 2008;101(2):165–169.
- Roemeling S, Roobol MJ, de Vries SH, et al. Active surveillance for prostate cancers detected in three subsequent rounds of a screening trial: characteristics, PSA doubling times, and outcome. Eur Urol. 2007;51(5):1244–1250.
- Khatami A, Aus G, Damber JE, et al. PSA doubling time predicts the outcome after active surveillance in screening-detected prostate cancer: results from the European randomized study of screening for prostate cancer, Sweden section. Int J Cancer. 2007;120(1):170–174.
- Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126–131.
- Choo R, Klotz L, Danjoux C, et al. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167(4):1664–1669.
- Klotz L. Active surveillance for prostate cancer: for whom? J Clin Oncol. 2005;10;23(32):8165–8169.
- Zhang L, Loblaw A, Klotz L. Modeling prostate specific antigen kinetics in patients on active surveillance. J Urol. 2006;176(4 Pt 1):1392–1397; discussion 1397–1398.
- Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010;184(1):131–135.
- Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol. 2011;185(3):869–875.
- Wolters T, Roobol MJ, van Leeuwen PJ, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol. 2011;185(1):121–125.
- Loblaw A, Zhang L, Lam A, et al. Comparing prostate specific antigen triggers for intervention in men with stable prostate cancer on active surveillance. J Urol. 2010;184(5):1942–1946.
- Vickers AJ, Savage C, O’Brien MF, et al. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J Clin Oncol. 2009;27(3):398–403.
- Hambrock T, Somford DM, Huisman HJ, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259(2):453–461.
- Attard G, Clark J, Ambroisine L, et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene. 2008;27(3):253–263.
- Clark JP, Cooper CS. ETS gene fusions in prostate cancer. Nat Rev Urol. 2009;6(8):429–439.
- Gopalan A, Leversha MA, Satagopan JM, et al. TMPRSS2-ERG gene fusion is not associated with outcome in patients treated by prostatectomy. Cancer Res. 2009;69(4);1400–1406.
- Donovan MJ, Khan FM, Fernandez G, et al. Personalized prediction of tumor response and cancer progression on prostate needle biopsy. J Urol. 2009;182(1):125–132.
Evidence-based Practice Center Presentation II: Definitions of Observational Strategies and the Factors That Affect the Use of Active Surveillance
Stanley Ip, M.D.; Mei Chung, Ph.D., M.P.H.; Issa Dahabreh, M.D., M.S.;
Winifred W. Yu, Ph.D., M.S.; Ethan M. Balk, M.D., M.P.H.;
Joseph Lau, M.D.
Introduction
Active surveillance and watchful waiting are two observational follow-up strategies that forego immediate therapy in patients with prostate cancer. Two key questions asked by the conference planning committee were (1) how are these strategies defined, and (2) what factors influence the offer of active surveillance by physicians, the acceptance of active surveillance, and the adherence to active surveillance by patients?
Objectives
The objectives were to review the various definitions and protocols of active surveillance and other observational management strategies in research studies (Key Question 2) and to examine the factors that affect their use in clinical practice (Key Question 3).
Review Methods
We searched MEDLINE® and the Cochrane Database of Systematic Reviews for relevant English-language publications, from inception through August 2011. We used search terms related to prostate cancer, active surveillance, watchful waiting, expectant management, and other related strategies. For the review of definitions of observational strategies, we considered studies that reported data on observational management strategies (i.e., no immediate active treatment), enrolled patients based on predefined eligibility criteria, and used prespecified protocols for follow-up.
It was clear at the start of the review that the two terms (active surveillance and watchful waiting), in addition to others, have been used by investigators to denote various and often inconsistent general strategies. Therefore, for the purpose of this review, we divided protocols into those where the intent of subsequent treatment had been clearly described as curative, and those where the intent of subsequent treatment was either unclear or primarily palliative, regardless of the terminology originally used by the study investigators. We extracted data on parameters monitored as triggers for recommending treatment and the definitions of prostate cancer progression.
For the question on the use of observational management strategies, three types of studies were eligible: (1) studies that used quantitative methods to analyze databases or cohorts of patients to identify predictors of the offer of, acceptance of, or adherence to observational strategies; (2) studies that used qualitative research methods (e.g., focus groups or surveys) to obtain information on factors that affect the use of observational strategies; and (3) experimental studies evaluating the effect of tools such as decision aids on the use of observational strategies.
Results
Definitions of Observational Management Strategies
Fifteen unique cohorts reported selection criteria and follow-up protocols for monitoring triggers other than symptom progression for curative treatment of prostate cancer.1–15 Other than the restriction to men with clinically localized prostate cancer (stage T1 or T2), the exact eligibility criteria had little in common across cohorts. The most commonly used patient selection criteria were based on Gleason score, prostate-specific antigen (PSA) value, and number of biopsy cores positive for cancer. All 15 cohorts included regular PSA testing in the follow-up protocol, but there was no uniform monitoring frequency; many also included regular digital rectal examinations and rebiopsies, also at various frequencies.
Seven unique cohorts of other observational strategies where subsequent treatment was of palliative intent were formed during the current PSA-screening era.7,16–21 The commonly used patient selection criteria were based on PSA (five cohorts), disease stage (four cohorts), age (four cohorts), Gleason score (four cohorts), and bone scan findings (four cohorts). We compared the 15 unique cohorts reporting protocols with curative intent with the 7 cohorts of other observational strategies. Compared with other observational strategies, the protocols with curative intent more commonly had selection criteria based on Gleason score thresholds. They also often used selection criteria based on the number or percentage of cores positive for cancer, whereas none of the other observational strategies used such criteria. Both sets of strategies used PSA-based criteria, but PSA thresholds in curative-intent cohorts were generally lower (typically 10–15 ng/ml) compared with other observational strategies (either 15 or 50 ng/ml). Protocols with curative intent had more clearly defined follow-up protocols compared with other observational strategies, with explicit indications for treatment including increase in Gleason scores, number and percentage of positive cores (on rebiopsy), and/or PSA values. In contrast to other observational strategies, protocols with curative intent generally performed prostate rebiopsies but did not include imaging tests as part of their follow-up procedures. Other observational strategies typically included bone scans and chest radiography, but not rebiopsy.
Factors That Affect the Use of Active Surveillance
For the question on active surveillance practice, studies generally did not directly analyze the offer of, acceptance of, and adherence to active surveillance. Instead, most studies reported analyses of men who were either not treated or not initially treated. In most cases, we could not determine whether these men were on an active monitoring protocol with triggers for curative treatments. The common method for analyzing “adherence to active surveillance” in the literature is the use of the outcome “interruption of active surveillance” to seek definitive treatment. This approach does not distinguish between men who meet predefined criteria of the active surveillance protocol (indicative of disease progression) that call for curative treatment and men who elect to stop active surveillance and pursue curative treatment for other reasons (i.e., without having met disease progression criteria). Although the former could be considered “adherent” (the person is following the protocol), the latter would be considered “not adherent.”
Only two studies specifically examined men who were enrolled in an active monitoring protocol with triggers for curative treatments (as opposed to other non-active surveillance observational management strategies).22,23 The study by van As et al. found that free-to-total PSA ratio and T stage were independent predictors of time to radical treatment in patients on the protocol, but initial PSA, PSA density, Gleason score, number of positive cores, and prostate volume were not.23 The study by Mills et al. found that decreased baseline anxiety and higher socioeconomic status were both associated with a decreased probability of willingness to consent to active surveillance randomization (i.e., these men refused randomization and proactively selected active surveillance).22
Some findings from the remaining 35 heterogeneous studies concerning observational management strategies in men with prostate cancer include:
- Physician recommendations have been reported to be important elements in the decisionmaking process of men with localized prostate cancer.
- The following patient and clinical variables have been reported to be associated with an increased probability for a patient to receive observational management: older age, presence of comorbidities, lower Gleason score, lower tumor stage, lower PSA values at diagnosis, and membership in a lower risk group.
- The following patient and clinical variables have been reported to be associated with an increased probability for a patient to interrupt observational management strategies to seek definitive treatments: younger age, higher tumor stage, higher diagnostic PSA, higher PSA velocity, membership in a higher risk group, and increased anxiety.
- The desire to avoid treatment-related side effects is reported to be a predictor of electing observational management.
Conclusions
There is no standardized definition of active surveillance. The selection criteria and follow-up protocols with curative intent used similar monitoring elements, but the triggering parameters for curative treatments differed across protocols. Older age, presence of comorbidities, lower Gleason score, lower tumor stage, lower PSA value at diagnosis, and lower risk group are associated with increased likelihood of not receiving initial active treatments. It is plausible that similar factors would also affect the receipt of active surveillance, but these associations have not been formally examined.
References
- Patel MI, DeConcini DT, Lopez-Corona E, et al. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol. 2004;171(4):1520–1524.
- Al Otaibi M, Ross P, Fahmy, N et al. Role of repeated biopsy of the prostate in predicting disease progression in patients with prostate cancer on active surveillance. Cancer. 2008;113(2):286–292.
- Ercole B, Marietti SR, Fine J, et al. Outcomes following active surveillance of men with localized prostate cancer diagnosed in the prostate specific antigen era. J Urol. 2008;180(4):1336–1339.
- Eggener SE, Mueller A, Berglund RK, et al. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J Urol. 2009;181(4):1635–1641.
- Soloway MS, Soloway CT, Williams S, et al. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int. 2008;101(2):165–169.
- Dall’era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112(12):2664–2670.
- Hardie C, Parker C, Norman A, et al. Early outcomes of active surveillance for localized prostate cancer. BJU Int. 2005;95(7):956–960.
- Ross AE, Loeb S, Landis P, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol. 2010;28(17):2810–2816.
- Choo R, DeBoer G, Klotz L, et al. PSA doubling time of prostate carcinoma managed with watchful observation alone. Int J Radiat Oncol Biol Phys. 2001;50(3):615–620.
- Adamy A, Yee DS, Matsushita K, et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol. 2011;185(2):477–482.
- Metcalfe C, Tilling K, Davis M, et al. Current strategies for monitoring men with localised prostate cancer lack a strong evidence base: observational longitudinal study. Br J Cancer. 2009;101(3):390–394.
- San Francisco IF, Werner L, Regan MM, et al. Risk stratification and validation of prostate specific antigen density as independent predictor of progression in men with low risk prostate cancer during active surveillance. J Urol. 2011;185(2):471–476.
- Kakehi Y, Kamoto T, Shiraishi T, et al. Prospective evaluation of selection criteria for active surveillance in Japanese patients with stage T1cN0M0 prostate cancer. Jpn J Clin Oncol. 2008;38(2):122–128.
- Miocinovic R, Jones JS, Pujara AC, et al. Acceptance and durability of surveillance as a management choice in men with screen-detected, low-risk prostate cancer: improved outcomes with stringent enrollment criteria. Urology. 2011;77(4):980–984.
- van den Bergh RC, Vasarainen H, van der Poel HG, et al. Short-term outcomes of the prospective multicentre “Prostate Cancer Research International: Active Surveillance” study. BJU Int. 2010;105(7):956–962.
- Bangma CH, Hop WCJ, Schroder FH. Serial prostate specific antigen measurements and progression in untreated confined (stages T0 to 3NxM0, grades 1 to 3) carcinoma of the prostate. J Urol. 1995;154(4):1403–1406.
- Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178(6):2359–2364.
- Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate Cancer Intervention Versus Observation Trial: VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;30(1):81–87.
- Stratton MS, Reid ME, Schwartzberg G, et al. Selenium and inhibition of disease progression in men diagnosed with prostate carcinoma: study design and baseline characteristics of the “Watchful Waiting” Study. Anticancer Drugs. 2003;14(8):595–600.
- McIntyre IG, Clarke RB, Anderson E, et al. Molecular prediction of progression in patients with conservatively managed prostate cancer. Urology. 2001;58(5):762–766.
- Anai S, Nakamura K, Chang MN, et al. The feasibility of expectant management with inner-city men with newly diagnosed localized prostate cancer. J Health Care Poor Underserved. 2008;19(1):164–170.
- Mills N, Metcalfe C, Ronsmans C, et al. A comparison of socio-demographic and psychological factors between patients consenting to randomisation and those selecting treatment (the ProtecT study). Contemp Clin Trials. 2006;27(5):413–419.
- van As NJ, Norman AR, Thomas K, et al. Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol. 2008;54(6):1297–1305.
Presenting Treatment Options to Patients With Localized Prostate Cancer
Jenny Donovan, Ph.D.
Treatment options for patients diagnosed with clinically localized prostate cancer were transformed following the introduction of prostate-specific antigen (PSA) testing in the mid-1980s. As incidence rates of prostate cancer rose steeply, so did rates of radical prostatectomy and radiotherapy, with radical prostatectomy rising to become the treatment of choice for 30% of all incident prostate cancers in the United States by 1990.1 During those decades, clinicians in the United States and the United Kingdom overwhelmingly recommended radical intervention to patients: urologists favored radical surgery, and radiation oncologists favored external beam radiotherapy.2–4 Watchful waiting was recommended mainly for older men and those with short life expectancies (≤10 years).
