FY 2012 President's Budget Request
DEPARTMENT OF HEALTH AND HUMAN SERVICES
NATIONAL INSTITUTES OF HEALTH
National Institute of Diabetes and Digestive and Kidney Diseases
FY 2012 Budget
Print Version (PDF 465 KB, 28 pages)
NATIONAL INSTITUTES OF HEALTH
National Institute of Diabetes and Digestive and Kidney Diseases
For carrying out section 301 and title IV of the Public Health Services Act with respect to diabetes and digestive and kidney diseases $1,837,957,000.
Amounts Available for Obligation 1/
(Dollars in thousands)| Source of Funding | FY 2010 Actual | FY 2011 CR | FY 2012 PB |
|---|
| Appropriation | 1,808,100 | 1,808,100 | 1,837,957 |
| Type 1 Diabetes 2/ | 150,000 | 150,000 | 150,000 |
| Subtotal, adjusted appropriation | 1,958,100 | 1,958,100 | 1,987,957 |
| Real transfer under Director's one-percent transfer authority (GEI) | 1,160 | 0 | 0 |
| Real transfer under Secretary's one-percent transfer authority | (270) | 0 | 0 |
| Comparative Transfers to NLM for NCBI and Public Access | (759) | (1,538) | 0 |
| Comparative transfer under Director's one-percent transfer authority (GEI) | (1,160) | 0 | 0 |
| Subtotal, adjusted budget authority | 1,957,071 | 1,956,562 | 1,987,957 |
| Unobligated balance lapsing | (85) | 0 | 0 |
| Total obligations | 1,956,986 | 1,956,562 | 1,987,957 |
1/ Excludes the following amounts for reimbursable activities carried out by this account (dollars in thousands):
FY 2010 - $5,363 FY 2011 - $10,000 FY 2012 - $10,000
Excludes $1,555 Actual in FY 2010; $3,000 Estimate in FY 2011 and FY 2012 for royalties.
2/ Type 1 Diabetes Special Statutory Authority in Accordance with P.L. 107-360 and P.L. 110-275.
Budget Mechanism - Total 1/
(Dollars in thousands)| MECHANISM | FY 2010 No. | FY 2010 Amount | FY 2011 CR No. | FY 2011 CR Amount | FY 2012 PB No. | FY 2012 PB Amount | Change vs. FY 2010 No. | Change vs. FY 2010 Amount |
|---|
| Research Grants | | | | | | | | |
| Research Projects Noncompeting | 2,083 | $851,634 | 2,177 | $878,109 | 2,073 | $894,682 | (10) | $43,048 |
| Research Projects Administrative Supplements | 106 | 20,695 | 106 | 20,695 | 62 | 17,355 | (44) | (3,340) |
| Competing Renewal | 279 | 140,660 | 257 | 133,955 | 248 | 132,490 | (31) | (8,170) |
| Competing New | 505 | 192,822 | 469 | 184,985 | 461 | 182,963 | (44) | (9,859) |
| Competing Supplements | 2 | 108 | 0 | 0 | 0 | 0 | (2) | (108) |
| Subtotal, Competing | 786 | $333,590 | 726 | $318,940 | 709 | $315,453 | (77) | ($18,137) |
| Subtotal, RPGs | 2,869 | $1,205,919 | 2,903 | $1,217,744 | 2,782 | $1,227,490 | (87) | $21,571 |
| SBIR/STTR | 129 | $48,610 | 127 | $47,658 | 129 | $48,242 | 0 | ($368) |
| Research Project Grants | 2,998 | $1,254,529 | 3,030 | $1,265,402 | 2,911 | $1,275,732 | (87) | $21,203 |
| Research Centers | | | | | | | | |
| Specialized/Comprehensive | 89 | $99,670 | 89 | $99,670 | 89 | $100,657 | 0 | $987 |
| Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Medicine | 0 | 49 | 0 | 49 | 0 | 49 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers | 89 | $99,719 | 89 | $99,719 | 89 | $100,706 | 0 | $987 |
| Other Research | | | | | | | | |
| Research Careers | 507 | $72,946 | 507 | $74,946 | 507 | $75,675 | 0 | $2,729 |
| Cancer Education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative Clinical Research | 0 | 4,932 | 0 | 4,932 | 0 | 4,981 | 0 | 49 |
| Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 96 | 40,486 | 96 | 41,486 | 96 | 41,891 | 0 | 1,405 |
| Other Research | 603 | $118,364 | 603 | $121,364 | 603 | $122,547 | 0 | $4,183 |
| Total Research Grants | 3,690 | $1,472,612 | 3,722 | $1,486,485 | 3,603 | $1,498,985 | (87) | $26,373 |
| Research Training | FTTPs | | FTTPs | | FTTPs | | | |
| Individual Awards | 252 | $10,859 | 252 | $11,022 | 252 | $11,354 | 0 | $495 |
| Institutional Awards | 917 | 46,848 | 917 | 47,589 | 917 | 49,054 | 0 | 2,206 |
| Total Research Training | 1,169 | $57,707 | 1,169 | $58,611 | 1,169 | $60,408 | 0 | $2,701 |
| Research and Development Contracts | 217 | $180,959 | 218 | $161,816 | 218 | $178,436 | 1 | ($2,523) |
| (SBIR/STTR) | 2 | $94 | 2 | $94 | 2 | $94 | 0 | $0 |
| | FTEs | | FTEs | | FTEs | | FTEs | |
| Intramural Research | 362 | $179,761 | 364 | $181,968 | 364 | $181,769 | 2 | $2,008 |
| Research Management and Support | 263 | 66,032 | 263 | 67,682 | 263 | 68,359 | 0 | 2,327 |
| Total, NIDDK | 625 | $1,957,071 | 627 | $1,956,562 | 627 | $1,987,957 | 2 | $30,886 |
1/ All items in italics are "non-adds"; items in parentheses are subtractions
Budget Mechanism - Type 1 Diabetes Only 1
(Dollars in thousands)| MECHANISM | FY 2010 Actual No. | FY 2010 Actual Amount | FY 2011 CR No. | FY 2011 CR Amount | FY 2012 PB No. | FY 2012 PB Amount | Change vs. FY 2010 No. | Change vs. FY 2010 Amount |
|---|
| Research Grants | | | | | | | | |
| Research Projects Noncompeting | 39 | $34,638 | 42 | $37,621 | 33 | $36,795 | (6) | $2,157 |
| Research Projects Administrative Supplements | 2 | 6,865 | 2 | 6,865 | 2 | 6,131 | 0 | (734) |
| Competing Renewal | 3 | 3,922 | 4 | 5,229 | 4 | 5,531 | 1 | 1,609 |
| Competing New | 16 | 14,995 | 22 | 20,619 | 23 | 21,811 | 7 | 6,816 |
| Competing Supplements | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal, Competing | 19 | $18,917 | 26 | $25,848 | 27 | $27,342 | 8 | $8,425 |
| Subtotal, RPGs | 58 | $60,420 | 68 | $70,334 | 60 | $70,268 | 2 | $9,848 |
| SBIR/STTR | 9 | $5,218 | 7 | $4,167 | 7 | $4,167 | (2) | ($1,051) |
| Research Project Grants | 67 | $65,638 | 75 | $74,501 | 67 | $74,435 | 0 | $8,797 |
| Research Centers | | | | | | | | |
| Specialized/Comprehensive | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Clinical Research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative Medicine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Other Research | | | | | | | | |
| Research Careers | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Cancer Education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative Clinical Research | 0 | 4,932 | 0 | 4,932 | 0 | 4,981 | 0 | 49 |
| Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority Biomedical Research Support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 0 | 874 | 0 | 874 | 0 | 883 | 0 | 9 |
| Other Research | 0 | $5,806 | 0 | $5,806 | 0 | $5,864 | 0 | $58 |
| Total Research Grants | 67 | $71,444 | 75 | $80,307 | 67 | $80,299 | 0 | $8,855 |
| Research Training | FTTPs | | FTTPs | | FTTPs | | | |
| Individual Awards | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Institutional Awards | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total Research Training | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Research and Development Contracts | 9 | $77,777 | 8 | $68,895 | 8 | $68,895 | (1) | ($8,882) |
| (SBIR/STTR) | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| | FTEs | | FTEs | | FTEs | | FTEs | |
| Intramural Research | 0 | $0 | 0 | $0 | 0 | $0 | 0 | $0 |
| Research Management and Support | 0 | 779 | 0 | 798 | 0 | 806 | 0 | 27 |
| Total, NIDDK | 0 | $150,000 | 0 | $150,000 | 0 | $150,000 | 0 | $0 |
1/ All items in italics are "non-adds"; items in parentheses are subtractions
Major Changes in the Fiscal Year 2012 Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2012 budget request for NIDDK, which is $30.886 million more than the FY 2010 level, for a total of $1,987.957 million.
