FACs staining for intracellular protein
Title |
FACs staining for intracellular protein |
Date Submitted |
May 5, 2012 |
Submitted by - |
Efthymiou, Anastasia - anastasia.efthymiou@nih.gov |
Adapted from - |
Gibco Protocol |
Contributors - |
Efthymiou, Anastasia |
Affiliation(s) - |
NIH CRM - NIAMS – Laboratory of Stem Cell Biology |
Introduction:
 |
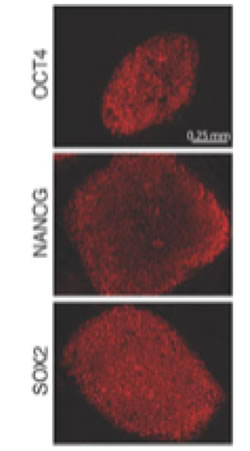 |
Staining for intracellular OCT4, NANOG, and SOX2 for FACs analysis1
Protocol:
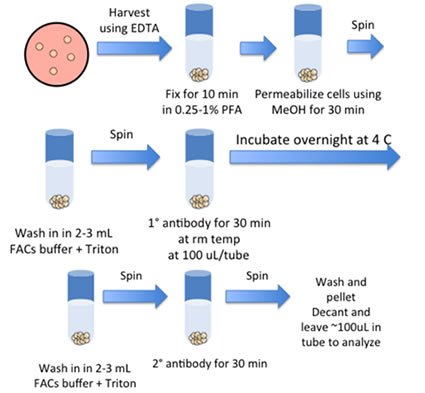
- Harvesting Undifferentiated Cells for FLOW
- Add 1mL of trypsin/EDTA + 2% chick serum/well of a 6-well plate and incubate for 10 min at 37 C. (*For differentiated cells add 1mL of Dispase for 10 min, remove, add trypsin)
- Scrape cells and pipette up/down to break up
- Dilute with FACS buffer and filter the suspension through 100, 80 or 40 micron filter.
- Spin down cells, 5 min, 1000 rpm
- Pour off supernatant and add 1mL of PBS
- Add paraformaldehyde so that the final concentration is 0.25-1% and fix for 10 min in 37 C water bath.
- Spin down. Resuspend in 2mLs of FACS buffer. (Cells can be placed at 4 degrees for storage if needed)
- Spin down cells and resuspend in 1mL of ice-cold 90% methanol. (Alternatively add 100% cold methanol to PBS + fixative to make 90% final concentration)
- Incubate on ice for 30 min. (Proceed with staining or store cells at –20C in 90% Methanol)
- Pellet cells.
- Wash cells by adding 2-3mLs of FACS buffer + Triton. Pellet cells.
- Pour off supernatant and add 100uL of pre-diluted primary Ab at 1:50.
- Inc. overnight at 4 C
- Wash cells by adding 2-3mLs of FACS buffer + Triton. Pellet cells.
- Pour off supernatant and add 100uL of pre-diluted secondary Ab (1:500).
- Incubate at RT for 30 min in the dark.
- Wash and pellet cells. Pour off supernatant to leave ~100ul in tube (Cells can be diluted if needed). Transfer on ice to Flow lab to be analyzed.
Materials:
- Begin with 5 x 10^5 – 1 x 10^6 cells/tube
Trypsin
FACS buffer EDTA
Methanol 2% hick serum Primary Ab 6-well tissue culture dish Secondary Ab PBS Triton 0.25-1% PFA
- FACS Buffer
PBS (w/o Ca/Mg++) + 2% FBS +0.1% NaN3
*0.5% BSA can be substituted for FBS
Troubleshooting:
References:
- Dirk Hockemeyer, Frank Soldner, et al.Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biot. 27 851-857 (2009).
Begin