At the turn of the century, however, concerns began to be raised about whether this strategy of intensive detection and immediate radical intervention was leading to overdiagnosis and overtreatment.5,6 The capacity for radical interventions to produce serious adverse events also was documented.7 Important long-term outcome studies showed that watchful waiting could lead to survival outcomes comparable with those from attempted cure.8–10 Groups began to explore whether prostate cancer could be stratified into different levels of risk, and safe but less radical forms of treatment could be devised. Several definitions of “no immediate intervention” emerged, ranging from delayed intervention and timing of palliative treatment to active monitoring/surveillance programs.11
Currently, men with prostate cancer can be offered and accept treatments from the most radical to the least invasive/intensive without clear evidence to guide them. Randomized controlled trials (RCTs) initiated in the PSA era including the Prostate Cancer Intervention Versus Observation Trial,12 Prostate Testing for Cancer and Treatment (ProtecT),13 and Standard Treatment Against Restricted Treatment14 provide better comparative evidence, but most patients have to rely on a variety of sources of information about treatments—for example, the media (including professional and commercial documents on the Internet), family doctors, and friends and relatives, as well as specialist clinicians. Uncertainty around treatment and outcome for PSA-detected prostate cancer means that many patients face the dilemma of having to weigh the risks of immediate radical intervention with the potential for cure but also adverse events, versus the opportunity to avoid the risks of radical intervention but undergo regular testing and take the chance of incurring increased and possibly only palliative treatment in older age. Various nomograms and algorithms can indicate probabilities of the occurrence of these events, but for any single patient the essential dilemma of having to make a choice between treatments without robust evidence remains, often causing considerable anxiety.15,16 There is little guidance about how treatment options are best presented to patients.
When the United Kingdom National Institute for Health Research’s ProtecT trial was initiated in 1999, there were many who believed that men with PSA-detected prostate cancer (and their clinicians) would not accept randomization between radical surgery, radical conformal radiotherapy, and conservative management. As a consequence, a feasibility study was undertaken with a nested RCT to investigate recruitment to a three-arm or two-radical-arm trial.17 Integrated qualitative research (interviews with patients and clinicians and recordings of recruitment appointments) was undertaken to explore patients’ treatment preferences, the presentation of study information by recruiters (urologists and nurses), and the interpretation of the information by trial participants.18
The study information was considered fully by the ProtecT trial management group and ethics committees, and meetings were held with recruiters to agree on the information to be presented, with a checklist provided of topics to be covered. In the first few months, most patients rejected randomization and opted for radical surgery.19 Scrutiny of recruitment appointments showed that surgery was always presented first, in considerably greater detail and with more enthusiasm than radiotherapy, and the arm that should have been referred to as “conservative management” was called “watchful waiting.”19 Interviews with patients revealed several crucial issues: that watchful waiting was unacceptable as it was seen to be a form of neglect; that terms used by recruiters such as “trial” and “random” were confusing; and that the purpose of the study was not well described or understood.19 The trial management group responded quickly: the protocol for the conservative option was refined and renamed “active monitoring” to reflect the regular PSA tests and review appointments needed by patients; recruiters were instructed to present active monitoring first so that it was fully explained; and they were provided with tips about how to avoid problematic terminology and better describe the purpose of randomization.19 Randomization rates rose from 30% to 70% of eligible participants,19 and recruitment to the full-scale three-arm treatment trial was then able to be completed.13 The ProtecT study has shown that the presentation of study information clearly influenced randomization rates and the treatments selected,19 and further research has provided evidence about treatment preferences20 and the impact on patient engagement of styles of information provided.21
Publications most recently from the Scandinavian Prostate Cancer Group Study 4 treatment trial,22 European screening trial (European Randomized Study of Prostate Cancer),23 and U.S. screening trial (Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial)24 have added very important information to our understanding of prostate cancer, but they have not resolved the key dilemma of how to balance detecting and treating life-threatening prostate cancer while avoiding overdiagnosis and unnecessary treatment. While we await evidence from the RCTs under way, patients and clinicians still have to choose between treatment options. There is now evidence that great care needs to be taken in interactions with patients because the way in which treatments are presented by clinicians can be highly influential, and also that the impact of these influences can only be fully understood by listening to patients’ interpretations. The lack of definitive evidence about the most effective and appropriate treatment, particularly for a man diagnosed with the most common low-grade and small-volume PSA-detected prostate cancer, means that clear and fair presentation of information about treatments is crucial if men are to make informed choices.
References
- Collin SM, Martin RM, Metcalfe C, et al. Prostate-cancer mortality in the USA and UK in 1975–2004: an ecological study. Lancet Oncol. 2008;9(5):445–452.
- Donovan JL, Frankel SJ, Faulkner A, et al. Dilemmas in treating early prostate cancer: the evidence and a questionnaire survey of consultant urologists in the United Kingdom. BMJ. 1999;318(7179):299–300.
- Fowler FJ Jr, McNaughton Collins M, Albertsen PC, et al. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283(24):3217–3222.
- Hanna CL, Mason MD, Donovan JL, et al. Clinical oncologists favour radical radiotherapy for localized prostate cancer: a questionnaire survey. BJU Int. 2002;90(6):558–560.
- Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from the U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94(13):981–990.
- Chodak GW, Warren KS. Watchful waiting for prostate cancer: a review article. Prostate Cancer Prostatic Dis. 2006;9(1):25–29.
- Wilt TJ, MacDonald R, Rutks I, et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–448.
- Albertsen PC, Fryback DG, Storer BE, et al. Long-term survival among men with conservatively treated localized prostate cancer. JAMA. 1995;274(8):626–631.
- Johannson JE, Holberg L, Johansson S, et al. Fifteen-year survival in prostate cancer: a prospective population-based study in Sweden. JAMA. 1997;277(6):467–471.
- Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293(17):2095–2101.
- Martin RM, Gunnell D, Hamdy F, et al. Continuing controversy over monitoring men with localized prostate cancer: a systematic review of programs in the prostate specific antigen era. J Urol. 2006;176(2):439–449.
- Wilt TJ, Brawer MK. The Prostate Cancer Intervention Versus Observation Trial: a randomized trial comparing radical prostatectomy versus expectant management for the treatment of clinically localized prostate cancer. J Urol. 1994;152(5 Pt 2):1910–1914.
- Lane JA, Hamdy FC, Martin RM, et al. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. Europ J Cancer. 2010;46(17):3095–3101.
- Klotz L. Active surveillance for prostate cancer: a review. Curr Urol Rep. 2010;11(3):165–171.
- Steginga SK, Occhipinti S, Gardiner RA, et al. Prospective study of men’s psychological and decision-related adjustment after treatment for localized prostate cancer. Urology. 2004;63(4):751–756.
- Pickles T, Ruether JD, Weir L, et al.; SCRN Communication Team. Psychosocial barriers to active surveillance for the management of early prostate cancer and a strategy for increased acceptance. BJU Int. 2007;100(3):544–551.
- Donovan JL, Peters TJ, Noble S, et al.; ProtecT Study Group. Who can best recruit to randomized trials? Randomized trial comparing surgeons and nurses recruiting patients to a trial of treatments for localized prostate cancer (the ProtecT study). J Clin Epidemiol. 2003;56(7):605–609.
- Donovan J, Hamdy F, Neal D, et al.; ProtecT Study Group. Prostate Testing for Cancer and Treatment (ProtecT) feasibility study. Health Technol Assess. 2003;7(14):1–88
- Donovan J, Mills N, Smith M, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (Prostate Testing for Cancer and Treatment) study. BMJ. 2002;325(7367):766–770.
- Mills N, Donovan JL, Wade J, et al. Exploring treatment preferences facilitated recruitment to a randomized controlled trial. J Clin Epidemiol. 2011 Apr 6. Epub ahead of print.
- Wade J, Donovan JL, Lane JA, et al. It’s not just what you say, it’s also how you say it: opening the “black box” of informed consent appointments in randomised controlled trials. Soc Sci Med. 2009:68(11):2018–2028.
- Bill-Axelson A, Holmberg L, Ruutu M, et al.; SPCG-4 Investigators. Radical prostatectomy versus watchful waiting in early prostate cancer. New Engl J Med. 2011;364(18):1708–1717.
- Schröder FH, Hugosson J, Roobol MJ, et al.; ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European study. New Engl J Med. 2009;360(13):1320–1328.
- Andriole GL, Crawford ED, Grubb RL 3rd, et al.; PLCO Project Team. Mortality results from a randomized prostate-cancer screening trial. New Engl J Med. 2009;360(13):1310–1319.
Improving the Communication of the Benefits and Harms of Treatment Strategies
Richard M. Hoffman, M.D., M.P.H.
The optimal treatment strategy for localized prostate cancer is unclear because there is a dearth of clinical trial data on comparative survival benefits. However, treatment complications, which occur frequently and can be long lasting, are recognized to vary by modality.1 Therefore, a man facing the complex decision of whether and how to treat a localized prostate cancer should ideally be engaging in a shared decisionmaking process. Treatment decisions should reflect a man’s personal values for the various potential outcomes and his attitudes toward risk.2 To achieve this goal, clinicians should discuss the patient’s role in the decisionmaking process, the nature of the decision, treatment alternatives, the potential benefits and harms of the alternatives, and the probabilities for various outcomes, as well as assess the patient’s understanding of the discussion and his preferences.3 Unfortunately, patient preferences do not consistently reflect careful consideration of the evidence.4 Prostate cancer treatment decisions are most strongly influenced by physician recommendations, which are often for the therapy that the particular specialist provides.5,6 In addition, patients do not routinely seek treatment opinions from multiple specialists or discuss options with primary care providers.4,6 Decisions can be driven by feelings of fear and uncertainty, and unduly influenced by anecdotes, misperceptions,7 and family and friends.8
One strategy for delivering comprehensive, objective information to support decisionmaking is to provide a health decision aid, which can be written or electronic.2 These aids should describe alternative options, provide information on the probabilities of benefit and harm for each option, help patients clarify their values, and guide them toward achieving shared decisionmaking.2,9 However, although a systematic review published in 2001 concluded that the content of educational material on prostate cancer treatment was generally accurate, balanced, and readable, most materials failed to provide sufficient information about the risks and benefits of each treatment to support quality decisionmaking.10 Recently, the International Patient Decision Aids Standards (IPDAS) Collaboration developed an instrument to measure the quality of a decision aid that encompassed 10 dimensions.11 A systematic review of 55 decision aid trials, though, found that few of the IPDAS dimensions were consistently evaluated.12
The effectiveness of decision aids in supporting clinical decisionmaking has been evaluated by several systematic reviews.13,14 Overall, using decision aids for treatment or screening decisions improved knowledge, created more realistic expectations, reduced decisional conflict, increased the level of involvement in decisionmaking, and reduced the chance of being undecided.13 Prostate cancer treatment decision aids specifically increased knowledge, encouraged more active involvement in decisionmaking, reduced decisional conflict, and reduced the proportion of patients undergoing surgery.13–16 However, few trials were randomized, decision aids did not consistently present all treatment options or provide exercises to help patients clarify their preferences, and the effects of decision aids on treatment selection were inconclusive.14
Interpreting the literature on decision aids is also problematic because the primary alternative to active treatment is presented as watchful waiting, which has the often unwelcome connotation of just palliating progressive symptoms. Acceptable decision aids must define active surveillance as a strategy to defer active treatment, allowing patients to avoid treatment complications in the absence of clinical evidence of tumor progression.
Research on decisionmaking for treatment of localized prostate cancer should use decision aids that meet expected quality standards and accurately define active surveillance. These decision aids need to be rigorously evaluated in clinical practice, and investigators should address issues about content (e.g., including patient testimonials, displaying risk information, tailoring presentations), format (e.g., written, video, web-based), the logistics of implementing decision aids (e.g., timing, setting, responsible provider, costs), and acceptability by patients and providers. Appropriate outcomes (e.g., decision quality, treatment selection, treatment decision satisfaction/regret) should be measured with validated instruments. Study designs also should account for the repeated decisions occurring during active surveillance. Effective decision aids could better align patient preferences with treatment selection, an important goal of patient-centered healthcare, and potentially improve health outcomes.
References
- Wilt TJ, MacDonald R, Rutks I, et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–448.
- Barry MJ. Health decision aids to facilitate shared decision making in office practice. Ann Intern Med. 2002;136(2):127–135.
- Braddock CH 3rd, Edwards KA, Hasenberg NM, et al. Informed decision making in outpatient practice: time to get back to basics. JAMA. 1999;282(24):2313–2320.
- Zeliadt SB, Ramsey SD, Penson DF, et al. Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer. 2006;106(9):1865–1874.
- Fowler FJ Jr, McNaughton Collins M, Albertsen PC, et al. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283(24):3217–3222.
- Jang TL, Bekelman JE, Liu Y, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. 2010;170(5):440–450.
- Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: the influence of emotion, misconception, and anecdote. Cancer. 2006;107(3):620–630.
- Zeliadt SB, Penson DF, Moinpour CM, et al. Provider and partner interactions in the treatment decision-making process for newly diagnosed localized prostate cancer. BJU Int. 2011 Jan 18. Epub ahead of print.
- Rimer BK, Briss PA, Zeller PK, et al. Informed decision making: what is its role in cancer screening? Cancer. 2004;101(5 Suppl):1214–1228.
- Fagerlin A, Rovner D, Stableford S, et al. Patient education materials about the treatment of early-stage prostate cancer: a critical review. Ann Intern Med. 2004;140(9):721–728.
- Elwyn G, O’Connor AM, Bennett C, et al. Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi). PLoS One. 2009;4(3):e4705.
- O’Connor AM, Bennett C, Stacey D, et al. Do patient decision aids meet effectiveness criteria of the international patient decision aid standards collaboration? A systematic review and meta-analysis. Med Decis Making. 2007;27(5):554–574.
- O’Connor AM, Bennett CL, Stacey D, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2009;(3):CD001431.
- Lin GA, Aaronson DS, Knight SJ, et al. Patient decision aids for prostate cancer treatment: a systematic review of the literature. CA Cancer J Clin. 2009;59(6):379–390.
- Auvinen A, Hakama M, Ala-Opas M, et al. A randomized trial of choice of treatment in prostate cancer: the effect of intervention on the treatment chosen. BJU Int. 2004;93(1):52–56; discussion 56.
- Davison BJ, Degner LF. Empowerment of men newly diagnosed with prostate cancer. Cancer Nurs. 1997;20(3):187–196.
Active Surveillance for Early-Stage Prostate Cancer—The University of California, San Francisco Experience
Peter Carroll, M.D., M.P.H.