Research Project Grants (RPGs; +$21.203 million; total $1,275.732 million): NIDDK will continue to maintain an adequate number to support competing RPGs—709 awards in FY 2012, a decrease of 77 from FY 2010. About 2,073 noncompeting RPGs, totaling $912.037 million will also be made in FY 2012.
CER Study Toward Establishing Personalized Therapy for Type 2 Diabetes (+$22.681 million; total $23 million): A U01 cooperative agreement currently in the U34 planning grant stage would support a new, multicenter study of the comparative effectiveness of five common drugs used for therapy of type 2 diabetes, including a comparison of more aggressive initial therapy using two drugs started at once versus sequential therapy. This U01 could be funded in FY 2012.
Treatment Options for type 2 Diabetes in Adolescents and Youth (TODAY) Study (-$16.2 million; total $1.2 million): This nationwide research study, comparing more aggressive therapy to standard therapy for type 2 diabetes in youth, is ending its randomized clinical trial phase; any follow-up would be done as part of a new study.
Follow-up to Hyperglycemia Adverse Pregnancy Outcomes (HAPO) Study (+$4.715 million; total $5.0 million): An additional U01 may be funded in FY 2012 for this initiative following children born to mothers who participated in the HAPO Study.
Lifestyle Interventions in Overweight and Obese Pregnant Women Consortium (+$2.0 million; total $2.0 million): This new initiative will support studies testing behavioral or lifestyle interventions in overweight and obese pregnant women, in order to improve weight and metabolic outcomes in both the women and their offspring.
Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) Consortium (-$0.381 million; total $2.0 million): This initiative will continue to support additional clinical, epidemiological, and behavioral studies of adolescent bariatric surgery.
The Frequent Hemodialysis Network (-$1.67 million): The data analysis phase of this Network is ending in FY 2012.
Complementary and Alternative Medicine for Urological Symptoms (CAMUS) (-$0.6 million): The data analysis phase of this multi-center clinical trial, which compares effects of two plant-based therapies, an active drug, and placebo on progression of benign prostatic hyperplasia, is ending in FY 2012.
Summary of Changes
(Dollars in thousands)
FY 2010 Actual: $1,957,071
FY 2012 Estimate: 1,987,957
Net Change: $30,886
| CHANGES | 2012 Estimate FTEs | 2012 Estimate Budget Authority | Change from FY 2010 FTEs | Change from FY 2010 Budget Authority |
|---|
| A. Built-in: | | | | |
| 1. Intramural Research: | | | | |
| a. Annualization of January 2010 pay increase | | $69,527 | | $417 |
| b. January FY 2012 pay increase | | 69,527 | | 0 |
| c. One less day of pay (n/a for 2011) | | 69,527 | | (268) |
| d. Payment for centrally furnished services | | 28,798 | | 285 |
| e. Increased cost of laboratory supplies, materials, and other expenses | | 83,444 | | 836 |
| Subtotal | | | | $1,270 |
| 2. Research Management and Support: | | | | |
| a. Annualization of January 2010 pay increase | | $33,844 | | $204 |
| b. January FY 2012 pay increase | | 33,844 | | 0 |
| c. One less day of pay (n/a for 2011) | | 33,844 | | (131) |
| d. Payment for centrally furnished services | | 3,807 | | 38 |
| e. Increased cost of laboratory supplies, materials, and other expenses | | 30,708 | | 299 |
| Subtotal | | | | $410 |
| Subtotal, Built-in | | | | $1,680 |
Summary of Changes... continued| CHANGES | 2012 Estimate Base No. | 2012 Estimate Base Amount | Change from FY 2010 No. | Change from FY 2010 Amount |
|---|
| B. Program: | | | | |
| 1. Research Project Grants: | | | | |
| a. Noncompeting | 2,073 | $912,037 | (10) | $39,708 |
| b. Competing | 709 | 315,453 | (77) | (18,137) |
| c. SBIR/STTR | 129 | 48,242 | 0 | (368) |
| Total | 2,911 | $1,275,732 | (87) | $21,203 |
| 2. Research Centers | 89 | $100,706 | 0 | $987 |
| 3. Other Research | 603 | 122,547 | 0 | 4,183 |
| 4. Research Training | 1,169 | 60,408 | 0 | 2,701 |
| 5. Research and development contracts | 218 | 178,436 | 1 | (2,523) |
| Subtotal, Extramural | | $1,737,829 | | $26,551 |
| | FTEs | | FTEs | |
| 6. Intramural Research | 364 | $181,769 | 2 | $738 |
| 7. Research Management and Support | 263 | 68,359 | 0 | 1,917 |
| Subtotal, program | 627 | $1,987,957 | | $29,206 |
| Total changes | | | 2 | $30,886 |
History of FTEs and Budget Authority: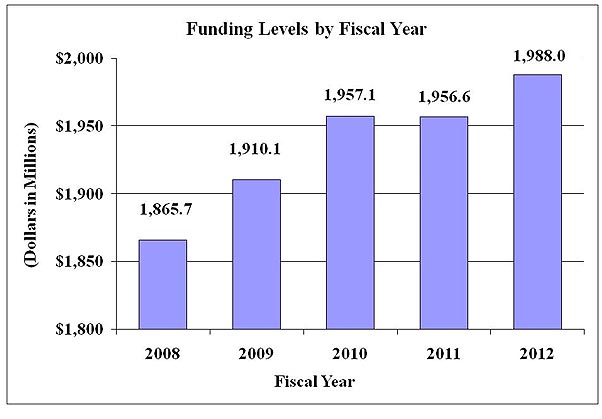
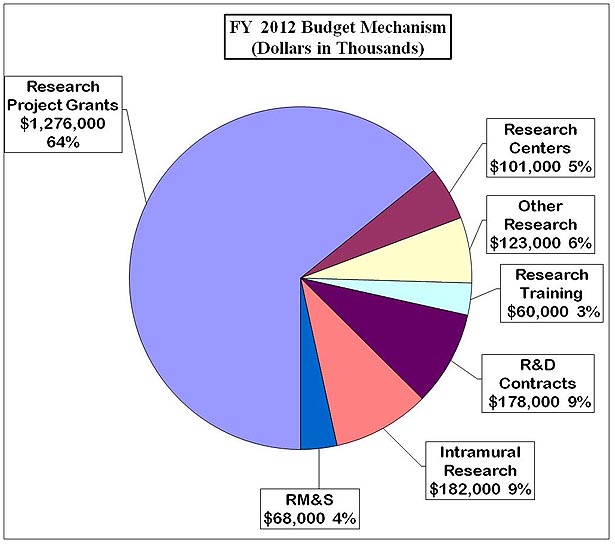
 Distribution by Mechanism
Distribution by Mechanism:
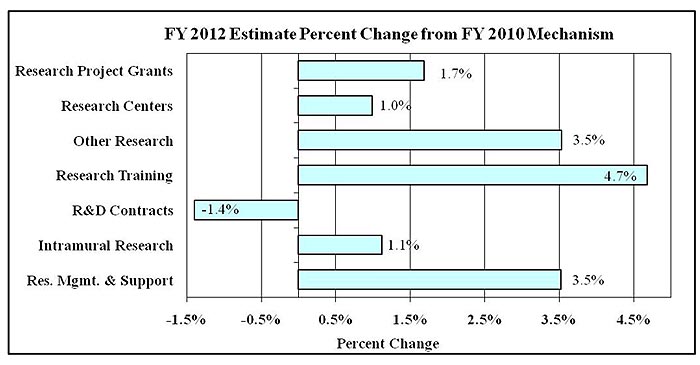
 Percent Change by Mechanism
Percent Change by Mechanism:
Budget Authority by Activity
(Dollars in thousands)| Detail: Extramural Research | FY 2010 Actual 1/ FTEs | FY 2010 Actual 1/ Amount | FY 2011 CR FTEs | FY 2011 CR Amount | FY 2012 PB FTEs | FY 2012 PB Amount | Change vs. FY 2010 FTEs | Change vs. FY 2010 Amount |
|---|
| Diabetes, Endocrinology, and Metabolic Diseases | | $640,444 | | $638,661 | | $651,340 | | $10,896 |
| Digestive Diseases and Nutrition | | 499,858 | | 498,467 | | 508,363 | | 8,505 |
| Kidney, Urologic, and Hematologic Diseases | | 421,755 | | 420,582 | | 428,932 | | 7,177 |
| Type 1 Diabetes | | 150,000 | | 150,000 | | 150,000 | | 0 |
