This page is for historical and reference purposes only. Updates to content and links will not be maintained. Current information about NIDDK's Kidney, Urological and Hematology programs can be found at http://www2.niddk.nih.gov/Research/ScientificAreas/Kidney/, http://www2.niddk.nih.gov/Research/ScientificAreas/Urology/ and http://www2.niddk.nih.gov/Research/ScientificAreas/Hematology/.
The American Society of Nephology will meet in Philadelphia, PA on November 8-13, 2011.
Meeting Posters (PDF, 2.08 MB) Printer friendly version of the contents on this page
Funding History and Research Opportunities
NIDDK Funding History and Opportunities
The National Institutes of Health (NIH) comprises 27 separate Institutes and Centers and is the largest biomedical research center in the world. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) was established by Congress in 1950. Within the NIDDK, the Division of Kidney, Urology and Hematology (KUH) manages programs in kidney research.
In 2010, NIDDK awarded >$250M for kidney research.
Exploratory Research (R21) Program
NEW! The NIDDK will no longer accept applications in response to the NIH Parent R21 Funding Opportunity Announcement (PA-10-069). NIDDK is accepting applications in response to:
- Pilot & Feasibility Clinical Research Grants in Kidney Diseases (R21) PAR-11-352
- Secondary Analyses in Obesity, Diabetes, Digestive & Kidney Diseases (R21) PA-09-313
Collaborative Research
Complex biomedical science often requires the expertise of collaborating investigators working together as an investigative team. Collaborative research can be supported by several different types of grant mechanisms:
- R01 with a Principal Investigator and one or more key personnel or collaborators
- Multi-PI R01 with multiple Principal Investigators collaborating and sharing credit and responsibility
- Center (P20/P30/P50) supporting a focused set of core activities
- Program Project Grant (P01) designed to support a broadly based interrelated research program that has multiple distinct but synergistic research projects built around a unifying central theme
- Resource-Related Research Project Grant (R24) provides flexible support for an interdisciplinary research team focused on answering a single critically important research question
Diabetic Complications Consortium (formerly AMDCC)
Given the strong evidence that diabetic complications are linked via dysregulation of common pathways, there is a strong need to facilitate the sharing of ideas, information, and reagents between research communities investigating similar pathologic mechanisms in different organs. Like the AMDCC, the DCC will continue to promote this communication and collaboration via an ANNUAL SCIENTIFIC MEETING and FUNDING OPPORTUNITIES. Unlike the AMDCC, the DCC has a BROADER SCIENTIFIC SCOPE and supports not only animal model development but all types of basic and translational studies. Full details at www.diacomp.org.
Kidney Research National Dialogue (KRND)
Kidney disease is a costly and major public health problem. To best position our research community to identify the most robust paths forward, we have initiated an interactive web-based dialogue on kidney health and disease to elicit a wide range of ideas and opinions. This dialogue consists of three sequential phases. FIRST, participants identify critically important questions or objectives that could be addressed through basic and/or clinical research. The questions and objectives should be compelling, answerable, and have the greatest promise to advance scientific knowledge or improve public health. SECOND, working group participants are asked to focus on the converging research questions/objectives and develop research strategies that address each of the research questions and objectives. All types of basic, translational, and clinical research should be considered. In some cases, fundamental groundwork may be needed before a larger question or objective can be approached. Thus, research strategies might include catalytic steps that surmount current barriers to progress. THIRD, content for the dialogue will be edited and incorporated into a blueprint for kidney research and posted for public comment. For more details contact KRND@nih.gov or kidneydialogue@nih.gov or GOOGLE "KUH KRND".
Generation of new mouse strains for analysis of the developing urogenital system
The goal of the Genito-Urinary Developmental Anatomy Project (GUDMAP) consortium is to develop a detailed molecular anatomy of the mammalian urogenital
system (UGS), to inform and stimulate research, and to better understand human disease. As part of this multi-center effort, GUDMAP2 members will generate novel transgenic mouse strains of broad interest to the community.
Gene nominations for targeting are NOW being sought from the research community.
GUDMAP2 will give preference to gene targets expressed in critical cell types of the lower UGS, but will consider cell-type specific marking throughout the urinary system. Once generated and transgene activity is confirmed, mice will be made available to nominating investigators and deposited in a Mouse Mutant Repository Center (MMRC) for broader distribution to the community. Full details at www.gudmap.org.