The widespread use of serum prostate-specific antigen (PSA) and extended pattern biopsy for the early detection of prostate cancer has led to profound stage and grade migration with the attendant consequences of overdetection and overtreatment. Active surveillance is an alternative to immediate treatment in well-selected patients.1,2
At the University of California, San Francisco to date, more than 650 men have been followed on an active surveillance regimen, which includes period serum PSA, clinical examination, ultrasound imaging, and repeat biopsy.3 Of these men, 80% met criteria as having low-risk disease, 18% as intermediate-risk disease, and 2% as having high-risk disease. Mean age at entry was 62 years, and mean PSA was 6.1 ng/ml.
Treatment-free survival (Figure 1) at 5 years is 64%, with upgrading at biopsy the most significant predictor of treatment. Overall survival at 5 years is 97%. No man has died of prostate cancer. Results at radical prostatectomy for those found to have progressed are no different from those found in a parallel cohort treated with surgery at the time of diagnosis.4,5 The most common indication for treatment has been a change in Gleason score. Changes in serum PSA have not predicted upgrading.6–8 Active surveillance appears to be a safe alternative to immediate treatment in properly selected—and followed—men with prostate cancer. Although men with very low-risk disease appear to be excellent candidates for surveillance, such an option may be appropriate for men with slightly higher risk disease.9 Prospective evaluation of predictors of treatment/risk (serum, tissue biomarkers, imaging) is currently under way. In addition, such men may be candidates for novel, low-morbidity treatment strategies (lifestyle, pharmacologic) that delay or prevent progression.
Figure 1. Treatment-Free Survival
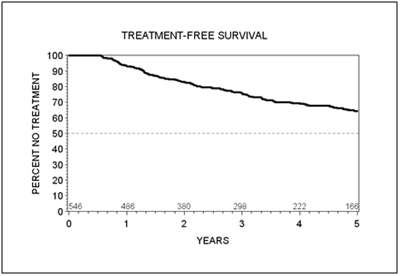
References
- Dall’Era MA, Carroll PR. Outcomes and follow-up strategies for patients on active surveillance. Curr Opin Urol. 2009;19(3):258–262.
- Whitson JM, Porten SP, Carroll PR. Prostate cancer: reducing overtreatment: active surveillance in low-risk disease. Nat Rev Urol. 2011;8(3):124–125.
- Dall’Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112(12):2664–2670.
- Conti SL, Dall’Era M, Fradet V, et al. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181(4):1628–1633; discussion 1633–1634.
- Dall’Era MA, Cowan JE, Simko J, et al. Surgical management after active surveillance for low-risk prostate cancer: pathological outcomes compared with men undergoing immediate treatment. BJU Int. 2010 Aug 26. Epub ahead of print.
- Porten SP, Whitson JM, Cowen JE, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol. 2011;29(20):2795–2800.
- Whitson JM, Porten SP, Hilton JF, et al. The relationship between prostate specific antigen change and biopsy progression in patients on active surveillance for prostate cancer. J Urol. 2011;185(5):1656–1660.
- Whitson JM, Carroll PR. Active surveillance for early-stage prostate cancer: defining the triggers for intervention. J Clin Oncol. 2010;28(17):2807–2809.
- Cooperberg MR, Cowan JE, Hilton JF, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol. 2011;29(2):228–234.
Factors Influencing Patients’ Acceptance and Adherence to Active Surveillance
David F. Penson, M.D., M.P.H.
Clinical decisionmaking in localized prostate cancer is a complicated, multidimensional process in which men often consider their own personal preferences, the advice of their healthcare providers, the opinions of their family and friends, and outside information sources. They synthesize all of this within the framework of their own unique socioeconomic situation, their social support network, and their preconceived impressions of their health and the healthcare system. Our prior systematic review of the literature on decisionmaking in prostate cancer identified a number of consistent themes men consider when choosing therapy.1 These include cancer eradication, treatment side effects (and their impact on quality of life), and out-of-pocket economic costs. Importantly, the way the decision is framed by the healthcare provider plays a critical role in decisionmaking as well. The last point has a strong influence on patient acceptance of active surveillance as a therapeutic strategy for localized prostate cancer.
Previous studies have documented that the specialty of the counseling physician strongly influences the primary recommendation for treatment—urologists tend to recommend radical prostatectomy, whereas radiation oncologists tend to recommend radiation therapy.2 To this end, if there were “invested” providers who advocated for active surveillance in the setting of localized prostate cancer, acceptance of active surveillance likely would increase. Evidence supporting this hypothesis comes from qualitative research performed by Davison et al.3 They interviewed 25 men with low-risk prostate cancer who opted for active surveillance and found that physician recommendation played an important role in a patient’s accepting active surveillance as a management strategy. Specifically, the way the physician described the cancer influenced the patient’s perception of the seriousness of his condition and set the tone for the decisionmaking process. Study participants also noted that initial physician reassurance regarding active surveillance as a viable treatment choice and obtaining additional provider opinions that confirmed active surveillance as an option also increased their acceptance of active surveillance. A full list of the factors identified in the study that influenced men to go on active surveillance is shown in the table below.
Table 1. Factors Influencing Men To Go on Active Surveillance
| Patient perception of prostate cancer |
| Physician recommendation |
| Decision control: Who made the decision? |
| Avoid the side effect of therapy |
| Seeking information to make a treatment decision |
| Advice from family and friends |
| Pre-existing medical conditions |
| Age |
| Coping strategies for active surveillance |
Although there is no doubt that all of these factors play a role in the acceptance of active surveillance, one factor in particular bears special mention: seeking outside information to make a treatment decision. Our group assessed the relationship between information seeking and treatment choice in 804 men with newly diagnosed localized prostate cancer from three clinical sites on the west coast.4 Men electing active surveillance were statistically significantly influenced by media stories and reports about prostate cancer (odds ratio = 1.86, confidence interval 1.12–13.10). This illustrates a possible “leverage point” that public health advocates can possibly use to increase acceptance of active surveillance in the general population.
Once a man elects active surveillance, he must deal with a number of issues. Some might believe that men on active surveillance are more likely to experience anxiety and/or fear of disease recurrence. Recent studies indicate that this may not actually be the case. A study by van den Bergh et al. assessed uncertainty, anxiety, and depression in 129 European men who elected active surveillance for localized prostate cancer.5 Among these men, 81% to 93% of the patients scored better than reference values for these outcomes, indicating that men who stayed on active surveillance protocols did not experience increased disease-specific anxiety. Of course, this likely represents an element of selection bias, as patients who experience increased anxiety are more likely to undergo an aggressive intervention. Acknowledging this, these data dispel the notion that active surveillance itself causes increased anxiety.
Little is known about what influences adherence to active surveillance protocols. A qualitative study by Oliffe and colleagues sheds light on ways that men cope with the uncertainty of active surveillance and provides some insight into factors that may influence adherence.6 They interviewed 25 men on active surveillance and identified the two most common strategies these men used to cope with uncertainty. First, the men tended to frame their prostate cancer as benign through stoicism. This, in turn, underscored their determination to “live a normal life.” Second, men often committed to “doing something extra” to complement active surveillance protocols. Importantly, they often involved their wives and focused on diet as an adjunct therapy. It seems that two of the factors that influence acceptance of active surveillance as a viable therapy—patient perception of disease and advice/support from family—also influence adherence.
References
- Zeliadt SB, Ramsey SD, Penson DF, et al. Why do men choose one treatment over another?: a review of patient decision making for localized prostate cancer. Cancer. 2006;106(9):1865–1874.
- Fowler FJ Jr, McNaughton Collins M, Albertsen PC, et al. Comparison of recommendations by urologists and radiation oncologists for treatment of clinically localized prostate cancer. JAMA. 2000;283(24):3217–3222.
- Davison BJ, Oliffe JL, Pickles T, et al. Factors influencing men undertaking active surveillance for the management of low-risk prostate cancer. Oncol Nurs Forum. 2009;36(1):89–96.
- Ramsey SD, Zeliadt SB, Arora NK, et al. Access to information sources and treatment considerations among men with local stage prostate cancer. Urology. 2009;74(3):509–515.
- van den Bergh RC, Essink-Bot ML, Roobol MJ, et al. Anxiety and distress during active surveillance for early prostate cancer. Cancer. 2009;115(17):3868–3878.
- Oliffe JL, Davison BJ, Pickles T, et al. The self-management of uncertainty among men undertaking active surveillance for low-risk prostate cancer. Qual Health Res. 2009;19(4):432–443.
Regional, Provider, and Economic Factors Associated With the Choice of Active Surveillance in the Treatment of Men With Localized Prostate Cancer
Ann S. Hamilton, Ph.D.
The physician has been shown to play a major role in the choice of active surveillance.1 However, active surveillance appears to be underused among eligible patients (with only 9% of eligible patients opting for active surveillance in the Cancer of the Prostate Strategic Urologic Research Endeavor study), and the role of the physician in the treatment decisionmaking process requires further study.2 Similarly, no treatment within the first 6 months after diagnosis was found for 9% of men with clinically localized prostate cancer from a 2002 patterns of care study based on the National Cancer Institute’s Surveillance, Epidemiology and End Results cancer registries.3 Earlier studies of men diagnosed between 1994 and 2002 indicated that men relying on primary care physicians as opposed to specialists were more likely to be followed by what was termed “expectant management.”4 Since then, the indications for surveillance as a treatment option have changed, with the earlier approach suggested for elderly men without curative intent. More recently, active surveillance is indicated for younger men with low-risk disease; it involves very rigorous follow-up and includes curative intent.5 Thus, the viewpoint of physicians and their understanding of this option may be changing.
The variation in use of surveillance (with various definitions) according to provider, regional factors, and rural/urban residence has been assessed from the Breast and Prostate Cancer Data Quality and Patterns of Care (PoC-BP) study, which was funded by the National Program of Cancer Registries of the Centers for Disease Control and Prevention (CDC) and involved researchers from CDC and seven states (California, Georgia, Kentucky, Louisiana, Minnesota, North Carolina, and Wisconsin). Conducted in 2007–2009, the PoC-BP study used registry data from cancer cases diagnosed in 2004 and included reabstraction of cancer data from hospitals and outpatient facilities (i.e., pathology laboratories, radiation facilities, free-standing surgery centers, long-term care facilities, physicians’ offices). Information on the demography of the cancer patient, characteristics of the cancer, work-up information, and data on the first course of cancer-directed treatment (i.e., therapy regimen that was given or planned at the time of the initial cancer diagnosis, before disease recurrence or progression) and its outcome were collected.
From all participating registries, 11,679 cases of invasive prostate cancer (International Classification of Diseases for Oncology, 3rd Edition, code C61.9) were randomly selected across strata defined by race/ethnicity and state-specific factors such as Appalachian versus non-Appalachian region, type of facility, and patient volume of the facility. The methods and quality of the data have been previously described.6 Linkages with hospital and physician provider files to obtain health system measures were completed. Census tract of residence was linked to variables associated with socioeconomic status and access to care including urbanization (urban [100% urban], rural [100% rural], urban-rural mix), working class (<66% in working class vs. ≥66%), and poverty level (20%+ below poverty vs. <20% below poverty level). The data were weighted by the sampling fractions using SAS Proc Survey to represent the source population.
Abstracts were completed for 77.2% (9,017) of the selected cases, and 8,376 of them had clinically localized disease (after excluding those with T3 or T4 disease, positive nodes, metastases, or missing data required for risk-group definition). Among these patients, 13.9% did not receive therapy in the first 6 months after diagnosis (Table 1). If no mention was made of active surveillance, watchful waiting, expectant management, or other surveillance plan in the records reviewed, they were classified as having “no plan/no therapy” (9.3%); if a specific surveillance plan was mentioned, they were classified as having “active surveillance” (4.6%).
Both types of surveillance were more likely to occur among those age 75 and older than among those diagnosed at younger ages (Table 1). Nonwhites were more likely than whites to not receive therapy within 6 months; this was due largely to having no plan rather than being followed by active surveillance. Men with lower clinical risk were more likely to receive no therapy due to both active surveillance and having no specific plan.
Little difference was seen in the percentage receiving surveillance of either type by urban/rural residence; however, having no plan was more common among those from areas with a greater percentage in the working class and from areas where 20% or more were below the poverty level than from higher socioeconomic status areas (Table 1). The average number of physicians (both primary care physicians and urologists) was higher in areas where men received active surveillance compared with areas where men received no therapy without a specific plan (Table 2).