| Subtotal, Extramural | | $1,712,057 | | $1,707,710 | | $1,738,635 | | $26,578 |
| Intramural Research | 362 | $179,761 | 364 | $181,968 | 364 | $181,769 | 2 | $2,008 |
| Research Management & Support 2/ | 263 | $65,253 | 263 | $66,884 | 263 | $67,553 | 0 | $2,300 |
| TOTAL 3/ | 625 | $1,957,071 | 627 | $1,956,562 | 627 | $1,987,957 | 2 | $30,886 |
1/ Includes Real Transfers and Comparable Adjustments as detailed in the "Amounts Available for Obligation" table.
2/ Research Management & Support excludes Type 1 Diabetes.
3/ Includes FTEs which are reimbursed from the NIH Common Fund for Medical Research.

Appropriations History 1/| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation |
|---|
| 2003 | $1,706,292,000 | $1,704,647,000 | $1,737,347,000 | $1,733,347,000 |
| Rescission | | | | ($10,617,000) |
| 2004 | $1,820,007,000 | $1,820,007,000 | $1,833,007,000 | $1,832,457,000 |
| Rescission | | | | ($10,654,000) |
| 2005 | $1,876,196,000 | $1,876,196,000 | $1,889,100,000 | $1,877,696,000 |
| Rescission | | | | ($14,112,000) |
| 2006 | $1,872,146,000 | $1,872,146,000 | $1,917,919,000 | $1,872,146,000 |
| Rescission | | | | ($17,221,000) |
| 2007 | $1,844,298,000 | $1,844,298,000 | $1,857,753,000 | $1,855,868,000 |
| Rescission | | | | $0 |
| 2008 | $1,858,045,000 | $1,881,893,000 | $1,897,784,000 | $1,886,199,000 |
| Rescission | | | | ($30,331,000) |
| Supplemental | | | | $9,077,000 |
| 2009 | $1,858,487,000 | $1,767,071,000 | $1,755,881,000 | $1,911,338,000 |
| Rescission | | | | $0 |
| 2010 | $1,931,494,000 | $1,974,251,000 | $1,940,518,000 | $1,958,100,000 |
| Rescission | | | | $0 |
| 2011 | $2,007,589,000 | | $2,004,674,000 | |
| Rescission | | | | |
| 2012 | $1,987,957,000 | | | |
1/ Includes Type 1 Diabetes Special Statutory Authority Funds.
Justification of Budget Request
National Institute of Diabetes and Digestive and Kidney Diseases
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
Budget Authority (BA)| | FY 2010
Actual | FY 2011
Continuing
Resolution | FY 2012
Budget
Request | FY 2012 +/-
FY 2010 |
|---|
| BA | $1,957,071,000 | $1,956,562,000 | $1,987,957,000 | +$30,886,000 |
| FTEs | 625 | 627 | 627 | +2 |
| Type 1 Diabetes: | -$150,000,000 | -$150,000,000 | -$150,000,000 | |
| Labor/HHS: | $1,807,071,000 | $1,806,562,000 | $1,837,957,000 | |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director’s Overview
The mission of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) is to support and conduct research to combat diabetes and other endocrine and metabolic diseases, liver and other digestive diseases, nutritional disorders, obesity, and kidney, urologic and hematologic diseases. These diseases are chronic, common, costly, and consequential for patients, their families, and our Nation. Diabetes afflicts an estimated 25.6 million people in the U.S., greatly increasing the risk for many serious complications, such as heart disease and kidney failure.[1] Estimates of chronic kidney disease show that more than 23 million Americans are affected, and approximately 550,000 have irreversible kidney failure.[2] Many urologic diseases are also highly prevalent.[3] Digestive diseases account for an estimated 104.7 million visits to ambulatory care centers and 13.5 million hospitalizations per year.[4] Obesity affects approximately one-third of U.S. adults and about 17 percent of children and adolescents.[5] Obesity is a strong risk factor for type 2 diabetes, nonalcoholic steatohepatitis (NASH), and many other diseases. Cystic fibrosis and other genetic diseases within NIDDK’s purview are less widespread, but still devastating in their impacts. Building on emerging opportunities from past research investments, NIDDK will continue to pursue basic, clinical, and translational research; research training and career development; and healh information dissemination, with continued focus on preserving balance in its investigator-initiated research portfolio.
Obesity: Epidemic obesity is contributing to higher rates of type 2 diabetes, the leading cause of kidney failure. To address complex factors that promote overweight, NIDDK co-chairs the NIH Obesity Research Task Force, and continues to support a multidimensional research portfolio on obesity. Studies are uncovering new biologic pathways that regulate appetite and metabolism, with implications for future therapy, and finding promising treatments for obesity-related conditions like NASH, such as the antioxidant vitamin E.[6]
Type 1 Diabetes: This form of diabetes, which most often develops in childhood or adolescence, places a huge burden on children and their families, but research has led to marked improvements in health and longevity. For example, long-term survival of those with type 1 diabetes has improved dramatically in the last 30 years.[7] Research efforts supporting these improvements include a major clinical trial showing that intensive glucose control dramatically delays or prevents eye, nerve, kidney, and cardiovascular complications of type 1 diabetes, which led to a paradigm shift in the way the disease is controlled.[8] Because major forms of diabetes share many devastating complications, type 1 diabetes research also improves health outcomes of people with more common type 2 diabetes.