37 Month Time Limit for NIH Re-submission Applications
Kidney and Urology Funding Announcements
Basic Research
- Grants for Research in Glomerular Diseases (R01) PA-10-113
- Calcium Oxalate Stone Diseases (R01) PA-09-213
- Advances in Polycystic Kidney Disease (R01) PA-09-202
- Basic and Clinical Studies of Congenital Urinary Tract Obstruction (R01) PA-09-226
- Enhancing Zebrafish Research with Research Tools and Techniques (R01) PAR-11-131
Translational Research
- Grants for Research in Glomerular Diseases (R01) PA-10-113
- Development of Assays for High-Throughput Drug Screening (R01) PA-10-213
- Development and Validation of Disease Biomarkers (R01) PA-09-204
- Non-Invasive Methods for Diagnosis and Progression of Diabetes, Kidney, Urological, Hematological & Digestive Diseases & Hypertensive Disorders (R01) PA-09-181
Clinical Research
- Ancillary Studies of Acute Kidney Injury, Chronic Kidney Disease, and End Stage Renal Disease Accessing Information from Clinical Trials, Epidemiological Studies, and Databases (R01) PA-09-196
- Ancillary Studies to Major Ongoing NIDDK Clinical Research Studies to Advance Areas of Scientific Interest within the Mission of NIDDK (R01) PAR-09-247
- NIDDK Multi-Center Clinical Study Implementation Planning Grants (U34) PAR-10-197
- NIDDK Multi-Center Clinical Study Cooperative Agreement (U01) PAR-11-15
Upcoming NIDDK Meetings
- Meeting on Measurement of Urinary Symptoms (MOMUS)
November 14–15, 2011
Natcher Building (Main Auditorium), NIH Campus, Bethesda, MD
- Urology Program Directors Meeting
November 30–December 2, 2011
Ellicott City, MD
- Human Tissue for Diabetes Complications Research Workshop
December 12–13, 2011
Bethesda Hyatt, Bethesda, MD
- Workshop on Advances in Chronic Kidney Disease in Children and Adults: Findings from the Chronic Kidney Disease in Children (CKiD) Study and the Chronic Renal Insufficiency Cohort (CRIC) Study
January 23–24, 2012
- Reducing the Impact of Glomerular Disease: Pathophysiology, Diagnosis, Therapy, and Clinical Trial Design
April 17–18, 2012
- PKD-Centers Meeting
April 2012
Small Business
Small Business Funding Opportunities
Why Seek SBIR/STTR Funds?
- Over $1 billion are available across NIH
- They provide seed money for high-risk projects
- They promote and foster partnerships with collaborators - including academia!
- Intellectual property rights are normally retained by small business
- Funds are NOT A LOAN - no repayment!
- Large corporations look to small companies for initial development
Small Business Innovation Research (SBIR)
The SBIR program supports innovative research conducted by small businesses to develop products for commercialization. The PI must be employed by the small business, but a research institution may be involved.
http://www.zyn.com/sbir
http://grants.nih.gov/grants/oer.htm
Small Business Technology Transfer (STTR)
The STTR program supports innovative research for products that have the potential for commercialization. STTR projects must be conducted cooperatively by a small business and a research institution.