In summary, although active surveillance may be underutilized in general, when it is used, it occurs more commonly among those with lower risk tumors, which would be considered appropriate, and where the ratio of physicians per 100,000 men is higher. In contrast, receiving no therapy with no specific plan occurs more commonly in lower socioeconomic status areas. Additional results on association of surveillance type with physician specialty will be presented, as well as multivariable analyses to identify factors that independently predict receipt of either active surveillance or no therapy with no plan.
| Characteristics | All Patients Total % Distribution* | Patients Receiving No Therapy in 6 Months | ||
|---|---|---|---|---|
| % of Total With No Therapy† | % With Active Surveillance‡ | % With No Plan/No Therapy§ | ||
| Number | 8,376 | 1,211 | 392 | 819 |
| Weighted number | 24,513 | 3,406 | 1,133 | 2,273 |
| % of total | 100.0 | 13.9 | 4.6 | 9.3 |
| Age at diagnosis | ||||
| <60 | 26.4 | 9.0 | 2.0 | 7.0 |
| 60–64 | 17.5 | 8.9 | 2.7 | 6.2 |
| 65–69 | 20.2 | 12.4 | 3.0 | 9.4 |
| 70–74 | 17.0 | 15.2 | 3.9 | 11.3 |
| 75+ | 18.8 | 25.8 | 12.5 | 13.3 |
| Race/ethnicity | ||||
| White | 73.6 | 12.6 | 4.7 | 7.9 |
| African American | 17.4 | 15.9 | 3.7 | 12.2 |
| API, AI/AN | 2.5 | 17.2 | 6.1 | 11.1 |
| Hispanic | 6.6 | 21.0 | 5.1 | 15.9 |
| Insurance | ||||
| None | 1.4 | 11.3 | 3.0 | 8.3 |
| Private | 60.8 | 12.5 | 3.8 | 8.7 |
| Medicaid | 5.6 | 18.6 | 3.6 | 15.0 |
| Medicare/other public | 27.0 | 16.1 | 6.8 | 9.3 |
| Unknown | 5.3 | 14.8 | 4.8 | 10.0 |
| Clinical risk group | ||||
| Low | 42.0 | 18.8 | 6.8 | 12.0 |
| Intermediate | 41.2 | 9.9 | 3.2 | 6.7 |
| High | 16.7 | 11.5 | 2.7 | 8.8 |
| Rural/urban | ||||
| 100% urban | 50.2 | 15.6 | 5.0 | 10.6 |
| 100% rural | 14.3 | 14.4 | 5.5 | 8.9 |
| Urban/rural mix | 35.1 | 10.9 | 3.7 | 7.2 |
| Working class | ||||
| <66% working class | 45.8 | 12.7 | 4.5 | 8.2 |
| ≥66% working class | 53.8 | 14.7 | 4.7 | 10.0 |
| Poverty level | ||||
| <20% below poverty level | 82.4 | 12.8 | 4.5 | 8.3 |
| ≥20% below poverty level | 17.2 | 18.1 | 5.0 | 13.1 |
|
API, AI/AN = Asian Pacific Islander, American Indian/Alaska Native. *Totals may not equal 100% due to rounding and missing data. †No therapy includes those with active surveillance and no plan. ‡Active surveillance = active surveillance plan specifically mentioned in medical record. §No plan/no therapy = no mention of active surveillance and no therapy received in first 6 months after diagnosis. ¶Definitions of risk groups: Low = T1–T2a and Gleason score 2–6 and PSA <10 ng/ml; Intermediate: T2b–T2c or Gleason score 7 or PSA 10–20 ng/ml; High: Gleason score 8–10 or PSA >20 ng/ml. |
||||
| Initial Therapy | Mean Number of Office-Based Physicians per 100,000 Men | ||
|---|---|---|---|
| Primary Care Physicians (PCP) (95% CI) |
Urologists (95% CI) |
PCP + Urologists (95% CI) |
|
| Active surveillance | 102.0 (97.5–106.5) | 6.7 (6.2–7.2) | 108.7 (103.8–113.6) |
| No plan/no therapy | 98.8 (96.2–101.4) | 6.0 (5.7–6.4) | 104.8 (102.0–107.7) |
| CI = confidence interval. | |||
References
- Gorin MA, Soloway CT, Eldefrawy A, et al. Factors that influence patient enrollment in active surveillance for low-risk prostate cancer. Urology. 2011;77:588–591.
- Barocas DA, Cowan JE, Smith JA Jr, et al.; CaPSURE Investigators. What percentage of patients with newly diagnosed carcinoma of the prostate are candidates for surveillance? An analysis of the CaPSURE database. J Urol. 2008;180(4):1330–1335.
- Hamilton AS, Albertsen PC, Johnson TK, et al. Trends in the treatment of localized prostate cancer using supplemented cancer registry data. BJU Int. 2011;107(4):576–584.
- Jang TL, Bekelman JE, Liu Y, et al. Physician visits prior to treatment for clinically localized prostate cancer. Arch Intern Med. 2010;170(5):440–450.
- Brewster S. Low-risk localized prostate cancer: are we ready to tell patients that active surveillance is the preferred option? BJU Int. 2008;102(8):923–926.
- German R, Wike J, Bauer K, et al. Quality of cancer registry data: findings from CDC-NPCR’s Breast and Prostate Cancer Data Quality and Patterns of Care Study. J Registry Manage. 2011; in press.
Overview of Randomized Controlled Trials for Localized Prostate Cancer
Mack Roach III, M.D., FACR
The argument for active surveillance is influenced critically by whether treatment is proven to be effective. This point was emphasized in an open forum on prostate cancer screening published in the January 18, 2002, edition of the San Francisco Chronicle by Yamey and Wilkes.1 They wrote, “For a screening test to be valuable, there must be a proven treatment that will alter the course of the disease.... There is no research to show that among the various treatment options...any option is better.... In fact, not giving any treatment may be just as good.” It is this last comment that I address in my presentation. In Tables 1 through 4, I summarize most of the major phase III randomized trials addressing men with localized prostate cancer. Based on these data, the following conclusions can be drawn:
- Radical prostatectomy prolongs survival compared with “watchful waiting.”
- There is no role for androgen deprivation therapy before radical prostatectomy.
- Postoperative external beam radiation therapy reduces recurrences after radical prostatectomy and may improve survival.
- Higher dose external beam radiation therapy improves prostate-specific antigen control, but there is no overall advantage yet.
- External beam radiation therapy with androgen deprivation therapy is better than external beam radiation therapy alone for intermediate-risk and high-risk patients.
- High-risk patients benefit from long-term androgen deprivation therapy (more than 2 years), but those with intermediate-risk disease appear to require only 4 to 6 months.
- Androgen deprivation therapy with external beam radiation therapy is better than androgen deprivation therapy alone for men with locally advanced disease.
| First Author (Year) | Design | Conclusions | Comments |
|---|---|---|---|
| Bill-Axelson (2011)2 | RP vs. watchful waiting | RP associated with better survival | Most benefit for men <65 years of age |
| Studer (2006)3 | RP ± adjuvant ADT | Small improvement in overall survival | No improvement on cause-specific survival or quality of life |
| Klotz (1999)4 | RP vs. ADT + RP | Similar rate of PSA failure and no survival advantage | Does not support the use of ADT with RP |
| Aus (1998)5 | RP vs. ADT + RP | Similar rate of PSA failure and no survival advantage | Does not support the use of ADT with RP |
| Soloway (2002)6 | RP vs. ADT + RP | Similar rate of PSA failure and no survival advantage | Does not support the use of ADT with RP |
| Schulman (2000)7 | RP vs. ADT + RP | Similar rate of PSA failure and no survival advantage | Does not support the use of ADT with RP |
| Homma (1997)8 | RP vs. ADT + RP | Similar rate of PSA failure and no survival advantage | Does not support the use of ADT with RP |
| Van Poppel (1995)9 | RP vs. estramustine + RP | Similar rate of PSA failure and no survival advantage | Does not support use of estramustine with RP |
| RP = radical prostatectomy; PSA = prostate-specific antigen; ADT = androgen deprivation therapy. | |||
| First Author (Year) | Design | Conclusions | Comments |
|---|---|---|---|
| Thompson (2009)10 | pT3 ± adjuvant RT | Improved PSA control, clinical failure, and overall survival | Longest follow-up |
| Wiegel (2009)11 | pT3 ± adjuvant RT | Improved PSA control | Follow-up too short to address survival? |
| Bolla (2005)12 | pT3 or + margins ± adjuvant RT | Improved PSA control, clinical failure, and metastasis-free survival | Follow-up too short to address survival? |
| RT = radiation therapy; PSA = prostate-specific antigen. | |||
| First Author (Year) | Design | Conclusions | Comments |
|---|---|---|---|
| Kuban (2011)13 | 70 vs. 78 Gy EBRT | Patients with PSA >10 ng/ml or high risk benefit from 78 Gy | 78 Gy decreased PSA, clinical failure, and, in a post-hoc analysis, prostate cancer deaths compared with 70 Gy |
| Zietman (2010)14 | 70.2 Gy EBRT vs. 79.2 Gy with protons | 5-year bFFS: Low risk: 84% to >98% Intermediate risk: 79% to >91% |
No impact on survival yet |
| Peeters (2006)15 | 68 vs. 78 Gy EBRT | PSA control better in the 78 Gy arm | No impact on survival yet |
| Dearnaley (2007)16 | 64 vs. 74 Gy EBRT | Improved PSA control at 5 years with 74 Gy | No impact on survival yet |
| Beckendorf (2011)17 | 70 vs. 80 Gy EBRT | Improved 5-year PSA failure with 80 Gy benefit greatest if PSA >15 | No impact on survival yet |
| Sathya (2005)18 | 66 Gy EBRT vs. 40 Gy + 35 Gy Ir-192 implant | Improved PSA control with addition of higher doses with implant | No impact on survival yet |
| EBRT = external beam radiation therapy; PSA = prostate-specific antigen; bFFS = biochemical failure-free survival. | |||
| First Author (Year) | Design | Conclusions | Comments |
|---|---|---|---|
| Shipley (2011)19 | RT vs. RT + bicalutamide 150 mg for rising PSA after prostatectomy | Improved PSA control, reduced metastasis rate | Pending assessment of primary end point due to short follow-up |
| Jones (2011)20 | ± NHT 2 months prior and during RT (66 Gy) | Overall and cause-specific survival advantage | Benefit of ADT greatest for intermediate risk |
| Roach (2008)21 | RT ± ADT 2 months prior and during WPRT | Cause-specific survival advantage | High-risk patients require longer term ADT |
| Pilepich (2005)22 | RT ± long-term adjuvant ADT | Overall survival advantage | Essentially all subsets with high risk benefited |
| Hanks (2003)23 | RT + 4 or 28 months ADT | Survival advantage GS = 8–10 |
High-risk patients require longer-term ADT |
| Roach (2003)24 | RT + 4 months ADT started either before or after RT and ± WPRT | Improved PFS with WPRT and ADT started before RT | Trial to confirm value of WPRT (RTOG 0924) under way |
| RT = radiation therapy; PSA = prostate-specific antigen; NHT = neoadjuvant hormonal therapy; ADT = androgen deprivation therapy; GS = Gleason score; PFS = progression-free survival; WPRT = whole-pelvic radiation therapy; RTOG = Radiation Therapy Oncology Group. | |||
| First Author (Year) | Design | Conclusions | Comments |
|---|---|---|---|
| Armstrong (2011)25 | 70 Gy + 4 vs. 8 months neoadjuvant ADT | No advantage | Included mostly high-risk patients |
| Denham (2011)26 | 66 Gy to prostate ± 3 or 6 months ADT | ADT for 6 months improves overall survival | Need at least 6 months of ADT? |
| Bolla (2007)27 | 70 Gy (50 Gy WP) 6 months vs. 3-year HT |
Improved survival with 3 years | Long term > short term |
| D’Amico (2004)28 | 70 Gy ± 6 months ADT | Improved survival | Need at least 6 months of ADT? |
| Crook (2009)29 | 66–67 Gy + 3 months vs. 8 months ADT |
Overall, no advantage in disease-free survival | Improved disease-free survival in subset of high-risk patients on 8-month arm |
| Bolla (2002)30 | 70 Gy ± 3 years ADT | Improved survival for very high-risk patients | |
| ADT = androgen deprivation therapy; WP = whole pelvis; HT = hormone therapy. | |||
| First Author (Year) | Design | Conclusions | Comments |
|---|---|---|---|
| Widmark (2009)31 | ADT ± RT for locally advanced disease | Better survival with addition of RT | Used primarily anti-androgens |
| Mason (2010)32 | ADT ± RT for locally advanced disease | Better survival with addition of RT | Used LHRH drug |
| ADT = androgen deprivation therapy; RT = radiation therapy; LHRH = luteinizing hormone-releasing hormone. | |||
References
- Yamey G, Wilkes M. Prostate cancer screening: is it worth the pain? San Francisco Chronicle. January 18, 2001; 29.
- Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. New Engl J Med. 2011;364(18):1708–1717.
- Studer UE, Whelan P, Albrecht W, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol. 2006;24(12):1868–1876.
- Klotz LH, Goldenberg SL, Jewett M, et al. CUOG randomized trial of neoadjuvant androgen ablation before radical prostatectomy: 36-month post-treatment PSA results. Canadian Urologic Oncology Group. Urology. 1999;53(4):757–763.
- Aus G, Abrahamsson PA, Ahlgren G, et al. Hormonal treatment before radical prostatectomy: a 3-year followup. J Urol. 1998;159(6):2013–2016; discussion 2016–2017.
- Soloway MS, Pareek K, Sharifi R, et al.; Lupron Depot Neoadjuvant Prostate Cancer Study Group. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167(1):112–116.
- Schulman CC, Debruyne FM, Forster G, et al. 4-year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur Urol. 2000;38(6):706–713.
- Homma Y, Akaza H, Okada K, et al. Preoperative endocrine therapy for clinical stage A2, B, and C prostate cancer: an interim report on short-term effects. Prostate Cancer Study Group. Int J Urol. 1997;4(2):144–151.
- Van Poppel H, De Ridder DD, Elgamal AA, et al. Neoadjuvant hormonal therapy before radical prostatectomy decreases the number of positive surgical margins in stage T2 prostate cancer: interim results of a prospective randomized trial. The Belgian Uro-Oncological Study Group. J Urol. 1995;154(2 Pt 1):429–434.
- Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–962.
- Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009;27(18):2924–2930.
- Bolla M, Van Poppel H, Collette L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911). Lancet. 2005;366(9485):572–578.
- Kuban DA, Levy LB, Cheung MR, et al. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int J Radiat Oncol Biol Phys. 2011;79(5):1310–1317.
- Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28(7):1106–1111.
- Peeters ST, Heemsbergen WD, Koper PC, et al. Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24(13):1990–1996.
- Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(6):475–487.
- Beckendorf V, Guerif S, Le Prisé E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011;80(4):1056–1063.
- Sathya JR, Davis IR, Julian JA, et al. Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol. 2005;23(6):1192–1199.