Kidney Disease: NIDDK is actively pursuing a range of research avenues on kidney disease, acute kidney injury, chronic kidney disease in children, hemodialysis, and other areas. For example, researchers are learning how to identify genetic markers that may predict which individuals are at higher risk of developing kidney disease, especially in African Americans.[9] Translating knowledge into practice, NIDDK is working with community health centers to improve screening and management practices, to enable better identification and treatment of people with chronic kidney disease before they develop kidney failure.[10]
Genetic Technologies: Recent technological advances in analyzing the human genome have aided the discovery of genetic risk factors for several diseases in the NIDDK mission, including type 1 diabetes, type 2 diabetes, kidney disease, inflammatory bowel diseases, celiac disease, and NASH.[11] NIDDK is capitalizing on these findings to accelerate development of new diagnostic, therapeutic, and preventive approaches through such efforts as an NIDDK signature program on genome-wide association studies, as well as leadership of two initiatives within the NIH Genes, Environment, and Health Initiative.
NIDDK-supported researchers have also utilized genetic technologies for pioneering studies of gut microbial effects on conditions such as obesity and digestive diseases. Recent findings have highlighted how interactions between gut bacteria and biological pathways in intestinal cells affect appetite and metabolism, as well as identified new interactions among gut viral populations, microbial communities, and the human host that may affect health and disease.[12] These studies inform larger endeavors like the NIH Human Microbiome Project.
Therapeutic Development: NIDDK is advancing the development of new and better treatments for many diseases. Building on basic research showing that chronic inflammation associated with obesity contributes to type 2 diabetes, heart disease, and the metabolic syndrome, a clinical trial has shown benefits of a generic anti-inflammatory drug as a treatment for type 2 diabetes.[13] Another program is moving novel therapeutics from pre-clinical development in animal models to clinical trials for therapy of type 1 diabetes. Recent studies have also tested a promising treatment for acute liver failure resulting from causes other than acetaminophen toxicity.[14]
Informing Health Care: NIDDK research enhances health care decision-making efforts by identifying cost-effective ways to take findings from intervention studies into real-world clinical practice and community settings. An ongoing trial of lifestyle intervention to prevent complications of type 2 diabetes has shown improved control of glucose, blood pressure, and lipids with less use of medications.[15] NIDDK also supports comparative effectiveness research, ranging from identifying the best procedures for treating obesity and type 2 diabetes with bariatric surgery, to comparing strategies for engaging providers and patients in efforts to delay or prevent type 2 diabetes in women with gestational diabetes. Chronic diseases of global importance benefit from knowledge gained through NIDDK programs, such as international diabetes research consortia and the Hepatitis B Research Network.
New Investigators: NIDDK seeks to foster a diverse biomedical research community through programs that promote innovative ideas and support new or early-stage investigators. In FY 2010, NIDDK funded 140 new investigators, exceeding its target. NIDDK programs are also helping to attract young physician scientists to study diabetes and obesity.
NIDDK also disseminates science-based health information through its Weight-control Information Network; National Diabetes Education Program, co-sponsored with CDC; National Kidney Disease Education Program; information clearinghouses; and other venues. NIDDK seeks external strategic planning and resource allocation advice from investigators, professional organizations, patient advocates, the public, NIDDK’s National Advisory Council, intra-/inter-agency coordinating bodies, ad hoc planning groups, and scientific meetings.
Overall IC Budget Policy: The FY 2012 request for NIDDK is $1,987.957 million, an increase of $30.886 million or 1.6 percent over the FY 2010 level. NIDDK is providing a 1 percent inflationary increase for non-competing and competing grants. In addition, NIDDK has targeted a portion of funds for competing research project grants to support high priority projects outside the payline, including awards to new and early-stage investigators. NIDDK also seeks to balance support for solicitations to the extramural community and funding for investigator-initiated projects. In FY 2012, NIDDK will support new investigators on R01 equivalent awards at success rates equal to those of established investigators submitting new R01 equivalent applications. NIH will provide an increase of four percent for stipends levels under the Ruth L. Kirschstein National Research Service Award training program to continue efforts to attain the stipend levels recommended by the National Academy of Sciences. This will build on the two percent increase in stipend levels for FY 2011. Stipend levels were largely flat for several years, and the requested increase will help to sustain the development of a highly qualified biomedical research workforce. Intramural Research and Research Management and Support receive increases to offset the effect of anticipated price inflation. NIDDK will continue to support research consistent with the NIH Director’s Four Initiatives. Funds are included in R&D contracts to reflect NIDDK’s share of NIH-wide funding required to support several trans-NIH initiatives, such as the Therapies for Rare and Neglected Diseases program, the Basic Behavioral and Social Sciences Opportunity Network (OppNet), and support for a new synchrotron at the Brookhaven National Laboratory. For example, each IC that will benefit from the new synchrotron will provide funding to total NIH’s commitment to support this new technology--$10 million.
Program Descriptions and Accomplishments
Diabetes, Endocrinology, and Metabolic Diseases: The goals of this program are to increase understanding of diabetes and other diseases and disorders of the endocrine system and metabolism, and to develop and test potential prevention and treatment strategies. This program supports basic, clinical, and translational research, as well as research training, in the areas of type 1 and type 2 diabetes, cystic fibrosis, and other endocrine and metabolic disorders; obesity, neuroendocrinology, and energy balance; and development, metabolism, and basic biology of endocrine and metabolic tissues. Knowledge from this research is communicated to patients, health professionals, and the public through the National Diabetes Information Clearinghouse and the National Diabetes Education Program. In 2010, the HEALTHY study showed that a middle school-based intervention lowered the obesity rate in students at high risk for type 2 diabetes, although its impact on the overall rate of obesity and overweight did not differ from control schools. The Beta Cell Biology Consortium (BCBC), now in its third funding cycle, is building on past research accomplishments to develop cell-based and regenerative therapies for diabetes. Additionally, NIDDK is supporting a planning grant for a new comparative effectiveness clinical trial testing different medications, in combination with the drug metformin, for type 2 diabetes treatment. This type of head-to-head comparison trial was identified in the Diabetes Research Strategic Plan and at a meeting of the Diabetes Mellitus Interagency Coordinating Committee as being important for informing clinical management of type 2 diabetes. A new T2D-GENES Consortium was launched to identify genes or gene regions conferring type 2 diabetes risk in multiple ethnic groups. New initiatives are fostering research on adult human brown adipose tissue and its potential contributions to energy use and obesity, as well as innovative projects addressing major challenges in type 1 diabetes research and/or therapy. Finally, using ARRA funds, NIDDK promoted expansion and acceleration of research on diabetes, endocrinology, and metabolic diseases by awarding grants totaling more than $105.6 million in FY 2010. These included additional funding for the BCBC, as well as funds supporting several research projects on topics such as diabetes and obesity treatment relating directly to the NIH Director’s Four Initiatives.
Budget Policy: The FY 2012 budget estimate for this program is $651.340 million, an increase of $10.896 million or 1.7 percent over the FY 2010 level. With FY 2012 resources, NIDDK will continue major diabetes clinical trials and encourage and support development of major new investigator-initiated clinical studies. FY 2012 funds will also support research capitalizing on new opportunities to identify diabetes risk genes in minority populations, to advance progress toward developing new therapeutic approaches, and to support comparative effectiveness research. NIDDK will also continue to fund translational research in FY 2012 and support health information dissemination activities to bring scientific discoveries in diabetes and obesity to real-world medical practice and other community settings. In FY 2012, NIDDK will continue an initiative encouraging collaborative, multidisciplinary research teams to work on complex biomedical problems in diabetes, endocrinology, and metabolic diseases. NIDDK will also continue funding for research centers to advance research relevant to diabetes and to cystic fibrosis and other genetic metabolic diseases. NIDDK plans for FY 2012 include capitalizing on new findings relevant to brown fat and gestational diabetes and pursuing other efforts as part of an overall balanced research program.
