BIOMARKER
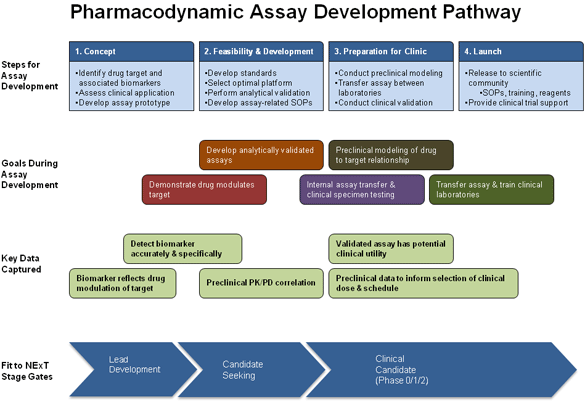
Pharmacodynamics (PD) is the study of the effects of a drug on a living organism. A pharmacologic response is assessed in terms of the duration of response, magnitude of action relative to the concentration of the drug at an active site, and the interactions between molecular targets. A goal of most anticancer drug development programs is to have one or more specific PD markers known as biomarkers that can be used to examine the link between a drug regimen, target effect, and biological tumor response. NCI is supporting the development and use of validated analytical tests for the monitoring of molecular response in target tissues after treatment with anticancer agents.
The strategy of the program is to use the principles routinely applied to clinical diagnostic tests to develop assays that will provide robust and accurate measurements of drug effects in patient specimens. Use of PD endpoints enhances the rationality and hypothesis-testing power throughout drug development from selection of lead compounds that affect the target in preclinical models to first-in-human trials. The coupling of drug development with focused PD assays provides critical data to make informed, early go/no-go decisions, select rational combinations of targeted agents, and optimize schedules of combinational regimens.
The introduction of PD testing has tremendous potential to improve the speed and success of drug discovery and development in oncology. Because development of targeted agents is often hampered by the inability to assess the effect of a compound on its target, the creation of go/no-go criteria based on biomarker measurements involves the development of assays that directly measure drug effect on a target.
Two biomarker programs have been set up to provide PD assay support for NExT drug development projects:
Imaging: The Cancer Imaging Program (CIP) facilities may allow non-invasive evaluation of the PD of a compound, as well as determination of overall biodistribution, to facilitate decisions based on measures of target inhibition in proof-of-concept animal models and first-in-human studies.
Clinical Laboratory Assays: NCI's Division of Cancer Treatment and Diagnosis has established a program to develop and validate cell-based (e.g., circulating tumor cells and biopsy samples) and macromolecular (DNA, RNA, protein) PD assays and transfer them to clinical laboratories in support of early clinical trials. This program will provide assay expertise for assay use in studies to select lead compounds.