Kenneth A. Jacobson, Ph.D., Chief
Daniel Appella, Ph.D.
Carole A. Bewley, Ph.D.
Paul Kovac, Ph.D.
Hans Luecke, Ph.D.
Jürgen Wess, Ph.D.
Research carried out by the Laboratory of Bioorganic Chemistry is designed to explore problems at the interface of chemistry and biology. Projects include studies on the mechanisms of interaction of pharmacologically active substances with biological systems, including systems relevant to diabetes, cancer, inflammatory diseases, tuberculosis, AIDS, Parkinson’s disease, Alzheimer's disease, and chronic pain. One goal of this area of research is to discover and develop new chemical agents, including synthetic molecules and natural products, as tools for the study of membrane and cytosolic functions of cells. Mechanisms of action or metabolism of such agents and their potential use as therapeutics are examined. An integral part of this research involves the development and application of modern techniques of organic and medicinal chemistry for the synthesis, separation, and spectral and biological investigation of new chemical agents, including bioactive natural products.
In addition, experiments in nuclear magnetic resonance spectroscopy and mass spectrometry are designed, developed, and carried out to elucidate structures of both small molecules and macro-molecules. One area of research involves the use of custom-designed peptide-nucleic acids as probes of protein and nucleic acid interactions. New bioorthogonal probes are being developed for the study of gene regulation through enzymes involved in histone modification. Utilizing the synthesis of complex carbohydrates, synthetic vaccines are developed for application to infectious diseases. New approaches to drug delivery, affinity labeling, enzyme catalysis, and receptor activation and new concepts of drug design are investigated.
Finally, modern techniques in molecular biology are used to study mechanisms of cell surface receptor activation and signal transduction, particularly with respect to adenosine, ATP, adrenergic, nicotinic, and muscarinic receptors and the ion channels and second messengers subserving such receptors. Transgenic mice are developed for in vivo investigation. Receptor mutagenesis studies and homology modeling, in conjunction with the use of recently determined X-ray crystallographic structures, have elucidated the requirements for molecular recognition in the ligand binding site and G protein interface, as well as structural aspects of receptor activation. These insights are being used to design new drug analogues with superior pharmacological properties and potentially fewer side effects.
Activities of Research Groups in LBC
Dan Appella, Synthetic Bioactive Molecules Group:
Synthetic multivalent scaffolds for the detection of pathogens, inhibition of protein-RNA and protein-protein complexes, and modulation of cell-surface receptors
The figure above represents a computational model of a PNA:DNA nanoscaffold (dark and light blue colors, respectively) with three ligands (cycloRGD, violet color) attached to the PNA. Each ligand is bound to one integrin receptor (gray color) to create a multivalent assembly where the entire complex simultaneously binds to three receptors. The final complex strongly inhibits metastasis of melanoma cells. The cartoon in the lower left corner represents the PNA:DNA complex where three ligands are covalently attached to the PNA. The nucleobases of the PNA (not shown) are non-covalently bound to the complementary nucleobases on the DNA.