- Shipley WU, Hunt D, Lukka HR, et al. Initial report of RTOG 9601, a phase III trial in prostate cancer: effect of anti-androgen therapy (AAT) with bicalutamide during and after radiation therapy (RT) on freedom from progression and incidence of metastatic disease in patients following radical prostatectomy (RP) with pT2-3,N0 disease and elevated PSA levels. American Society of Clinical Oncology 2011 Genitourinary Cancers Symposium. Orlando, Florida, February 17–19, 2011. J Clin Oncol. 201(Suppl 7; Abst 1).
- Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. New Engl J Med. 2011;365(2):107–118.
- Roach M 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26(4):585–591.
- Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma—long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61(5):1285–1290.
- Hanks GE, Pajak TF, Porter A, et al.; Radiation Therapy Oncology Group. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: the Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21(21):3972–3978.
- Roach M 3rd, DeSilvio M, Lawton C, et al.; Radiation Therapy Oncology Group. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol. 2003;21(10):1904–1911.
- Armstrong JG, Gillham CM, Dunne MT, et al. A randomized trial (Irish Clinical Oncology Research Group 97–01) comparing short versus protracted neoadjuvant hormonal therapy before radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2010 August 24. Epub ahead of print.
- Denham JW, Steigler A, Lamb DS, et al. Short-term neoadjuvant androgen deprivation and radiotherapy for locally advanced prostate cancer: 10-year data from the TROG 96.01 randomised trial. Lancet Oncol. 2011;12(5):451–459.
- Bolla M, de Reijke TM, Van Tienhoven G, et al.; EORTC Radiation Oncology Group and Genito-Urinary Tract Cancer Group. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516–2527.
- D’Amico AV, Manola J, Loffredo M, et al. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: a randomized controlled trial. JAMA. 2004;292(7):821–827.
- Crook J, Ludgate C, Malone S, et al. Final report of multicenter Canadian phase III randomized trial of 3 versus 8 months of neoadjuvant androgen deprivation therapy before conventional-dose radiotherapy for clinically localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73(2):327–333.
- Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–106.
- Widmark A, Klepp O, Solberg A, et al.; Scandinavian Prostate Cancer Group Study 7; Swedish Association for Urological Oncology 3. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–308.
- Mason MD, Warde PR, Sydes MR, et al. Intergroup randomized phase 3 study of androgen deprivation therapy (ADT) + radiation therapy (RT) in locally advanced prostate cancer (CaP) (NCIC-CTG, SWOG, MRC-UK, and INT: T94-0110; NCT00002633). Int J Radiat Oncol Biol Phys. 2010;78(3 Suppl 1):S2.
Results From the Scandinavian Prostate Cancer Group 4 Trial (SPCG-4)
Lars Holmberg, M.D., Ph.D.; Anna Bill-Axelson, M.D., Ph.D.; Hans Garmo, Ph.D.; Juni Palmgren, Ph.D., M.Sc.; Gunnar Steineck, M.D., Ph.D.; Hans-Olov Adami, M.D., Ph.D.; Jan-Erik Johansson, M.D., Ph.D., for the SPCG-4 Trialists
Introduction
The Scandinavian Prostate Cancer Group 4 (SPCG-4) trial started in 1989 when there was no conclusive evidence from previous studies to introduce radical treatment for early prostate cancer.1 The trial aimed to test whether radical removal of the prostate could lower prostate cancer mortality in men with clinically localized disease with what, in those days, was considered medium- and low-risk prostate cancer. During the course of the trial, studies of side effects and quality of life in long-term survivors have come increasingly into focus. The trial started well before prostate-specific antigen (PSA) testing became widespread in the studied Nordic population, but with the knowledge that diagnostic intensity was increasing and a swing from conservative to active management was happening without empirical evidence of benefit. By today’s standards, none of the participating centers were high-volume prostatectomy surgical units.
Methods
The study design and methods of estimation of main effects on overall, cause-specific, and distant metastasis-free survival have been described in a series of publications.2–5 From 1989 to 1999, 695 men with early prostate cancer were randomized to watchful waiting or to radical prostatectomy. Men were eligible for inclusion if they were younger than age 75, were deemed to have a life expectancy of more than 10 years, and had a localized well- or moderately well-differentiated tumor of stage T1b–T2 and a PSA level of less than 50 ng/ml. Follow-up is complete through December 2009. A team of study pathologists reviewed biopsy and radical prostatectomy specimens. Throughout the study, an independent review committee blinded to study arm allocation classified causes of death. The reports include relative risk and absolute risk difference estimates with 95% confidence intervals.
The methods for assessing symptom burden and self-assessed quality of life have been described in detail earlier.6–8 Physical symptoms, symptom-induced stress, and self-assessed quality of life were evaluated by study-specific questionnaires. In a first follow-up, all living Swedish men enrolled in the SPCG-4 trial 1989 through February 1996 were approached.6 In a second long-term follow-up, all 400 living men randomized to the SPCG-4 trial in Sweden and Finland from 1989 to 1999 were included.8 For this second follow-up, a population sample of 300 men without prostate cancer were in an age- and region-matched control group. Longitudinal data were provided for men in the SPCG-4 trial participating in both assessments.
Results
The long-term follow-up of survival and recurrence is based on 367 deaths, of which 136 were attributed to prostate cancer.5 Stable 15-year estimates show a difference between the study arms of 6.1 percentage points in prostate cancer mortality (95% confidence interval [CI], 0.2–12.0), corresponding to a relative risk with surgery of 0.62 (95% CI, 0.44–0.87). These results have remained stable over the follow-up and have been shown in exploratory subgroup analyses to be confined primarily to men younger than age 65, but also observed among men with a low-risk prostate cancer. Tumor size, PSA levels, and Gleason grade at time of diagnosis all have prognostic impact but do not modify the effect of radical prostatectomy.9 During a median follow-up of 12 years, the overall cost in the radical prostatectomy group was 34% higher (p <0.01) than in the watchful waiting group, corresponding to €6,123 in Sweden. The difference was driven almost exclusively by the cost of the surgical procedure.10
In men who underwent radical prostatectomy and had extracapsular tumor growth in the operative specimen, the risk of death from prostate cancer was about seven times that of men without extracapsular tumor growth. In a study of PSA as a marker of progressive disease in men in the watchful waiting group, both PSA value at baseline and the rate of PSA change were associated with the development of lethal prostate cancer.11 However, the accuracy of classifying the disease as either indolent or aggressive was low, regardless of the cut-off point chosen for initial PSA level or rate of change in PSA level.
In the first follow-up of symptoms and quality of life, erectile dysfunction was more common after radical prostatectomy, although urinary obstruction was less common than among men on watchful waiting. The prevalence of depression, well-being, and subjective quality of life were similar in the two groups.6 In the long-term follow-up, the prevalence of erectile dysfunction was 84% and 80% in the radical prostatectomy and the watchful waiting group, respectively. The corresponding figures for urinary leakage were 41% and 11%, respectively. Distress from these symptoms was significantly more severe among men allocated to radical prostatectomy than among those allocated to watchful waiting. Men who provided information at two points of follow-up reported an increase in bothersome symptoms and a reduction in quality of life. However, the level of self-assessed quality of life was similar between the groups. The prevalence of symptoms and the level of anxiety were considerably higher in the SPCG-4 groups than among population controls, for example, with a relative risk of 1.42 for presence of anxiety in the SPCG-4 groups compared with controls.8
Discussion
The SPCG-4 trial is the first study to provide randomized evidence that radical treatment of early prostate cancer confers a survival benefit. Several factors limit the direct generalizability of these results to the present situation. The SPCG-4 trial was undertaken among men with clinically detected prostate cancer. Today, PSA screening introduces long lead times and possibly diagnosis of cancers from a qualitatively different biological domain. The surgery in SPCG-4 was done in the pioneering period of the present surgical technical development and, by today’s standards, by low-volume surgeons. The comparison group was managed by watchful waiting, not active surveillance.
Nevertheless, the study provides clinically highly relevant information from many points of view. In many regions of the world, PSA testing is still not prevalent for policy reasons or will not be feasible for a very long time. Among men with a clinically detected prostate cancer, no routinely assessed clinical parameters (besides, tentatively, age) modify the effect of radical prostatectomy. In a watchful waiting group, PSA does not seem to be a safe indicator of whom to treat early. As for the effect modification by age, we caution that this is a finding in an exploratory subgroup analysis; a more detailed investigation shows that there is no sharp cut-off of the effect by age 65 but rather indicates a slowly diminishing survival benefit in the age span 65–75.12 The poor prognosis among men with extracapsular growth indicates that they may be suitable candidates for postoperative adjuvant treatment.
The quality-of-life studies show that the effects of leaving the tumor in situ with ensuing higher risk of being treated with androgen deprivation impacts quality of life similarly to the sequelae of radical prostatectomy.7 Thus, it is important for decisionmaking and patient information that choice of therapy is guided by complete information and understanding of patient preferences because the interventions involve complex scenarios that are not directly comparable.
References
- Iversen P, Madsen PO, Corle DK. Radical prostatectomy versus expectant treatment for early carcinoma of the prostate. Twenty-three year follow-up of a prospective randomized study. Scand J Urol Nephrol Suppl. 1995;172:65–72.
- Holmberg L, Bill-Axelson A, Helgesen F, et al. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med. 2002;347(11):781–789.
- Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977–1984.
- Bill-Axelson A, Holmberg L, Filén F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian Prostate Cancer Group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144–1154.
- Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–1717.
- Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347(11):790–796.
- Johansson E, Bill-Axelson A, Holmberg L, et al. Time, symptom burden, androgen deprivation, and self-assessed quality of life after radical prostatectomy or watchful waiting: the randomized Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4) clinical trial. Eur Urol. 2009;55(2):422–430.
- Johansson E, Steineck G, Holmberg L, et al. Long-term quality of life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomized trial. Lancet Oncol.; in press.
- Holmberg L, Bill-Axelson A, Garmo H, et al. Prognostic markers under watchful waiting and radical prostatectomy. Hematol Oncol Clin North Am. 2006;20(4):845–855.
- Andersson SO, Andrén O, Lyth J, et al. Managing localized prostate cancer by prostatectomy or watchful waiting: cost analysis of a randomized trial (SPCG-4). Scand J Urol Nephrol. 2011;45(3):177–183.
- Fall K, Garmo H, Andrén O, et al. Prostate-specific antigen levels as a predictor of lethal prostate cancer. J Natl Cancer Inst. 2007;99(7):526–532.
- Bill-Axelson A. Localized Prostate Cancer: Results From a Randomized Clinical Trial. [Thesis]. Uppsala, Sweden: Acta Universitatis Upsaliensis; 2005. (Digital Comprehensive Summaries of Uppsala Dissertations From the Faculty of Medicine, 25:1651–6206).
The Prostate Cancer Intervention Versus Observation Trial (PIVOT): U.S. Department of Veterans Affairs/National Cancer Institute/Agency for Healthcare Research and Quality Cooperative Studies Program #407: A Randomized Trial Comparing Radical Prostatectomy to Observation for Men With Clinically Localized Prostate Cancer
Timothy J. Wilt, M.D., M.P.H., for the PIVOT Study Group
Background
The comparative effectiveness of surgery versus observation for men with localized prostate cancer detected since the initiation of prostate-specific antigen (PSA) testing is not known.
Methods
Between November 1994 and December 2002, we randomly assigned 731 men (mean age 67, median PSA = 7.8 ng/ml) with clinically localized (T1–T2NxMO) prostate cancer to radical prostatectomy or observation. We achieved complete follow-up through January 2010. The primary end point was all-cause mortality, and the secondary end point was prostate cancer mortality.
Results
During the median follow-up of 10.0 years (interquartile range 7.3 years to 12.6 years), 171 (47.0%) of 364 men in the radical prostatectomy group and 183 (49.9%) of 367 men in the observation group died (hazard ratio [HR] = 0.88; 95% confidence interval [CI] = 0.71–1.08; p = 0.22; absolute risk reduction (ARR) = 2.9%; 95% CI = –4.3–10.1). Overall, 52 men (7.1%) had a death attributed to prostate cancer or prostate cancer treatment. Among men in the radical prostatectomy group, 21 (5.8%) died of prostate cancer or prostate cancer treatment versus 31 (8.4%) men in the observation group (HR = 0.63; 95% CI = 0.36–1.09; p = 0.09; ARR = 2.7%; 95% CI = –1.1–6.5). In predefined subgroups, all-cause mortality did not differ by treatment assignment according to age, race, comorbidity, health status, or tumor histology categories. A reduction in all-cause mortality was limited to men with baseline PSA >10 (p = 0.043 for interaction) and borderline reduction in those with intermediate-risk prostate cancer (p = 0.073 for interaction). Prostate cancer mortality was rare in men with baseline PSA values ≤10 ng/ml and those with low-risk prostate cancer, and did not differ by treatment assignment (p ≥0.1 for all groups). Radical prostatectomy reduced prostate cancer mortality among men with PSA values >10 ng/ml (5.6% vs. 12.8%; p = 0.02) and those with high-risk prostate cancer (9.1% vs. 17.4%; p = 0.04).
Conclusions
In men with localized prostate cancer detected during the early PSA era, radical prostatectomy compared with observation produced reductions in all-cause and prostate cancer mortality that were not significant and less than 3% in absolute terms through 12 years. While a larger reduction may occur in men with higher PSA or higher risk disease, surgery did not reduce mortality compared with observation in men with PSA values 10 ng/ml or less or men with low-risk prostate cancer.
Impact of Different Management Strategies on Quality of Life in Localized Prostate Cancer
Mark S. Litwin, M.D., M.P.H.