Program Portrait: Beta Cell Biology Consortium (BCBC)
| FY 2010 Level: | $ 3.0 million | | FY 2012 Level: | $14.8 million | | Change: | +$11.8 million |
The BCBC is an international group of researchers collaboratively studying how insulin-producing beta cells develop and function, with an ultimate goal of developing cell-based or regenerative therapies for replacing damaged or destroyed beta cells in people with type 1 diabetes or severe type 2 diabetes. Additionally, the BCBC is generating key research resources, such as mouse models and antibodies, which are being used by the Consortium and the broad scientific community. Research conducted by the successful BCBC has increased understanding of the developmental pathways required to produce a fully functioning pancreatic islet; the nature of stem/progenitor cells during normal pancreatic development and in the adult pancreatic islet; and the mechanisms of beta cell regeneration in the adult animal and human islet. The third funding cycle of the BCBC was launched in August 2010 to build on these new insights and discoveries. During BCBC 3.0, the Consortium will increase its focus on translational outcomes and also tackle scientific issues that are barriers toward developing new cell-based and regenerative therapies. Toward these goals, the BCBC is working to reconstruct human type 1 diabetes in the mouse to produce a better animal model in which to study this disease and evaluate potential cell-based therapies. The BCBC will also place a greater emphasis on studies of human cells and tissues to move new discoveries forward as quickly as possible. Efforts will be increased to generate beta cells from human embryonic stem cells, to increase the human beta cell mass, and to uncover the mechanism to reprogram human adult cells into beta cells. Furthering basic research on beta cells through the BCBC will enhance efforts to produce an abundant supply of beta cells for transplantation and/or efforts to promote the generation of new beta cells within the body, paving the way toward new cell-based or regenerative therapies for people with diabetes. |
Digestive Diseases and Nutrition: The goals of this program are to increase understanding of digestive diseases, nutrition, and obesity, and to develop and test strategies for disease prevention and treatment. This program supports basic, clinical, and translational research, as well as research training, encompassing fundamental studies of the digestive system; disease-targeted research involving the esophagus, stomach, small intestine, large intestine and anorectum, liver and biliary system, and pancreas; studies relevant to nutrition; and research on obesity. Insights gleaned from scientific efforts are communicated to patients, health professionals, and the public through NIDDK’s National Digestive Diseases Information Clearinghouse and Weight-control Information Network. In 2010, NIDDK expanded the Hepatitis B Research Network, which is finalizing protocols to test treatments for chronic hepatitis B infection in at-risk populations, such as Asian Americans and Pacific Islanders. NIDDK also merged and expanded existing consortia to form a network conducting studies on severe forms of childhood liver injury, including a new focus on liver disease associated with cystic fibrosis. To further advance research on adult-to-adult living donor transplantation, NIDDK established new clinical centers to continue a study of risks and benefits associated with this procedure. NIDDK also extended a clinical outcomes research initiative and the largest national database on gastrointestinal endoscopy. Additionally, NIDDK extended support for the Nonalcoholic Steatohepatitis Clinical Research Network to complete clinical trials in adults and children and continue collection of biospecimens and data. In a major new initiative, NIDDK funded projects of the Gastrointestinal Stem Cell Consortium to improve understanding of intestinal biology and function, as well as aid therapeutic development. Studies by the Acute Liver Failure Study Group have been key to developing new regulations regarding acetaminophen and showing the benefits of N-acetylcysteine for treating acute liver failure due to other causes. Finally, using ARRA funds, NIDDK promoted expansion and acceleration of research on digestive diseases, obesity, and nutrition by awarding grants totaling more than $70.9 million in FY 2010.
Budget Policy: The FY 2012 budget estimate for this program is $508.363 million, an increase of $8.505 million or 1.7 percent over the FY 2010 level. In FY 2012, NIDDK will continue major clinical research networks to help understand and treat liver diseases, including hepatitis B, drug-induced liver injury, and nonalcoholic steatohepatitis. NIDDK will support a network to study immunosuppression in pediatric liver transplant recipients. Among its obesity-related efforts in FY 2012, NIDDK will support major ongoing observational studies to assess the health risks and benefits of weight-loss surgery in extremely obese adults and adolescents, as well as an ongoing trial evaluating the long-term health effects of weight loss in obese adults with type 2 diabetes (Look AHEAD). NIDDK will also use FY 2012 funds to support Digestive Diseases Research Core Centers, and to sustain a consortium that is conducting cutting-edge genetic research on inflammatory bowel diseases (IBD). New research on intestinal stem cells that can benefit a variety of digestive diseases will continue in FY 2012, along with other efforts as part of an overall balanced research program.
Program Portrait: The Inflammatory Bowel Diseases (IBD) Genetics Consortium
| FY 2010 Level: | $3.6 million | | FY 2012 Level: | $3.6 million | | Change: | $0.0 million |
Inflammatory bowel diseases (IBD) are complex diseases that may arise from a combination of genetic and other factors, resulting in inappropriate immune responses to otherwise harmless gut microbes. The two major types of IBD are Crohn’s disease and ulcerative colitis, which can affect both adults and children. NIDDK’s IBD Genetics Consortium is a team of researchers based at several sites in the U.S. and Canada that uses methods such as genome-wide association studies to identify genetic risk factors that predispose individuals to developing IBD. Through collaborations among the Consortium and other international groups, more than 100 IBD susceptibility genes and genomic regions have been identified. The identification of these genetic risk factors provides insights into disease processes and diagnostic tests, as well as new therapeutic targets. In recent years, the Consortium has increased its efforts to recruit individuals from minority populations and those with defined subtypes of IBD, such as early-onset IBD in children, to yield informative data on disease development in these groups. Recently, the Consortium has identified additional genomic regions associated with adult ulcerative colitis and pediatric forms of IBD. Some of the IBD susceptibility genes identified by the Consortium code for molecules that are targets of drug therapies shown to effectively treat IBD, such as infliximab and rosiglitazone. The Consortium is continuing its search for genetic risk factors that contribute to IBD, in order to inform knowledge of disease development and enable the translation of these genetic discoveries into better treatments for IBD. |
Kidney, Urologic, and Hematologic Diseases: The goals of this program are to increase the understanding of diseases and disorders of the kidneys, urinary tract, and blood (hematologic), and to develop and test potential prevention and treatment strategies. Basic, clinical, and translational research, as well as research training, is supported in the areas of chronic kidney disease (CKD), diabetic kidney disease, end-stage renal disease (ESRD or kidney failure), polycystic kidney disease, and many other kidney diseases; urinary incontinence, benign prostatic hyperplasia, interstitial cystitis/painful bladder syndrome, stones, impotence, congenital urologic disorders, and urinary tract infections; and disorders of the blood and blood-forming organs, including sickle cell disease, Cooley's anemia, hemochromatosis, and the anemia of inflammation and of chronic disease. Science-based information is communicated to patients, health professionals, and the public through NIDDK’s National Kidney and Urologic Diseases Information Clearinghouse and National Kidney Disease Education Program (NKDEP). In 2010, NIDDK launched an initiative to support interdisciplinary teams studying causes of lower urinary tract symptoms. NIDDK also initiated a program to boost innovative basic and translational research on hematologic disease. Research efforts related to CKD included continuation of a CKD biomarker consortium, as well as a broader, large-scale epidemiologic study, to find new predictive markers for disease progression and associated cardiovascular, infectious, and gastrointestinal complications. Additionally, NIDDK continued a multi-center consortium to study how acute kidney injury leads to or worsens chronic kidney injury and initiated a study to understand why arteriovenous fistulas do not always develop to be functionally suitable for hemodialysis. NIDDK also held scientific meetings on genetic risk factors for kidney disease in African Americans, translation of CKD research into improved clinical outcomes, novel therapies to enhance end-stage renal disease patient survival, clinical trial design for acute kidney injury, and global studies of CKD. In addition, NIDDK will host a conference in FY2011 that focuses on new, innovative interventions that hold promise in slowing the inexorable progression of CKD to ESRD. The NKDEP also expanded its efforts to reduce the burden of CKD through collaborations with other federal agencies, particularly CMS and CDC. Finally, using ARRA funds, NIDDK promoted expansion and acceleration of research on kidney, urologic, and hematologic diseases, in many cases related directly to the NIH Director’s Four Initiatives, by awarding grants totaling more than $65.4 million in FY 2010.