http://www.zyn.com/sbir
http://grants.nih.gov/grants/oer.htm
Select NIDDK-Supported Small Business Projects
- Predicting kidney stones in relatives of stone formers
- Measurement of GFR
- Computerized Clinical Decision Support
- Probiotic Use in CKD
- Commercialization of embryonic kidney stem cell lines, products and kits
- Bioartifical renal epithelial cell systems (See INNOVATIVE BIOTHERAPIES: Poster # TH-PO175 & TH-PO176)
- Test for Salt-Sensitivity in Essential Hypertension
- Prevention-Treatment of Diabetic Nephropathy
- Intravital kidney multiphoton microscopy assays of therapeutic agents
- Renal perfusion quantification
- Tracking Transplant Centers Performance
Training and Career Development
Post-Doctoral Training
Training & Career Development Timeline
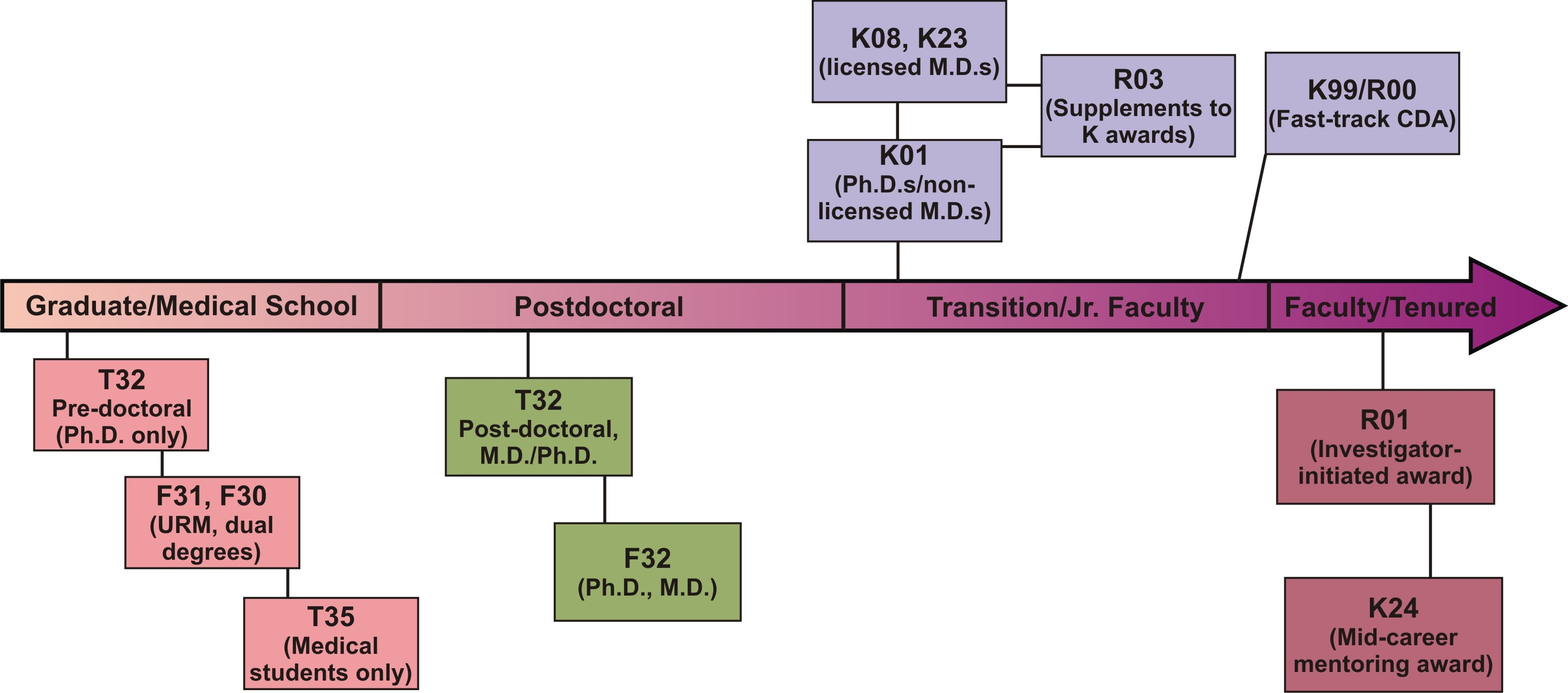
Loan Repayment Program
The goal of the Loan Repayment Program is to ease the debt burden clinical scientists may have incurred while attending medical school and a residency program. The NIDDK has two loan repayment programs: one for clinicians and one for pediatricians. In addition, the National Center on Minority Health and Health Disparities sponsors two other loan repayment programs for clinicians: one for those involved in health disparities research and another for clinical researchers from disadvantaged backgrounds. Competitive applicants must demonstrate their commitment to a research career and have a debt-to-salary ratio of at least 20 percent. The Loan Repayment Program may repay up to $35,000 a year toward each participant’s outstanding eligible educational loan debt, depending on total eligible repayable debt. For more details about eligibility and to apply online, visit http://www.lrp.nih.gov.
Career Development Awards*
- K01 (Mentored Research Scientist Development Awards)
Support Ph.D. scientists who have at least 3 to 5 years of postdoctoral training and who need to transition to independence.
- K08 (Mentored Clinical Scientist Development Awards)
Aimed at physician-scientists to transition them to independence.