The clinical indolence of prostate cancer begets uncertainty regarding the role of initial therapy. Men diagnosed with low-risk prostate cancer are typically offered three treatment options: radical prostatectomy, radiation therapy, or active surveillance (formerly called “watchful waiting”). Because affected men are much more likely to die with than of their disease, the decision between active treatment and active surveillance may hinge on factors other than expected cause-specific survival. As Harry Herr noted in 1987, “The goal of any treatment strategy for cancer is to improve not only patient survival but also quality of that survival.”1
Research on health-related quality of life (HRQOL) in men with early-stage prostate cancer has focused primarily on the impact of active treatment with sparse attention to active surveillance. Although little is known about the psychosocial impact of active surveillance, one study found that patient uncertainty over treatment outcomes, support from physicians, and concerns about side effects often influence men to choose watchful waiting.2 Yet many men perceive watchful waiting as “doing nothing,” which they consider inherently unacceptable.3–10 In some studies, men on watchful waiting report higher levels of stress and worse HRQOL than those receiving active treatment.11 The few studies focused specifically on active surveillance indicate that the main predictor of abandoning active surveillance for active treatment is patient anxiety, a central domain of HRQOL.12–14 One recent study found that men on active surveillance use two strategies to overcome active surveillance-related angst. First was considering the cancer benign and living a normal life, and second was doing something extra, which included diet modifications and complementary and alternative medicine.15 Overall, the emergent literature suggests that barriers to choosing active surveillance are anxiety, uncertainty, and lack of education.12–14,16–18
Prospective Randomized Controlled Trials
The only large, prospective randomized controlled trial published to date in which HRQOL has been compared between men receiving active treatment versus active surveillance is the Scandinavian Prostate Cancer Group Study 4 (SPCG-4),19 which has shown a durable and significant survival benefit of prostatectomy over watchful waiting.20 Comparing the surgery and watchful waiting groups in the first 5 years after randomization, HRQOL differences were mainly limited to more erectile dysfunction (80% vs. 45%) and urinary leakage (49% vs. 21%) but less urinary obstruction (28% vs. 44%) in men undergoing prostatectomy. Bowel function, anxiety, depression, well-being, and overall HRQOL were similar in the two groups. However, subsequent analyses at 6–8 years have shown that watchful waiting is associated with worse late deterioration in other domains of HRQOL, such as anxiety and depression.21
Prospective National Observational Trials
Two multicenter cohort studies have been particularly fruitful in prostate cancer. Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) is a national, longitudinal disease registry of more than 10,000 men with prostate cancer in community and academic settings across the United States, led by Dr. Peter Carroll at the University of California, San Francisco, which has provided valuable insights into evolving practice patterns, technology diffusion, outcomes, and severity of disease from data collected.22–24
As reported in the recent CaPSURE review by Porten et al., CaPSURE enrollees who underwent radical prostatectomy had lower scores on both disease-specific and general HRQOL instruments immediately postoperatively, which improved significantly at 1 year after treatment and continued to improve in the domain of sexual function in the second year.25 Men who were treated with external beam radiation therapy, active surveillance, or primary androgen deprivation therapy had scores that were relatively stable, except for sexual function, which declined with time. Overall, patients who underwent surgery had the greatest decline initially and also the greatest degree of recovery. Most men experienced the greatest recovery of both urinary and sexual functions within 2 years of treatment, with little change in reported HRQOL scores after 3 years.26 Those who received multimodal therapy appeared to have greater declines in urinary and sexual functions than those who were treated with monotherapy.27
Prostate Cancer Outcomes and Satisfaction With Treatment Quality Assessment is a consortium of nine academic centers, led by Dr. Martin Sanda at Harvard Medical School, which has tracked more than 1,800 patients and spouses for several years with a specific focus on quality-of-life outcomes after surgery, external beam radiation, or interstitial brachytherapy.28 As Figure 1 illustrates in part, Sanda found that “patients in the brachytherapy group reported having long-lasting urinary irritation, bowel and sexual symptoms, and transient problems with vitality or hormonal function. Adverse effects of prostatectomy on sexual function were mitigated by nerve-sparing procedures. After prostatectomy, urinary incontinence was observed, but urinary irritation and obstruction improved, particularly in patients with large prostates. No treatment-related deaths occurred, and serious adverse events were rare. Treatment-related symptoms were exacerbated by obesity, a large prostate size, a high prostate-specific antigen score, and older age. Black patients reported lower satisfaction with the degree of overall treatment outcomes. Changes in quality of life were significantly associated with the degree of outcome satisfaction among patients and their spouses or partners.”
Retrospective National Observational Trials
Building on Fowler’s early cross-sectional work in Medicare patients,29 Carroll’s national CaPSURE registry,23,24 Talcott’s early prospective single-institution series,30,31 and the availability of validated instruments,32–34 Potosky and colleagues at the National Cancer Institute undertook the Prostate Cancer Outcomes Study (PCOS). Drawing subjects from Surveillance, Epidemiology and End Results (SEER) registries across the United States, PCOS was the first nationally representative, population-based, longitudinal cohort study examining outcomes and HRQOL from the patient perspective in men who had undergone active treatment for prostate cancer.
Initial PCOS results indicated that 2 years after radical prostatectomy, more than 90% of men were continent, and about half were (by some definition) potent.35 This represented a dramatic improvement over the era before Walsh and Donker reported their now-classic description of the cavernous erectile nerves.36
Figure 1. Changes in Quality of Life After Primary Treatment for Prostate Cancer
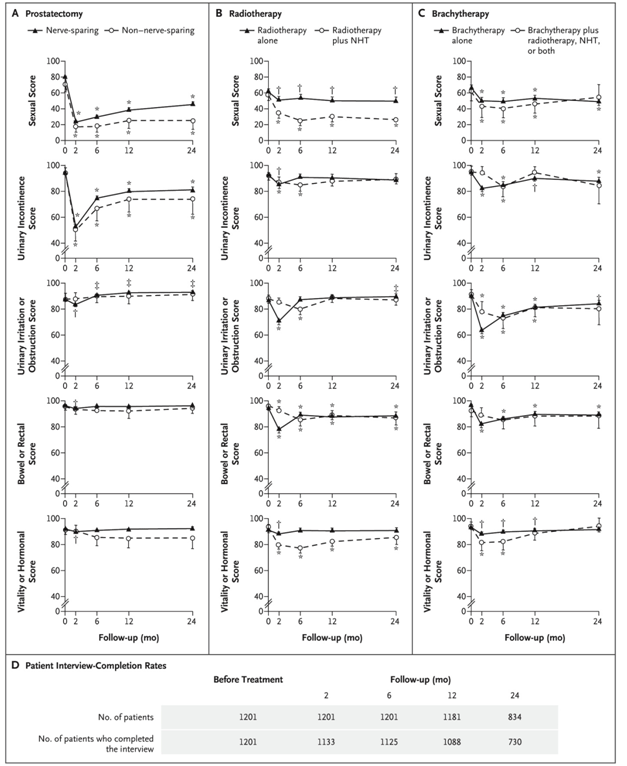
NHT = neoadjuvant hormonal therapy.
From Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261. Reprinted with permission. Copyright © 2008 Massachusetts Medical Society. All rights reserved.
Subsequent PCOS results revealed that 5 years after treatment, men who had undergone surgery reported stable urinary function, but those who had undergone radiation experienced progressive urinary impairment. Just as striking was the finding that 3–5 years after treatment, those who had been irradiated saw a much sharper decline in sexual function than did those who had been operated on,37 thus providing empirical evidence for the widely held observation that time homogenizes sexual outcomes after surgery or radiation. This may be due to the effects of aging, cumulative radiation injury, postoperative nerve recovery, or all three.
Penson’s 2005 update to the 5-year PCOS data featured several important observations for men undergoing radical prostatectomy.38 First, significant urinary leakage, uncommon although not trivial, remains fairly constant between 11% at 2 years and 14% at 5 years postoperatively. Associated urinary distress is commensurate with leakage, occurring in only 13% of surgical cases. Second, Penson reiterated our understanding of how vastly different sexual outcomes are between urologists in general and those in referral centers with high-volume subspecialty practices.39 Third, sildenafil appears to aid in the postoperative return of erections for men who are potent at baseline and who undergo bilateral nerve sparing. Fourth, for the majority of men, functional outcomes remain fairly stable between 2 and 5 years after surgery. Finally, even though only 28% of respondents overall report erections firm enough for intercourse, almost twice as many (54%) state that they are sexually active at least once a month. This clarifies that the nature of sexual function in prostate cancer survivors includes activities beyond coitus alone, an observation that has clinical relevance for men whose sexual partner is unable or unwilling to have intercourse.
Single-Institution Series
In a prospective longitudinal study of recovery profiles in 475 men before and through 4 years after prostatectomy, external beam radiation, or interstitial brachytherapy, Gore and colleagues compared changes in mean HRQOL scores and the probability of regaining baseline HRQOL across treatment groups (Figure 2).40 Urinary incontinence was more common after prostatectomy, while voiding and storage urinary symptoms were more prevalent after brachytherapy. Sexual dysfunction profoundly affected all treatment groups, with a relatively low likelihood of regaining baseline function among prostatectomy subjects. Bowel dysfunction was more common after radiation. Capturing baseline function prior to treatment permitted comparison of interval mean scores with pretreatment function.
Wei and colleagues reported results from a cross-sectional survey of 902 men who underwent radical prostatectomy, external beam radiation, or brachytherapy at an academic medical center and from 112 age-matched controls.41 The authors summarized their HRQOL results: “Compared with controls, each therapy group reported bothersome sexual dysfunction; radical prostatectomy was associated with adverse urinary HRQOL; external beam radiation was associated with adverse bowel HRQOL; and brachytherapy was associated with adverse urinary, bowel, and sexual HRQOL. Hormonal adjuvant symptoms were associated with significant impairment. More than 1 year after therapy, several HRQOL outcomes were less favorable among subjects after brachytherapy than after external radiation or radical prostatectomy. Progression-free subjects reported better sexual and hormonal HRQOL than subjects with increasing prostate-specific antigen.” Figure 3 highlights these results.
Figure 2. Longitudinal Mean Scores for Health-Related Quality of Life Across Treatment Groups
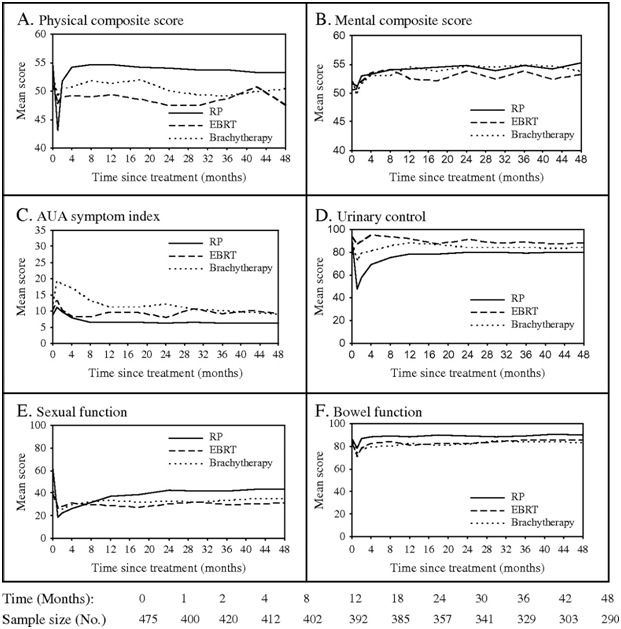
RP = radical prostatectomy; EBRT = external beam radiation therapy; AUA = American Urological Association.
From Gore JL, Kwan L, Lee SP, et al. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101(12):888–892. Reprinted with permission. Copyright © 2009 Oxford University Press. All rights reserved.
Figure 3. Severity of Overall Urinary, Bowel, and Sexual Bother After Localized Prostate Cancer Therapy and in Age-Matched Controls
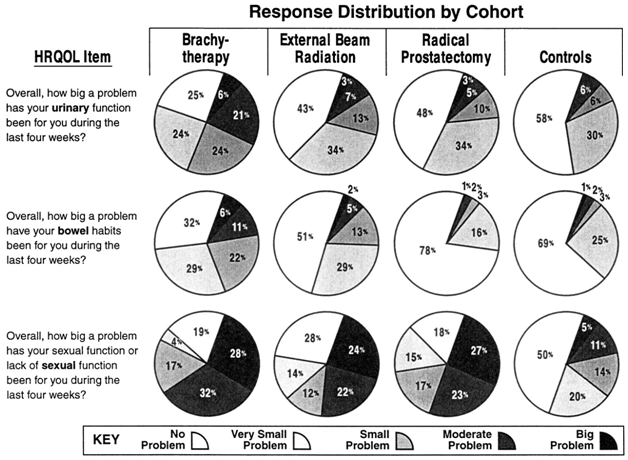
From Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20(2):557–566. Reprinted with permission. Copyright © 2002 American Society of Clinical Oncology. All rights reserved.
Special Populations
Results from low-income, underserved populations with prostate cancer have been reported primarily in men enrolled in a large, state-funded program, Improving Access, Counseling, and Treatment for Californians With Prostate Cancer (IMPACT). IMPACT enrollees’ HRQOL scores are significantly worse at baseline in all domains of the 12-Item Short Form Survey from the RAND Medical Outcomes Study than men in the general population. Subsequently, sexual bother worsens significantly but bowel domains improve.42,43 Nurse case management appears to improve patients’ HRQOL.44 Enrollees with low self-efficacy fare worse over a range of psychosocial outcomes and both general and disease-specific HRQOL.45
Conclusions
Physicians interacting with prostate cancer patients should advise them that treatment is unlikely to affect general HRQOL, but it may be associated with clinically significant changes in sexual, urinary, or bowel function. Treatment decisions should continue to be individualized. Any survival gains from surgery or radiation must be balanced with expected decrements in some areas of function and bother. With richer information on HRQOL, in addition to duration of survival, patients will be able to make better informed decisions and feel more comfortable proceeding with therapy or observation for localized prostate cancer.
References
- Herr HW. Strategies for the management of recurrent and advanced urologic cancers. Quality of life. Cancer. 1987;60(3 Suppl):623–630.