Budget Policy: The FY 2012 budget estimate for this program is $428.932 million, an increase of $7.177 million or 1.7 percent over the FY 2010 level. In FY 2012, NIDDK will continue support for ongoing major clinical studies of CKD in adults and children and fund new research to identify and validate biomarkers and risk assessment tools for patients with this condition. NIDDK also plans to continue to sponsor planning grants to conduct translational research on the effectiveness of interventions shown in clinical trials to prevent, treat, and manage CKD. In FY 2012, NIDDK will continue treatment trials for polycystic kidney disease (HALT-PKD study) and continue support for the Consortium for Radiologic Imaging Studies of PKD; results of these studies will help to define measures of kidney disease progression. Centers focused on kidney, urologic, and hematologic research will receive continued funding, as will research on acute kidney injury and a study of arteriovenous fistulas. NIDDK will continue support for the Systolic Blood Pressure Intervention Trial (led by NHLBI) and for other efforts as part of an overall balanced research portfolio.
Program Portrait: Multi-disciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network
| FY 2010 Level: | $7.7 million | | FY 2012 Level: | $7.5 million | | Change: | -$0.2 million |
People with interstitial cystitis/painful bladder syndrome (IC/PBS) or chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) suffer from recurring discomfort or pain in the bladder and lower urinary tract and the surrounding pelvic region, as well as other symptoms. Diagnosis is difficult, and effective therapies remain elusive for Americans living with these conditions. To encourage a comprehensive understanding of urologic chronic pelvic pain that could lead to new therapeutic strategies, NIDDK has established the Multi-disciplinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network. In this Network, researchers at six “Discovery Sites” conduct innovative, collaborative studies of IC/PBS and CP/CPPS and the potential relationships between these conditions and other chronic pain disorders, such as fibromyalgia, while scientists at two “Core Sites” coordinate data collection, analyze tissue samples, and provide technical support. The Network also has an Ancillary Studies Program through which researchers not currently funded as part of the Network may initiate collaborative studies with Network investigators—thus leveraging this investment in research on IC/PBS and other urologic chronic pelvic pain conditions. Data and samples collected from the MAPP Research Network will also be saved in the NIDDK Central Repositories. The Network capitalizes on research suggesting that clues to the cause(s) of both IC/PBS and CP/CPPS may lie outside the bladder, a promising new direction that could elucidate these challenging pain conditions. More information on the MAPP Research Network is available on its web site at http://www.mappnetwork.org/. |
Special Statutory Funding Program for Type 1 Diabetes Research: Complementing efforts of the Diabetes, Endocrinology, and Metabolic Diseases program, the Special Program’s goal is to foster improved treatment, prevention, and cure of type 1 diabetes and its complications through basic, clinical, and translational research framed around six scientific goals: 1) identifying genetic and environmental causes of type 1 diabetes ($26.000 million); 2) preventing or reversing the disease ($34.105 million); 3) developing cell replacement therapy ($19.150 million); 4) preventing or reducing hypoglycemia ($26.900 million); 5) preventing or reducing health complications ($20.600 million); and 6) attracting new talent and applying new technologies to research ($23.245 million) (FY 2012 estimate dollars). Although focused on type 1 diabetes, aspects of this research are relevant to other autoimmune disorders, as well as type 2 diabetes. Both type 1 and type 2 diabetes share impaired function of insulin-producing beta cells of the pancreas along with potential complications, such as heart disease, stroke, blindness, kidney failure, nerve damage, and lower limb amputations. A recent comparative effectiveness research study, conducted by a Network led by NEI, found that a drug, in combination with laser therapy, was substantially better than laser therapy alone or laser therapy with a different drug at treating diabetic macular edema and improving vision.[16] The Beta Cell Biology Consortium is working to generate mouse models with components of a human immune system so that human beta cell function can be evaluated in a context of human immunity and autoimmunity. The Diabetes Research in Children Network is collaborating with Type 1 Diabetes TrialNet on a clinical trial testing whether early and intensive blood sugar control can protect insulin-producing beta cells. The Environmental Determinants of Diabetes in the Young (TEDDY) study completed enrollment of over 8,000 infants at high genetic risk for type 1 diabetes and is following them until age 15 to identify environmental triggers of the disease. The SEARCH for Diabetes in Youth study, a joint effort by NIDDK and CDC, provided the first national estimates of rates of childhood diabetes and found that children with rarer forms of diabetes are often misdiagnosed as type 1 or type 2 diabetes, and thus are not treated properly. A new initiative calls for research to overcome key roadblocks to treatment and cure of type 1 diabetes.
Budget Policy: The FY 2012 budget estimate for the Special Statutory Funding Program for Type 1 Diabetes Research is $150 million, the same as FY 2010. NIDDK administers the program, but because of its trans-HHS nature, the resources are disbursed among multiple NIH Institutes and Centers as well as CDC. Among ongoing efforts that will continue with FY 2012 funds are an ambitious study that aims to identify environmental causes of type 1 diabetes in genetically susceptible individuals (The Environmental Determinants of Diabetes in the Young study) ($6 million), the Clinical Islet Transplantation Consortium ($3 million), and the Beta Cell Biology Consortium, which aims to regenerate or replace the insulin-producing cells of the pancreas ($14.8 million). Research on development of an “artificial pancreas” will be expanded in FY 2012 through initiatives funding small business research to develop new therapeutics and monitoring technologies for type 1 diabetes ($2 million) and clinical research on closed loop technologies ($20 million). FY 2012 funds will also foster research and early career development for pediatric endocrinologists and behavioral scientists studying new approaches to treat, prevent, and cure type 1 diabetes ($13 million).