Carole Bewley, Section on Natural Products:
Discovery and engineering of natural products and proteins as inhibitors and probes of HIV entry, protein-carbohydrate interactions, and bacterial pathogens
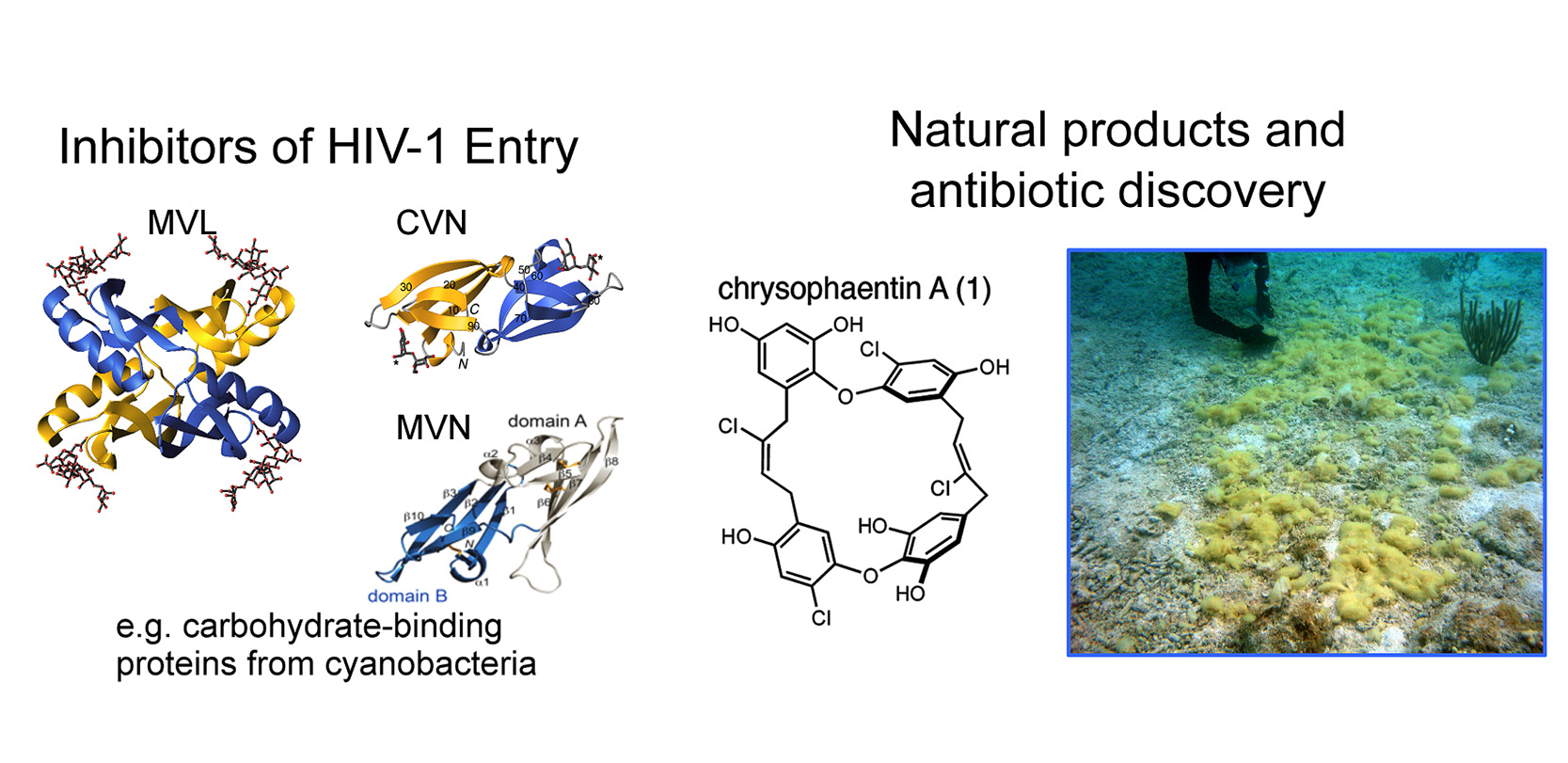
Left: Three-dimensional structures of proteins from cyanobacteria that bind avidly to carbohydrate structures that are uniquely displayed on the surface of many bacteria and viruses. Each of these proteins inhibits HIV from entering a target cell.
Right: Chemical structure of chrysophaentin A, representative of a new chemical class with antibiotic properties against drug-resistant bacteria. An underwater photo of the source organism is shown on the right.
Kenneth Jacobson, Section on Molecular Recognition:
Engineering of selective ligands for mechanistic probing and therapeutic modulation of adenosine and P2Y nucleotide receptors
The degree of sequence identity of twelve purine and pyrimidine G protein-coupled receptors was compared. These comprise four adenosine receptors and eight nucleotide (P2Y) receptors. Subtypes that are the current principal focus of the Section on Molecular Recognition for ligand design and structural probing are circled in orange.
Paul Kovac, Section on Carbohydrates:
Conjugate vaccines from synthetic fragments that mimic structure of bacterial carbohydrate antigens
Schematic representation of a neoglycoconjugate vaccine made by sequential, controlled conjugation of two synthetic antigens, a carbohydrate and a peptidoglycan, to a recombinant tetanus toxoid. The construct depicted, synthesized in the Section, has been shown to protect mice from cholera infection.