- Chapple A, Ziebland S, Herxheimer A, et al. Is “watchful waiting” a real choice for men with prostate cancer? A qualitative study. BJU Int. 2002;90(3):257–264.
- O’Rourke ME, Germino BB. Prostate cancer treatment decisions: a focus group exploration. Oncol Nurs Forum. 1998;25(1):97–104.
- Maliski SL, Kwan L, Elashoff D, et al. Symptom clusters related to treatment for prostate cancer. Oncol Nurs Forum. 2008;35(5):786–793.
- Berry DL, Ellis WJ, Woods NF, et al. Treatment decision-making by men with localized prostate cancer: the influence of personal factors. Urol Oncol. 2003;21(2):93–100.
- Denberg TD, Melhado TV, Steiner JF. Patient treatment preferences in localized prostate carcinoma: the influence of emotion, misconception, and anecdote. Cancer. 2006;107(3):620–630.
- O’Rourke ME. Narrowing the options: the process of deciding on prostate cancer treatment. Cancer Invest. 1999;17(5):349–359.
- Steginga SK, Occhipinti S. The application of the heuristic-systematic processing model to treatment decision making about prostate cancer. Med Decis Making. 2004;24(6):573–583.
- Navon L, Morag A. Advanced prostate cancer patients’ ways of coping with the hormonal therapy’s effect on body, sexuality, and spousal ties. Qual Health Res. 2003;13(10):1378–1392.
- Maliski SL, Rivera S, Connor S, et al. Renegotiating masculine identity after prostate cancer treatment. Qual Health Res. 2008;18(12):1609–1620.
- Litwin MS, Lubeck DP, Spitalny GM, et al. Mental health in men treated for early stage prostate carcinoma: a posttreatment, longitudinal quality of life analysis from the Cancer of the Prostate Strategic Urologic Research Endeavor. Cancer. 2002;95(1):54–60.
- el-Geneidy M, Garzotto M, Panagiotou I, et al. Delayed therapy with curative intent in a contemporary prostate cancer watchful-waiting cohort. BJU Int. 2004;93(4):510–515.
- Latini DM, Hart SL, Knight SJ, et al. The relationship between anxiety and time to treatment for patients with prostate cancer on surveillance. J Urol. 2007;178(3 Pt 1):826–831; discussion 831–832.
- Patel MI, DeConcini DT, Lopez-Corona E, et al. An analysis of men with clinically localized prostate cancer who deferred definitive therapy. J Urol. 2004;171(4):1520–1524.
- Oliffe JL, Davison BJ, Pickles T, et al. The self-management of uncertainty among men undertaking active surveillance for low-risk prostate cancer. Qual Health Res. 2009;19(4):432–443.
- Bailey DE Jr, Wallace M, Mishel MH. Watching, waiting and uncertainty in prostate cancer. J Clin Nurs. 2007;16(4):734–741.
- Kronenwetter C, Weidner G, Pettengill E, et al. A qualitative analysis of interviews of men with early stage prostate cancer: the Prostate Cancer Lifestyle Trial. Cancer Nurs. 2005;28(2):99–107.
- Pickles T, Ruether JD, Weir L, et al. Psychosocial barriers to active surveillance for the management of early prostate cancer and a strategy for increased acceptance. BJU Int. 2007;100(3):544–551.
- Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347(11):790–796.
- Bill-Axelson A, Holmberg L, Filén F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian Prostate Cancer Group-4 randomized trial. J Natl Cancer Inst. 2008;100(16):1144–1154.
- Johansson E, Bill-Axelson A, Holmberg L, et al. Time, symptom burden, androgen deprivation, and self-assessed quality of life after radical prostatectomy or watchful waiting: the randomized Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4) clinical trial. Eur Urol. 2009;55(2):422–430.
- Lubeck DP, Litwin MS, Henning JM, et al. The CaPSURE database: a methodology for clinical practice and research in prostate cancer. CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1996;48(5):773–777.
- Lubeck DP, Litwin MS, Henning JM, et al. Measurement of health-related quality of life in men with prostate cancer: the CaPSURE database. Qual Life Res. 1997;6(5):385–392.
- Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: lessons from the Cancer of the Prostate Strategic Urologic Research Endeavor (CapSURE), a national disease registry. J Urol. 2004;171(4):1393–1401.
- Porten SP, Cooperberg MR, Konety BR, et al. The example of CaPSURE: lessons learned from a national disease registry. World J Urol. 2011;29(3):265–271.
- Huang GJ, Sadetsky N, Penson DF. Health related quality of life for men treated for localized prostate cancer with long-term followup. J Urol. 2010;183(6):2206–2212.
- Wu AK, Cooperberg MR, Sadetsky N, et al. Health related quality of life in patients treated with multimodal therapy for prostate cancer. J Urol. 2008;180(6):2415–2422; discussion 2422.
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261.
- Fowler FJ Jr, Barry MJ, Lu-Yao G, et al. Effect of radical prostatectomy for prostate cancer on patient quality of life: results from a Medicare survey. Urology. 1995;45(6):1007–1013.
- Talcott JA, Rieker P, Clark JA, et al. Patient-reported symptoms after primary therapy for early prostate cancer: results of a prospective cohort study. J Clin Oncol. 1998;16(1):275–283.
- Talcott JA, Rieker P, Propert KJ, et al. Patient-reported impotence and incontinence after nerve-sparing radical prostatectomy. J Natl Cancer Inst. 1997;89(15):1117–1123.
- Litwin MS, Hays RD, Fink A, et al. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36(7):1002–1012.
- Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273(2):129–135.
- Wei JT, Dunn RL, Litwin MS, et al. Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905.
- Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283(3):354–360.
- Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128(3):492–497.
- Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2004;96(18):1358–1367.
- Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the Prostate Cancer Outcomes Study. J Urol. 2005;173(5):1701–1705.
- Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346(15):1138–1144.
- Gore JL, Kwan L, Lee SP, et al. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101(12):888–892.
- Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20(2):557–566.
- Brar R, Maliski SL, Kwan L, et al. Changes in quality of life among low-income men treated for prostate cancer. Urology. 2005;66(2):344–349.
- Krupski TL, Fink A, Kwan L, et al. Health-related quality-of-life in low-income, uninsured men with prostate cancer. J Health Care Poor Underserved. 2005;16(2):375–390.
- Zavala MW, Maliski SL, Kwan L, et al. Longitudinal quality of life in low-income men in a state-funded prostate cancer treatment program. J Health Care Poor Underserved. 2008;19(1):200–214.
- Maliski SL, Kwan L, Krupski T, et al. Confidence in the ability to communicate with physicians among low-income men with prostate cancer. Urology. 2004;64:329–334.
Economic Analysis of Different Management Strategies for Localized Prostate Cancer
Daniella J. Perlroth, M.D.
Community management of low-risk localized prostate cancer is characterized by a proliferation of treatment options. This may partly reflect the paucity of high-quality evidence to guide treatment selection for most patients.1 Conservative management with active surveillance is considered a reasonable and recommended initial management for many patients with localized prostate cancer.2,3 Despite this, conservative management is less frequently used than active treatments in current clinical practice. The frequency of initial treatments provided within 9 months of diagnosis for patients with localized prostate cancer is shown in Figure 1. This illustrates that the use of conservative management as initial treatment has not increased during the recent period (2003–2007), at approximately 20% of patients. Notably, the largest increase in use was for intensity-modulated radiation therapy, from 8% to 14% as single therapy, and 7% to 16% when used in combination with brachytherapy.
Figure 1. Use of Primary Treatment Options for Localized Prostate Cancer by Year of Diagnosis (2003–2007) in SEER-Medicare Data
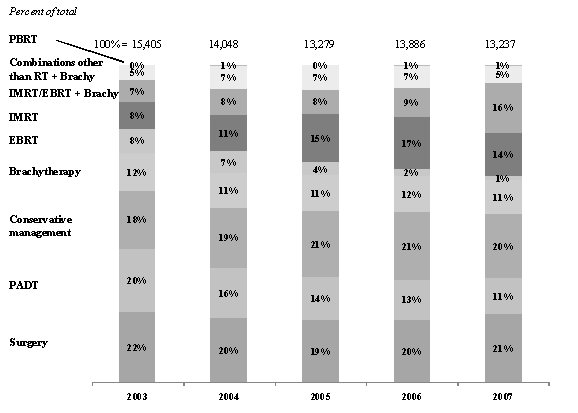
PBRT = proton beam radiation therapy; RT = radiation therapy; Brachy = brachytherapy; IMRT = intensity-modulated radiation therapy; EBRT = external beam radiation therapy; PADT = primary androgen deprivation therapy.
Initial treatment costs for prostate cancer were estimated to be $4.5 billion for 213,000 patients newly diagnosed in 2010, and forecast to increase to $6.02 billion for 283,000 patients by 2020.4 This would result in a total of $57.5 billion spent on initial treatment for prostate cancer over this decade. The majority of these expenditures will be for localized disease (80% to 90% of diagnosed patients), and most patients will have low- to intermediate-risk profiles (70% to 75%).5
The choice of initial management differs substantially in both treatment-related and long-term costs. Snyder et al. found wide variation in initial treatment costs for patients diagnosed in 2000 with localized prostate cancer based on Surveillance, Epidemiology and End Results (SEER) Medicare data, with initial (1-year) costs ranging from $4,270 (in 2000 U.S. dollars) for watchful waiting to $17,474 for radiation plus hormonal therapy; 5-year total costs were $9,130 for watchful waiting and $26,896 for hormonal therapy alone.6 The difference in costs for active treatments over watchful waiting dissipated by year 3 after diagnosis, except for patients receiving initial hormonal therapy whose costs remained elevated throughout the 5-year analytic period.
We performed a similar cost analysis for patients diagnosed with nonmetastatic prostate cancer between 1998 and 2006 based on analysis of a commercial database containing claims for 42 large employers (Ingenix in Eden Prairie, Minnesota).7 Initial treatment was assigned based on claims for treatment procedures present in the data within 6 months of diagnosis. Patients were required to have a new code for prostate cancer following a code for prostate biopsy, with a prior 1-year period devoid of prostate-cancer-related claims. Annual median costs by initial treatment groups incremental to conservative management were adjusted for comorbid conditions, age, year, region of diagnosis, preceding 12-month health expenditures, median household income, marital status, percent black population in patient’s three-digit ZIP Code, and a National Cancer Institute comorbidity index for prostate cancer.8 Median incremental annual costs of active treatments are reported in Table 1.
All active treatment groups cost more than conservative management, even over 5 years of analysis. The combined treatment group (the most frequent being radiation therapy plus brachytherapy) and intensity-modulated radiation therapy initial treatment incurred the highest initial cost; costs for primary androgen deprivation therapy remained consistently higher in all years following diagnosis than conservative management.
To compare long-term costs of initial treatment strategies, how long should the analytic period extend? A recent long-term prospective cohort study in the United States reported that the mean time to treatment for patients (mean age 72.7) initially managed with active surveillance was 3.9 years; at a mean follow-up of nearly 8 years, 49% had progressed to treatment.9 According to this study, most patients who will eventually be treated will do so within 5 years of diagnosis. Furthermore, both cost analyses described above find no difference in annual costs for initial active treatment compared with conservative management after the first several years from diagnosis (with the exception of primary androgen deprivation therapy), providing reassurance that a 5-year period of analysis is adequate for the goals of most cost estimation.
| Initial Treatment Group | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 |
|---|---|---|---|---|---|
| RP | 14,362 (13,295, 15,430)* |
–385 (–800, 30) |
–554 (–997, –112) |
15 (–750, 780) |
–89 (–1,237, 1,059) |
| Brachytherapy | 27,065 (25,522, 28,608)* |
–771 (–1,370, –172) |
–428 (–1,075, 217) |
–445 (–1,624, 733) |
1,008 (–890, 2,907) |
| EBRT | 17,869 (14,617, 21,210)* |
63 (–1,197, 1,325) |
–288 (–1,544, 968) |
–272 (2,273, 1,728) |
2,137 (–808, 5,084) |
| IMRT | 45,925 (43,154, 48,695)* |
–654 (–1,731, 422) |
362 (–1,006, 1,731) |
–810 (–3,901, 2,280) |
9,435 (1,662, 17,208) |
| Combined treatments† | 60,046 (57,167, 62,925)* |
799 (–337, 1,895) |
1,301 (54, 2,548)* |
1,752 (–566, 4,071) |
950 (–3,348, 5,250) |
| PADT | 35,450 (34,092, 36,869)* |
7,192 (6,652, 7,732)* |
3,398 (2,854, 3,942)* |
3,183 (2,282, 4,083)* |
3,173 (1,841, 4,505)* |
| Number | 5,512 | 5,512 | 3,611 | 2,519 | 1,708 |
| RP = radical prostatectomy; EBRT = external beam radiation therapy; IMRT = intensity-modulated radiation therapy; PADT = primary androgen deprivation therapy. *Statistically significant at the 5% level. †45% of patients in combined treatment group received radiation therapy plus brachytherapy. |
|||||
I modeled health expenditure savings based on the previous incremental cost estimates for treatment of a hypothetical cohort of patients diagnosed in 2010 with localized disease, assuming that active surveillance replaced current rates of treatment. Assuming equal cost across risk groups for localized disease, estimated savings from shifting patients with low risk, intermediate risk, and all risks of disease are reported in Table 2. Since local geographic treatment norms have been found to be the primary determinant of treatment choice, rather than disease or risk characteristics, the assumption of equal costs across risk groups may in fact be valid.10 This analysis concludes that 18% to 49% of 5-year treatment costs for localized disease could be saved should active surveillance become the initial management strategy of choice. Although this estimate may seem high, studies do report surprisingly high rates of initial active treatment (75%) for even very low-risk populations (prostate-specific antigen <4.0 ng/ml), where many are probably suitable for active surveillance.11 These estimates suffer from limitations of observational analysis, particularly the influence of immeasurable factors on treatment selection.
| Scenarios | |||||
|---|---|---|---|---|---|
| Active Treatment Group | Base Case | Increase in Localized Disease | Increase in Rates of Active Treatment | ||
| IMRT | 260 490 690 | 275 515 730 | 350 665 940 | ||
| Brachytherapy | 135 250 355 | 140 265 375 | 150 280 395 | ||
| Combined treatments | 235 445 625 | 250 470 660 | 190 280 505 | ||
| PADT | 210 395 560 | 220 420 590 | 285 535 755 | ||
| Total | 840 1,580 2,225 | 885 1,670 2,355 | 975 1,840 2,595 | ||
| IMRT = intensity-modulated radiation therapy; PADT = primary deprivation therapy. *Assumes 219,000 new cases of prostate cancer with 75% below age 75. Base case scenario assumes 80% of diagnosed cases are localized disease and 68% are locally treated (excluding PADT), with IMRT accounting for 20% of active treatments. Increase in localized disease scenario assumes 90% of cases are localized disease. Increase in rates of active treatment assumes 75% are locally treated and IMRT accounts for 33% of those treatments. |
|||||
References
- Wilt TJ, MacDonald R, Rutks I, et al. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–448.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. 2011; Version 1. Available at: nccn.org. Accessed August 10, 2011.
- Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304(21):2373–2380.
- Mariotto AB, Yabroff K, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128.
- Shao YH, Demissie K, Shih W, et al. Contemporary risk profile of prostate cancer in the United States. J Natl Cancer Inst. 2009;101(18):1280–1283.
- Snyder CF, Frick KD, Blackford AL, et al. How does initial treatment choice affect short-term and long-term costs for clinically localized prostate cancer? Cancer. 2010;116(23):5391–5399.
- Perlroth DJ, Goldman DP, Garber AM. The potential impact of comparative effectiveness research on U.S. health care expenditures. Demography. 2010;47 Suppl:S173–190.
- Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590.
- Shappley WV 3rd, Kenfield SA, Kasperzyk JL, et al. Prospective study of determinants and outcomes of deferred treatment or watchful waiting among men with prostate cancer in a nationwide cohort. J Clin Oncol. 2009;27(30):4980–4985.
- Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28(7):1117–1123.
- Shao YH, Albertsen PC, Roberts CB, et al. Risk profiles and treatment patterns among men diagnosed as having prostate cancer and a prostate-specific antigen level below 4.0 ng/ml. Arch Intern Med. 2010;170(14):1256–1261.
Evidence-based Practice Center Presentation III: Comparative Effectiveness of Active Surveillance Versus Radical Prostatectomy or Radiation Therapy in Men With Localized Prostate Cancer
Mei Chung, Ph.D., M.P.H.; Winifred W. Yu, Ph.D., M.S.; Stanley Ip, M.D.; Issa Dahabreh, M.D., M.S.; Ethan M. Balk, M.D., M.P.H.; Joseph Lau, M.D.
Introduction
The comparative effectiveness of active surveillance versus either immediate radical prostatectomy or radiation therapy for reducing morbidity and mortality among men with localized prostate cancer is not known. For this review, active surveillance was characterized as an observational management strategy with patients receiving deferred curative treatment based on cancer progression.
Objectives
The objectives were to review primary studies and selected relevant existing systematic reviews, evidence reports, and technology assessments that compared the effectiveness of active surveillance versus radical prostatectomy or radiation therapy in terms of clinical outcomes and costs (Key Question 4).
Review methods
We searched MEDLINE® and the Cochrane Database of Systematic Reviews for relevant English-language publications, from inception through August 2011. We also relied on two previously completed Agency for Healthcare Research and Quality (AHRQ) evidence reports on treatments for localized prostate cancer1,2 and two economic evaluations of these treatments prepared by the Institute for Clinical and Economic Evaluation.3,4 We supplemented the evidence summarized in these documents with comparative studies performed in a multicenter setting and with studies analyzing databases sourced from the U.S. population. The population of interest was men with clinically localized prostate cancer (T1 or T2), without known lymph nodes (N0-X) or metastases (M0-X). No more than 20% of the study sample could have more advanced-stage disease. For observational studies exclusively including patients with T1 or T2 disease, we required statistical adjustment for age. For studies also including patients with non-T1 or non-T2 disease (up to 20% of the total population), we also required adjustment for disease severity (e.g., stage or Gleason score). Studies had to compare observational management strategies (without androgen deprivation therapy) to active treatment, including radical prostatectomy, external beam radiation therapy, or brachytherapy, all with or without androgen deprivation therapy. Androgen deprivation therapy monotherapy was not considered an active treatment. Outcomes of interest included prostate cancer mortality, all-cause mortality, morbidity of primary treatment, metastatic disease, quality of life, satisfaction with treatment, and costs. We assessed the studies’ methodological quality and rated the strength of evidence regarding active surveillance versus active treatment using established AHRQ Evidence-based Practice Center methods.
Results
No study reported clinical outcomes specifically for active surveillance management strategies versus immediate definitive treatment. Therefore, the strength of evidence is insufficient regarding the comparative effectiveness of active surveillance versus immediate definitive treatment for men with localized prostate cancer.
Due to the lack of studies comparing active surveillance with immediate treatment, we evaluated studies that compared other observational management strategies (largely resembling watchful waiting) with immediate treatment.
Observational Management Strategies Versus Radical Prostatectomy
Two randomized controlled trials (RCTs) that were included in previous systematic reviews compared observational management strategies with radical prostatectomy: the Scandinavian Prostate Cancer Group Study 4 (SPCG-4)5 and the Veterans Administration Cooperative Urological Research Group (VACURG)6 trials. These trials enrolled mostly patients with prostate cancer diagnosed in the pre-prostate-specific antigen screening era. We did not identify any new RCTs, but we found two publications reporting recent data on clinical outcomes7 and treatment costs8 from the SPCG-4 trial. We also identified nine additional observational studies.9–17
Generally, the results from observational studies indicated that men treated with radical prostatectomy had lower all-cause and prostate-cancer-specific mortality rates than men on watchful waiting. These findings are consistent with the latest update from the SPCG-4 trial, which followed 695 men with localized prostate cancer for a median of 12.8 years.9–12 However, the VACURG trial followed 142 patients for a median of 23 years and found no difference in mortality between the watchful waiting and radical prostatectomy groups.6 The development of metastatic disease was assessed only by the SPCG-4 trial, which found a significant benefit for radical prostatectomy compared with watchful waiting.7 Morbidity of primary treatment was reported by two observational studies that suggested an increased risk for urethral stricture (and procedures to treat it) among patients treated with radical prostatectomy versus those managed with watchful waiting.13,14 The findings for quality of life reported in two observational studies varied across different domains of the quality-of-life measure.15,17 One study reported that the percentage of patients satisfied with treatment were similar for the watchful waiting and radical prostatectomy groups.16
Observational Management Strategies Versus Radiation Therapy
No RCTs comparing observational management strategies with radiation therapy were identified. For this comparison, we relied on an AHRQ report (which included nine observational studies),2 and seven additional observational studies identified through our update search.11,12,14–18 It should be noted that studies in the evidence report may have included some men treated with androgen deprivation therapy in the observational management arms, which would have been excluded based on this review’s eligibility criteria. Two of the seven observational studies in our update reported that men treated with radiation therapy had lower all-cause mortality rates than men on watchful waiting.11,12 One study reported prostate-cancer-specific mortality information and did not find a statistically significant difference between radiation therapy and observational management.12 No study reported on treatment comparisons for the development of metastatic disease. One study did not find a significant difference in treatment-related morbidity between observational management and brachytherapy or external beam radiation therapy.14 The findings for quality of life reported in three studies varied across different domains of the quality-of-life measure.15,17,18 One study reported that the proportion of patients satisfied with treatment was lower in the watchful waiting group than in the radiation therapy group.16
Observational Management Strategies Versus Combined Treatment Modalities or Active Treatments Considered in Aggregate
One observational study in our update reported that patients who received active treatment (radical prostatectomy, radiation therapy, and/or brachytherapy considered together) had significantly lower risks of all-cause and prostate-cancer-specific mortality compared with those on observation.11 Morbidity of primary treatment was reported by only one study, which found a higher rate of receiving treatments for urethral stricture in men treated with both external beam radiation therapy and brachytherapy compared with those on watchful waiting.14 No study reported incidence of metastatic disease or quality-of-life outcomes for this comparison.
Costs
We did not identify any primary study comparing the cost of active surveillance with active treatment strategies; economic modeling using U.S. prices suggests that active surveillance may be associated with higher costs compared with radical prostatectomy or brachytherapy, but lower costs compared with intensity-modulated radiation therapy or proton-beam radiation therapy.19
Two studies using observational data from the United States and one based on a subgroup of patients enrolled in the SPCG-4 trial reported on cost comparisons between observational management strategies (watchful waiting) and immediate active treatment for localized prostate cancer. Short- and long-term costs were higher for active treatment strategies (radical prostatectomy or radiation therapy) compared with watchful waiting; however, studies were small and used heterogeneous measurement methods.
Conclusions
No published studies have compared active surveillance (monitoring with the intent of curative intervention upon disease progression) versus immediate active treatment. Thus, the strength of evidence is insufficient regarding the comparative effectiveness of active surveillance versus immediate definitive treatment for men with localized prostate cancer. Randomized and observational studies suggest that active treatment may be more effective than watchful waiting for reducing overall and prostate-cancer-specific mortality. However, we caution that confounding bias is likely in observational studies of treatment effectiveness because patients managed with observational strategies and those who received active treatments differ in many characteristics that are also associated with the outcomes of interest. Ongoing clinical trials are expected to provide information on the comparative effectiveness of active surveillance compared with immediate active treatment, but they will require long-term follow-up.
References
- Wilt TJ, Shamliyan T, Taylor B, et al. Comparative effectiveness of therapies for clinically localized prostate cancer. AHRQ Comparative Effectiveness Reviews. Report No. 08-EHC010-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2008.
- Ip S, Dvorak T, Yu WW, et al. Comparative Evaluation of Radiation Treatments for Clinically Localized Prostate Cancer: An Update. Rockville, MD: Agency for Healthcare Research and Quality, Technology Assessment Program; August 13, 2010. Available at: cms.gov/mcd/viewtechassess.asp?where=index&tid=69. Accessed August 31, 2011.
- Ollendorf DA, Hayes J, McMahon P, et al. Management Options for Low-Risk Prostate Cancer: A Report on Comparative Effectiveness and Value. Boston, MA: Institute for Clinical and Economic Review; 2009.
- Ollendorf DA, Hayes J, McMahon P, et al. Active surveillance and radical prostatectomy for the management of low-risk, clinically-localized prostate cancer. Boston, MA: Institute for Clinical and Economic Review; September 11, 2009.
- Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2005;352(19):1977–1984.
- Iversen P, Madsen PO, Corle DK. Radical prostatectomy versus expectant treatment for early carcinoma of the prostate. Twenty-three year follow-up of a prospective randomized study. Scand J Urol Nephrol Suppl. 1995;172:65–72.
- Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364(18):1708–1717.
- Andersson SO, Andren O, Lyth J, et al. Managing localized prostate cancer by radical prostatectomy or watchful waiting: cost analysis of a randomized trial (SPCG-4). Scand J Urol Nephrol. 2011;45(3):177–183.
- Schymura MJ, Kahn AR, German RR, et al. Factors associated with initial treatment and survival for clinically localized prostate cancer: results from the CDC-NPCR Patterns of Care Study (PoC1). BMC Cancer. 2010;10:152.
- Hadley J, Yabroff KR, Barrett MJ, et al. Comparative effectiveness of prostate cancer treatments: evaluating statistical adjustments for confounding in observational data. J Natl Cancer Inst. 2010;102(23):1780–1793.
- Wong YN, Mitra N, Hudes G, et al. Survival associated with treatment vs observation of localized prostate cancer in elderly men. JAMA. 2006;296(22):2683–2693.
- Stattin P, Holmberg E, Johansson JE, et al. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst. 2010;102(13):950–958.
- Berge V, Thompson T, Blackman D. Additional surgical intervention after radical prostatectomy, radiation therapy, androgen-deprivation therapy, or watchful waiting. Eur Urol. 2007;52(4):1036–1043.
- Elliott SP, Meng MV, Elkin EP, et al. Incidence of urethral stricture after primary treatment for prostate cancer: data from CaPSURE. J Urol. 2007;178(2):529–534.
- Litwin MS, Lubeck DP, Spitalny GM, et al. Mental health in men treated for early stage prostate carcinoma: a posttreatment, longitudinal quality of life analysis from the Cancer of the Prostate Strategic Urologic Research Endeavor. Cancer. 2002;95(1):54–60.
- Hoffman RM, Hunt WC, Gilliland FD, et al. Patient satisfaction with treatment decisions for clinically localized prostate carcinoma. Results from the Prostate Cancer Outcomes Study. Cancer. 2003;97(7):1653–1662.
- Schapira MM, Lawrence WF, Katz DA, et al. Effect of treatment on quality of life among men with clinically localized prostate cancer. Med Care. 2001;39(3):243–253.
- Thong MS, Mols F, Kil PJ, et al. Prostate cancer survivors who would be eligible for active surveillance but were either treated with radiotherapy or managed expectantly: comparisons on long-term quality of life and symptom burden. BJU Int. 2010;105(5):652–658.
- Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304(21):2373–2380.
| Learn more about the NIH Consensus Development Program | ||
| Join the Consensus Development Program Information Network | ||
 |
Contact us with any questions or comments | |
| Elizabeth Neilson, Senior Advisor | ||
| neilsone@mail.nih.gov | 301-496-4999 | ||
Contact Consensus Program |
Privacy Notice |
Disclaimer |
Accessibility |
Contact NIH
![]()
![]()
![]()
![]()