Intramural Research: The goal of the Intramural Research Program (IRP) is to conduct basic, translational, and clinical biomedical research related to diabetes and other endocrine and metabolic diseases; digestive diseases, including liver diseases and nutritional disorders; obesity; kidney diseases; and hematologic diseases. Intramural research is conducted in the Institute’s laboratories and clinical facilities in Bethesda, Maryland, as well as in Phoenix, Arizona, where a long-standing research partnership with the Pima Indians in the region, who have the highest rates of type 2 diabetes in the world, has led to important scientific advances in type 2 diabetes and obesity. Research training is also an integral component of the IRP. Recently, the NIDDK IRP has conducted metabolic research to phenotype a wide range of patients in terms of their energy expenditure and oxidation rates, providing insights into critical individual differences in body weight regulation. Additionally, NIDDK IRP research has identified several genetic variants that contribute to obesity in Pima Indians. Other studies of obesity have investigated the roles of gut microbes and of increased energy expenditure through brown fat activation, as well as developed mathematical models of human metabolism to accurately simulate the body’s response to physical activity and various diets. To translate research insights into clinical practice, IRP research has developed a web-based simulation tool for personalized weight loss goal-setting and progress-tracking. Other recent IRP studies have utilized solution phase nuclear magnetic resonance spectroscopy to elucidate structures of immunomodulatory proteins, HIV proteins, the membrane fusion domain of the influenza coat protein hemagglutinin, and protein-DNA complexes, as well as an array of protein-protein complexes related to signal transduction. Another recent IRP study, showing that in human pancreatic islets the insulin gene regulatory region is capable of activating other genes located distantly on the same chromosome, has led to the important discovery that one of these genes is involved in the regulation of insulin secretion.[17]
Budget Policy: The FY 2012 budget estimate for this program is $181.769 million, an increase of $2.008 million or 1.1 percent over the FY 2010 level. With FY 2012 funds, the NIDDK IRP will continue a broad spectrum of research studies to strengthen understanding of basic biology and disease mechanisms, and evaluate potential therapeutic approaches. For example, in FY 2012, intramural scientists will continue research on obesity in the trans-NIH Metabolic Clinical Research Unit, as well as research relevant to diabetes; digestive diseases, including liver disease; kidney disease; and hematologic disease. The program will also continue to support research training.
Research Management and Support (RMS): RMS activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards, and research and development contracts. RMS functions also encompass strategic planning, coordination, and evaluation of the Institute’s programs, regulatory compliance, international coordination, and liaison with other federal agencies, Congress, and the public. Through its RMS activities, NIDDK has continued to fund meritorious basic, clinical, and translational research and research training efforts, and also continued its health information dissemination and education/outreach activities. Additionally, NIDDK’s strategic planning, evaluation, and other activities have continued; some of these are highlighted above. In addition, ARRA resources allowed the Institute to support substantially more meritorious scientific research this year than in the past, and NIDDK rose to the management challenges related to funding this research.
Budget Policy: The FY 2012 budget estimate for RMS is $67.553 million (excludes Type 1 Diabetes), an increase of $2.300 million or 3.5 percent over the FY 2010 level. NIDDK will continue effective research management and support so as to deploy research resources to the most meritorious and promising areas, and to communicate research opportunities and findings to investigators, health professionals, and the public.
Budget Authority by Object
(Dollars in thousands)| | | FY 2010 Actual | FY 2012 PB | Increase or Decrease |
|---|
| | Total compensable workyears: | | | |
| Full-time employment | 625 | 627 | 2 |
| | Full-time equivalent of overtime and holiday hours | 1 | 1 | 0 |
| | Average ES salary | $169,264 | $169,264 | $0 |
| | Average GM/GS grade | 11.9 | 11.9 | 0.0 |
| | Average GM/GS salary | $95,135 | $95,135 | $0 |
| | Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) | $92,421 | $93,900 | $1,479 |
| | Average salary of ungraded positions | 90,841 | 90,841 | 0 |
| | OBJECT CLASSES Personnel Compensation: | FY 2010 Actual | FY 2012 Estimate | Increase or Decrease |
| 11.1 | Full-time permanent | $33,953 | $34,101 | $148 |
| 11.3 | Other than full-time permanent | 31,621 | 31,809 | 188 |
| 11.5 | Other personnel compensation | 1,659 | 1,666 | 7 |
| 11.7 | Military personnel | 1,951 | 1,965 | 14 |
| 11.8 | Special personnel services payments | 12,867 | 12,936 | 69 |
| | Total, Personnel Compensation | $82,051 | $82,477 | $426 |
| 12.0 | Personnel benefits | $19,159 | $19,331 | $172 |
| 12.2 | Military personnel benefits | 1,552 | 1,563 | 11 |
| 13.0 | Benefits for former personnel | 0 | 0 | 0 |
| | Subtotal, Pay Costs | $102,762 | $103,371 | $609 |
| 21.0 | Travel and transportation of persons | $2,383 | $2,603 | $220 |
| 22.0 | Transportation of things | 234 | 262 | 28 |
| 23.1 | Rental payments to GSA | 1 | 1 | 0 |
| 23.2 | Rental payments to others | 0 | 0 | 0 |
| 23.3 | Communications, utilities and miscellaneous charges | 707 | 717 | 10 |
| 24.0 | Printing and reproduction | 680 | 693 | 13 |
| 25.1 | Consulting services | 1,043 | 1,142 | 99 |
| 25.2 | Other services | 17,830 | 15,637 | (2,193) |
| 25.3 | Purchase of goods and services from government accounts | 177,671 | 189,994 | 12,323 |
| 25.4 | Operation and maintenance of facilities | 3,224 | 3,500 | 276 |
| 25.5 | Research and development contracts | 91,336 | 91,767 | 431 |
| 25.6 | Medical care | 670 | 735 | 65 |
| 25.7 | Operation and maintenance of equipment | 4,239 | 4,868 | 629 |
| 25.8 | Subsistence and support of persons | 0 | 0 | 0 |
| 25.0 | Subtotal, Other Contractual Services | $296,013 | $307,643 | $11,630 |
| 26.0 | Supplies and materials | $17,066 | $18,332 | $1,266 |
| 31.0 | Equipment | 8,796 | 9,644 | 848 |
| 32.0 | Land and structures | 0 | 0 | 0 |
| 33.0 | Investments and loans | 0 | 0 | 0 |
| 41.0 | Grants, subsidies and contributions | 1,528,429 | 1,544,691 | 16,262 |
| 42.0 | Insurance claims and indemnities | 0 | 0 | 0 |
| 43.0 | Interest and dividends | 0 | 0 | 0 |
| 44.0 | Refunds | 0 | 0 | 0 |
| | Subtotal, Non-Pay Costs | $1,854,309 | $1,884,586 | $30,277 |
| | Total Budget Authority by Object | $1,957,071 | $1,987,957 | $30,886 |