- K23 (Mentored Patient-Oriented Research Career Development Awards)
Aimed at clinical investigators engaged in patient-based research.
- K24 (Investigator Awards in Patient-Oriented Research)
Support mid-career physicians in patient-oriented research with funded clinical investigations and who are mentoring young clinicians.
- K25 (Mentored Quantitative Research Career DevelopmentAwards)
Available to individuals with quantitative (E.g., engineering, mathematics, computer science, etc.) backgrounds who wish to pursue biomedical research.
Training-Related Program Announcements
Small Grant Program for NIDDK K01/K08/K23 Recipients (R03)
In the final two years of the career development grant, K recipients may apply for small grant funding for additional development support for their research.
NIDDK Education Program Grants (R25)
The R25 program provides support for educational opportunities (E.g., workshops, classes) to engage students from undergraduate to graduate in research areas relevant to NIDDK.
K99/R00 NIH Pathways to Independence
This is intended for talented postdoctoral candidates on the fast-track to a productive research career. Eligible applicants must have five-years or fewer of postdoctoral research experience and may not already have an independent faculty position. The first two years of the award, the K99 phase, is for mentored career-development. At the end of the second year, the applicant must have secured an independent tenure-track position to continue the final three years of the award as an R00. While this award does not require U.S. citizenship or permanent resident status, the applicant must be able to remain in the United States to conduct the full five years of the proposed work.
NEW! Recent revisions to the NIH appeals policy of the initial peer review process (NOT-OD-11-064)
Contacts
Kidney, Urology & Hematology (KUH) Staff
Telephone: (301) 594-7717
Director, KUH
Robert A. Star, M.D.
starr@extra.niddk.nih.gov
Deputy Director, Clinical; Pediatric Neph Program & Renal Centers
Marva Moxey-Mims, M.D.
mimsm@extra.niddk.nih.gov
Deputy Director, Basic; Renal Physiology Program
Christian J. Ketchum, Ph.D.
ketchumc@extra.niddk.nih.gov
Director, Minority Health Program
Lawrence Y. Agodoa, M.D.
agodoal@extra.niddk.nih.gov
Epidemiology Program and U.S. Renal Data System
Paul W. Eggers, Ph.D.
eggersp@extra.niddk.nih.gov
Clinical PKD & Inflammatory Renal Diseases Programs
Michael F. Flessner, MD, PhD
flessnermf@niddk.nih.gov
Kidney Development & Repair & Regeneration Programs
Deborah Hoshizaki, Ph.D.
hoshizakid@niddk.nih.gov
Acute Injury and HIV Nephropathy Program
Paul L. Kimmel, M.D.
kimmelp@extra.niddk.nih.gov
Clinical and Translational Urology Programs
Ziya Kirkali, M.D.
ziya.kirkali@nih.gov
Clinical Trials Program
John W. Kusek, Ph.D.
kusekj@extra.niddk.nih.gov
Urology and Kidney Cell Biology Programs
Christopher V. Mullins, Ph.D.
mullinsc@extra.niddk.nih.gov
National Kidney Disease Education Program (NKDEP)
Andrew S. Narva, M.D.
narvaa@extra.niddk.nih.gov
Kidney and Urology Training
Tracy L. Rankin, Ph.D.
rankint@mail.nih.gov
Genetics and Basic PKD Programs
Rebekah Rasooly, Ph.D.
rasoolyr@extra.niddk.nih.gov
Kidney Pathophysiology Program
Krystyna Rys-Sikora, Ph.D.
Krystyna.Rys-Sikora@nih.gov
NIH Center for Scientific Review (CSR)
The Center for Scientific Review (CSR) are organized into Integrated Review Groups (IRGs) representing a general scientific area. Study sections for kidney and urological research within Digestive, Kidney & Urological Sciences (DKUS) are:
- NEW! Kidney Molecular Biology & Genitourinary Organ Development Study Section [KMBD] Replaces Cellular & Molecular Biology of the Kidney (CMBK) study section. KMBD reviews applications involving basic & applied aspects of normal & abnormal renal physiology, cell biology, transport biology, including osmoregulation and osmosensing, hormone action & signal transduction, vascular biology, genetic disorders, cell-matrix interactions, biophysics, bioenergetics, and basic processes underlying upper & lower genitourinary organ development.