Hans Luecke, Gene Regulation Group:
Chemical tools and approaches to study epigenetic mechanisms of gene expression and to elucidate novel pathways controlled by lysine post-translational modification
Every cell in the human body contains our entire genome, yet proper function of different organs requires that a highly specific subset of these genes be expressed in distinct cell types. We are using chemical biological approaches to understand how a critical family of histone-modifying enzymes dynamically control and maintain the gene expression patterns required for normal human development and health.
Jürgen Wess, Section on Molecular Signaling:
G protein-coupled receptors (GPCRs): Structure-function analysis and studies with GPCR mutant mice
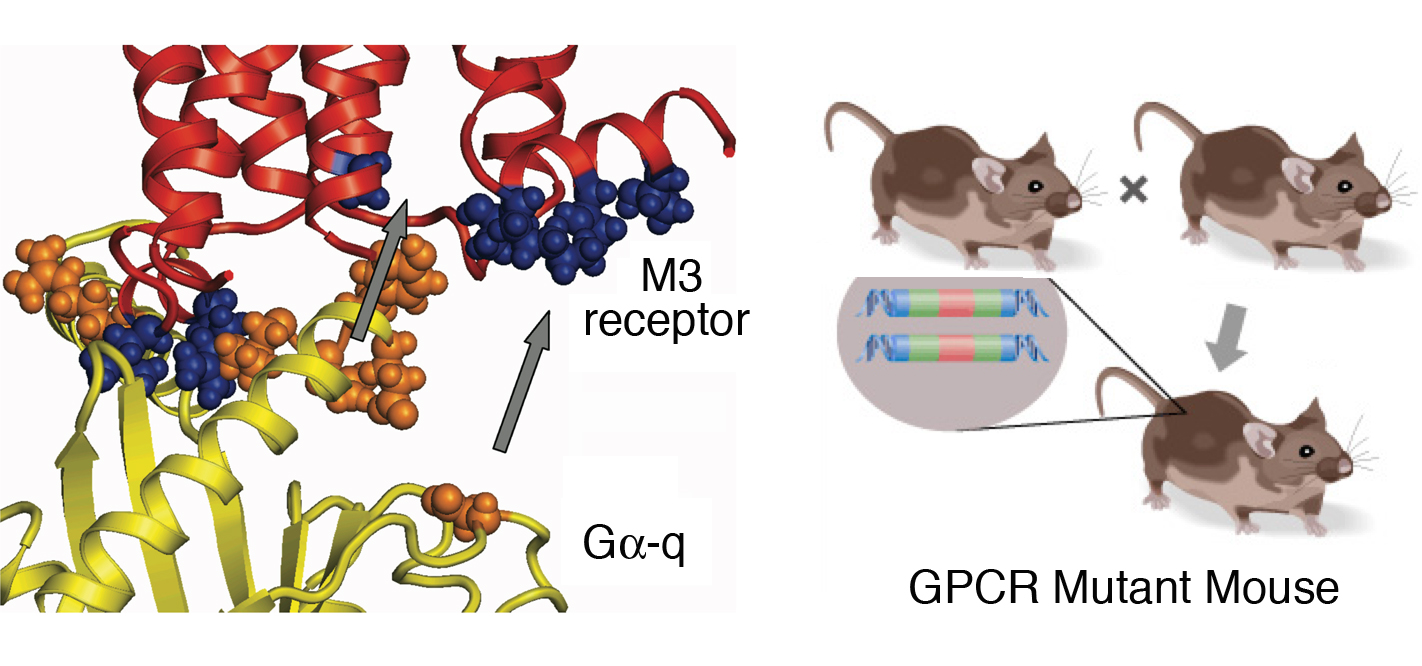
Left: Molecular model of the predicted interaction of a G protein (yellow) with the intracellular regions of the M3 muscarinic acetylcholine receptor.
Right: Schematic depiction of the generation of a mouse expressing homozygous mutations of a given G protein-coupled receptor (GPCR).