Includes FTEs which are reimbursed from the NIH Common Fund for Medical Research
Salaries and Expenses
(Dollars in thousands)OBJECT CLASSES
Personnel Compensation: | FY 2010 Actual | FY 2012 PB | Increase or Decrease |
|---|
| Full-time permanent (11.1) | $33,953 | $34,101 | $148 |
| Other than full-time permanent (11.3) | 31,621 | 31,809 | 188 |
| Other personnel compensation (11.5) | 1,659 | 1,666 | 7 |
| Military personnel (11.7) | 1,951 | 1,965 | 14 |
| Special personnel services payments (11.8) | 12,867 | 12,936 | 69 |
| Total Personnel Compensation (11.9) | $82,051 | $82,477 | $426 |
| Civilian personnel benefits (12.1) | $19,159 | $19,331 | $172 |
| Military personnel benefits (12.2) | 1,552 | 1,563 | 11 |
| Benefits to former personnel (13.0) | 0 | 0 | 0 |
| Subtotal, Pay Costs | $102,762 | $103,371 | $609 |
| Travel (21.0) | $2,383 | $2,603 | $220 |
| Transportation of things (22.0) | 234 | 262 | 28 |
| Rental payments to others (23.2) | 0 | 0 | 0 |
| Communications, utilities and miscellaneous charges (23.3) | 707 | 717 | 10 |
| Printing and reproduction (24.0) | 680 | 693 | 13 |
| Other Contractual Services: | | | |
| Advisory and assistance services (25.1) | 1,043 | 1,142 | 99 |
| Other services (25.2) | 17,830 | 15,637 | (2,193) |
| Purchases from government accounts (25.3) | 101,037 | 103,608 | 2,571 |
| Operation and maintenance of facilities (25.4) | 3,224 | 3,500 | 276 |
| Operation and maintenance of equipment (25.7) | 4,239 | 4,868 | 629 |
| Subsistence and support of persons (25.8) | 0 | 0 | 0 |
| Subtotal Other Contractual Services | $127,373 | $128,755 | $1,382 |
| Supplies and materials (26.0) | $17,053 | $18,318 | $1,265 |
| Subtotal, Non-Pay Costs | $148,430 | $151,348 | $2,918 |
| Total, Administrative Costs | $251,192 | $254,719 | $3,527 |
Details of Full-Time Equivalent Employment (FTEs)| OFFICE/DIVISION | FY 2010 Actual Civilian | FY 2010 Actual Military | FY 2010 Actual Total | FY 2011 CR Civilian | FY 2011 CR Military | FY 2011 CR Total | FY 2012 PB Civilian | FY 2012 PB Military | FY 2012 PB Total |
|---|
| Office of the Director | 132 | 0 | 132 | 132 | 0 | 132 | 132 | 0 | 132 |
| Division of Diabetes, Endocrinology, and Metabolic Diseases | 27 | 2 | 29 | 27 | 2 | 29 | 27 | 2 | 29 |
| Division of Digestive Diseases and Nutrition | 19 | 3 | 22 | 19 | 3 | 22 | 19 | 3 | 22 |
| Division of Kidney, Urologic, and Hematologic Diseases | 17 | 0 | 17 | 17 | 0 | 17 | 17 | 0 | 17 |
| Division of Nutrition Research Coordination | 7 | 2 | 9 | 7 | 2 | 9 | 7 | 2 | 9 |
| Division of Extramural Activities | 53 | 1 | 54 | 53 | 1 | 54 | 53 | 1 | 54 |
| Division of Intramural Research Programs | 350 | 12 | 362 | 352 | 12 | 364 | 352 | 12 | 364 |
| Total* | 605 | 20 | 625 | 607 | 20 | 627 | 607 | 20 | 627 |
| FTEs supported by funds from Cooperative Research and Development Agreements | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
* Includes FTEs which are reimbursed from the NIH Common Fund for Medical Research
Average GM/GS Grade by Year| FISCAL YEAR | Average GS Grade |
|---|
| 2008 | 11.8 |
| 2009 | 11.8 |
| 2010 | 11.9 |
| 2011 | 11.9 |
| 2012 | 11.9 |
Detail of Positions|
| GRADE | FY 2010 Actual | FY 2011 CR | FY 2012 PB |
|---|
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | 169,264 | 169,264 | 169,264 |
| GM/GS-15 | 42 | 41 | 40 |
| GM/GS-14 | 67 | 68 | 72 |
| GM/GS-13 | 84 | 85 | 86 |
| GS-12 | 63 | 60 | 59 |
| GS-11 | 40 | 42 | 43 |
| GS-10 | 0 | 0 | 0 |
| GS-9 | 33 | 34 | 33 |
| GS-8 | 21 | 18 | 16 |
| GS-7 | 18 | 20 | 21 |
| GS-6 | 3 | 4 | 4 |
| GS-5 | 4 | 2 | 3 |
| GS-4 | 0 | 0 | 0 |
| GS-3 | 0 | 1 | 2 |
| GS-2 | 1 | 2 | 0 |
| GS-1 | 1 | 0 | 0 |
| Subtotal | 377 | 377 | 379 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): | | | |
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 10 | 10 | 10 |
| Senior Grade | 4 | 4 | 4 |
| Full Grade | 4 | 5 | 5 |
| Senior Assistant Grade | 1 | 1 | 1 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 19 | 20 | 20 |
| Ungraded | 260 | 263 | 261 |
| Total permanent positions | 395 | 400 | 400 |
| Total positions, end of year | 657 | 661 | 661 |
| Total full-time equivalent (FTE) employment, end of year | 625 | 627 | 627 |
| Average ES salary | 169,264 | 169,264 | 169,264 |
| Average GM/GS grade | 11.9 | 11.9 | 11.9 |
| Average GM/GS salary | 95,135 | 95,135 | 95,135 |
Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research.
New Positions Requested| | FY 2012 Grade | FY 2012 Number | FY 2012 Annual Salary |
|---|
| Lead Information Technology Specialist | GS-2210-14 | 1 | $105,211 |
| Information Technology Specialists | GS-2210-13 | 1 | 89,033 |
| Total Requested | | 2 | $194,244 |
[1] Centers for Disease Control and Prevention. National Diabetes Fact Sheet, United States, 2010.
[2] Levey AS, et al. Ann Intern Med 150: 604-612, 2009; U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the U.S., National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010.
[3] NIDDK, NIH/DHHS. Kidney and urologic diseases statistics (http://kidney.niddk.nih.gov/statistics/), 2010.
[4] Everhart JE, ed. The Burden of Digestive Diseases in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Dept of Health and Human Services; 2008. NIH Pub 09-6433.
[5] Flegal KM, et al. JAMA 303: 235-241, 2010; and Ogden CL, et al. JAMA 303: 242-249, 2010.
[6] Perez-Tilve D et al. Nat Neurosci 13: 877-882, 2010; Vijay-Kumar M, et al. Science 328: 228-231, 2010; and Sanyal AJ et al. New Engl J Med 362:1675-1685, 2010.
[7] Pittsburgh Epidemiology of Diabetes Complications Study Group and adapted from Diabetes, 55: 1463-1469, 2006.
[8] The Diabetes Control and Complications Trial (DCCT) Research Group. New Engl J Med 329(14): 977-986, 1993; DCCT/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. New Engl J Med 353(25): 2643-2653. 2005; and DCCT/EDIC Research Group, et al. Arch Intern Med 169:1307-16, 2009.
[9] Kopp JB et al. Nat Genet 40: 1175-1184, 2008; Linda Kao WH et al. Nat Genet 40: 1185-1192, 2008; and Genovese G, et al. Science 329: 841-845, 2010.
[10] National Kidney Disease Education Program (http://www.nkdep.nih.gov/)
[11] Voight BF et al. Nat Genet 42: 579-589, 2010; Barrett JC et al. Nat Genet 41: 703-707, 2009; Genovese G, et al. Science 329: 841-845, 2010.; Imielinski M, et al. Nat Genet 41: 1335-1340, 2009.; McGovern DPB, et al. Nat Genet 42: 332-337, 2010.; Dubois PC, et al. Nat Genet 42: 295-302, 2010.; and Petersen KF, et al. N Engl J Med 362: 1082-1089, 2010.
[12] Vijay-Kumar M, et al. Science 328: 228-231, 2010; and Reyes A, et al. Nature 466: 334-338, 2010.
[13] Goldfine AB, et al. Ann Intern Med 152: 346-357, 2010.
[14] Lee WM, et al. Gastroenterology 137: 856-864, 2009.
[15] The Look AHEAD Research Group. Arch Intern Med 17: 1566-1575, 2010.
[16] Diabetic Retinopathy Clinical Research Network, Elman MJ, et al. Ophthalmology. 117(6):1064-1077, 2010.
[17] Xu Z, et al. Nature Structural & Molecular Biology, In press.
Page last updated: April 01, 2011