- Pathobiology of Kidney Disease [PBKD] Reviews applications involving basic & clinical studies of kidney disease including pathophysiology, diagnosis, consequences & treatment of acute & chronic disorders of the kidney, and consequences of kidney disease & failure, as well as studies of glomerulus normal structure & function.treatment of acute and chronic disorders of the kidney, and consequences of kidney disease and failure, as well as studies of the normal structure and function of the glomerulus.
- NEW! Urologic & Genitourinary Physiology & Pathology Study Section [UGPP] Replaces Urologic & Kidney Development & Genitourinary Diseases (UKGD) and incorporates Urological Sciences Small Business Activities [SBIR/STTR]. UGPP reviews applications involving physiological & pathophysiological processes of the lower urinary tract, male reproductive organs, female pelvic floor including urolithiasis, microbial infection & inflammation in the lower urinary tract.
Scientific Review Officers
KMBD
Ryan Morris, Ph.D.
morrisr@csr.nih.gov
PBKD
Atul Sahai, Ph.D
Atul.Sahai@nih.gov
UGPP
Ryan Morris, Ph.D.
morrisr@csr.nih.gov
NIDDK Review Branch
NIDDK Review Branch conducts review of the applications received in response to IC-specific Funding Opportunity Announcements (FOAs: RFAs and PARs):
- Clinical trials to prevent or slow chronic renal disease
- Epidemiology, prevention, and treatment of acute kidney injury
- Epidemiologic and genetic studies of ESRD patients
- Clinical trials to reduce mortality and morbidity in ESRD patients
- Epidemiology of chronic renal insufficiency, including CV disease
Clinical Translational Research
Clinical Trials and Epidemiological Studies
NIDDK supports a wide range of clinical trials and epidemiological studies on chronic kidney disease. While many of these programs are solicited by NIDDK through initiatives, investigators may also develop their own ideas.
Areas of General Interest
- Clinical trials to prevent or slow chronic renal disease
- Epidemiology, prevention, and treatment of acute kidney injury
- Epidemiologic and genetic studies of ESRD patients
- Clinical trials to reduce mortality and morbidity in ESRD patients
- Epidemiology of chronic renal insufficiency, including CV disease
Mechanisms of Support
- Investigator-initiated (R01, R21, R24)
- Institute-initiated research solicitations (U01, contracts)
- Investigator-Initiated Multi-Center Clinical Studies (U34/U01)
- PCORI: Pilot Projects PFA PI-12-001
If you have patients with Nephrotic Syndrome
NEPTUNE (Nephrotic Syndrome Study Network)
Multi-center collaboration of clinical translational and basic scientists along with the patient support groups NephCure and Halpin Foundations. Study patients with focal segmental glomerulosclerosis (FSGS), Minimal Change Disease (MCD), and Membranous Nephropathy (MN).
Objectives:
Recruit 450 participants (no upper or lower age limit) for:
- Collaborative, integrated, cost-effective investigational infrastructure for clinical and translational research in FSGS, MCD, and MN
Diagnosed with first UTI within 112 days prior to beginning study medication
- Longitudinal Observational Cohort Studies of patients with incipient biopsy proven FSGS, MCD or MN
- Pilot & Ancillary program using unique resources, clinical data, or specimens in NEPTUNE
- Training program for post-doctoral trainees and junior faculty
- Collaborate with the NIH Office of Rare Diseases Data Management Coordinating Center, Halpin and NephCure Foundations
- Contact Registry for Nephrotic SyndromeDiagnosed urinary tract infection (UTI) with either fever or associated symptoms
For more information: See http://NEPTUNE-STUDY.ORG
NEPTUNE Participating Sites:
Harbor UCLA, NYU, University of Toronto, Johns Hopkins, University of Miami, Case Western University, University of North Carolina, Montefiore Medical Center, Mayo Clinic, Cohen Children's Hospital (LIJ Hospital), Children's Hospital Los Angeles, University of Michigan, Temple University, University of Washington, NIDDK Intramural, University of Pennsylvania, Columbia University, Emory University
Ongoing Studies/Currently Recruiting
- he ASsessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Network is an epidemiological study of long term outcomes following episodes of AKI. Aims are to determine (a) whether AKI leads to a faster progression or greater risk of developing chronic kidney disease, and (b) whether AKI leads to a higher risk of death, cardiovascular events, and other adverse events.
- Prospective Study of Chronic Kidney Disease in Children (CKiD). Cooperative agreement-supported clinical study; 2 Clinical Coordinating Centers and 1 DCC.
- The Nephrotic Syndrome Study Network (NEPTUNE) is an integrated group of academic medical centers, patient support organizations and clinical research resources dedicated to advancing the understanding and treatment of Minimal Change Disease (MCD), Focal and Segmental Glomerulosclerosis(FSGS), and Membranous Nephropathy (MN).
- The Rare Kidney Stone Consortium is an intergrated group of academic medical centers, patient support organizations, and clinical research resources studying primary hyperoxaluria, cystinuria, APRT deficiency and Dent disease.
- Oral vs. IV Iron Therapy in Chronic Kidney Disease. Single center study (Indiana University) investigating whether IV iron therapy in CKD is associated with more rapid decline in GFR than chronic oral iron therapy.
- Blood Pressure in Dialysis (BID). Pilot clinical trial to test feasibility of randomizing hemodialysis patients to two levels of blood pressure control. Investigators: Drs. Phillip Zager (University of New Mexico Health Sciences Center), Dana Miskulin (Tufts Medical Center), and Jennifer Gassman (Cleveland Clinic Foundation)
Ongoing Studies/NOT Currently Recruiting
- Chronic Renal Insufficiency Cohort (CRIC) Study. Cooperative agreementsupported prospective epidemiological study: 7 centers and 1 DCC
- Consortium for Radiologic Imaging Studies in PKD II (CRISP II). Cooperative agreement-supported clinical study; 4 centers and 1 DCC
- HALT-PKD. Cooperative agreement-supported clinical study; 6 sites and 1 DCC
- Randomized Intervention for Vesicoureteral Reflux (RIVUR). Multi-center trial of Trimethoprim-Sulfamethoxazole prophylaxis compared to placebo in children with VUR (13 primary sites and 1 DCC). Age range 2 months – 6 years. Outcome measures will include frequency of UTI, changes in scarring measured by DMSA scan, and development of antimicrobial resistance. (www.ClinicalTrials.gov identifier NCT00405704)
- Angiotensin II Blockade in Chronic Allograft Nephropathy (ABCAN). Cooperative agreement-supported clinical trial; single center (Un. Minn)
- Assessing Long Term Outcomes of Living Donation (ALTOLD). Multi-center prospective cohort study will address whether kidney donation increases the risk of developing ESRD and/or increases the risk of developing CV disease. Participating sites: University of Minnesota (Hennepin County), Ohio State University, Mayo Clinic,University of Iowa, Johns Hopkins University, UCSF, University of Maryland.he ASsessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Network is an epidemiological study of long term outcomes following episodes of AKI. Aims are to determine (a) whether AKI leads to a faster progression or greater risk of developing chronic kidney disease, and (b) whether AKI leads to a higher risk of death, cardiovascular events, and other adverse events.
Repository
Central NIDDK Repositories
Data, Biosamples, and DNA for your research
The NIDDK Central Repositories store samples and data from NIDDK-funded clinical studies, which are made available to the community at the end of the study or when an interim phase is completed.
DNA and data - Go to the NIDDK Repository for instructions for using the Web-based application system (https://www.niddkrepository.org/niddk/jsp/request/instruction.jsp). Often takes less than 2 weeks to review and deliver data!
Samples - apply using an X01 (no funds; http://grants.nih.gov/grants/guide/pa-files/PAR-11-306.html). The next deadline is March 1, 2012. See https://www.niddkrepository.org/niddk/jsp/public/sampleInstruction.jsp for more details.
Since 2005, the Repository has distributed data to 126 requesters!
*The table above depicts a number of data requests from the NIDDK repository. The following studies are listed: AASK ATN, BACH, CRISP, EDIC_DCCT, GoKinD, HEMO, ICDB, MDRD, and MTOPS. Number of samples from studies requested are included.
| Study |
Approved Requests |
| AASK |
8 |
| ATN |
3 |
| BACH |
1 |
| CRISP |
6 |
| EDIC_DCCT |
26 |
| GoKinD |
9 |
| HEMO |
7 |
| ICDB |
3 |
| MDRD |
13 |
| MTOPS |
6 |