Conditioning pluripotent stem cell media with mouse embryonic fibroblasts (MEF-CM)
| Conditioning pluripotent stem cell media with mouse embryonic fibroblasts (MEF-CM) | Prepared by: |
|
Date Submitted |
April 4, 2012 |
|
Submitted by |
Mark Tomishima tomishim@mskcc.org |
|
Adapted from |
SKI Stem Cell Research Facility protocols |
|
Contributors |
Mark Tomishima |
|
Affiliation(s) |
Sloan-Kettering Institute |
Introduction:
There are a number of methods to expand human pluripotent stem cells (hPSCs) without feeders. I prefer to culture my hPSCs with mouse embryonic fibroblasts (MEFs) but there are occasions when feeder free growth is required (e.g., nucleofection, viral transduction or karyotyping to name a few). To expand cells without feeders, I prefer to use mouse embryonic fibroblast-conditioned media (MEF-CM). This is simply human pluripotent stem cell (hPSC) media placed on MEFs overnight. The MEFs secrete factors that contribute to hPSC maintenance during the overnight incubation, and before use FGF2 is added to the preparation.
Flowchart:
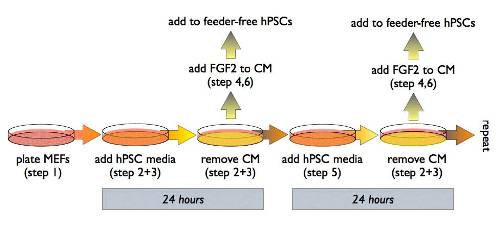
Producing MEF-conditioned media (MEF-CM)
- Two days before you need it, plate MEFs in DMEM+10%FBS at high density (50,000 cells/cm2). Let cells attach overnight.
- The next day, aspirate DMEM+10%FBS and wash once with PBS.
- Aspirate PBS, then add complete hPSC media and incubate overnight.
- After overnight incubation, remove conditioned media and place in a conical tube or other appropriate container, depending on the volume.
- Place fresh hPSC media back on the feeders and return to the incubator for the next day’s conditioned media.
- Add FGF2 to 10ng/ml final concentration to the conditioned media before use.
Notes:
Conditioned media can be stored frozen, although likely with some loss of activity. We do not keep it for longer than one month after freezing at -20°C although it is possible that it works longer. Always add fresh FGF2 before use. Some suggest filtering it through a 0.22µM filter. I do not do this and I think it might be a bad idea. While it can remove the few dead MEFs that float in these preparations, it likely also removes a lot of the protein from the CM, reducing its efficacy – at least in principle. Experience has demonstrated that the filtering is, at the very least, not necessary.
Additional hPSC media can be conditioned daily for up to two weeks using the same culture of high density MEF feeders.
Materials:
| Reagents | Supplier | Catalog Number | Notes |
|---|---|---|---|
| MEFs (CF-1, mitomycin-C treated) | GlobalStem, Inc. | GSC-6001M | |
| FGF2 | R&D Systems | 233-FB-001MG/CF | Dissolve in PBS with 0.1% BSA to 100 ng/ml |
| 45 µm nylon mesh strainer | BD Falcon | 352340 | |
| 1x DPBS | Life Technologies | 14190 |
hPSC media
| 800 ml DMEM/F12 | Life Technologies | 11330-032 |
| 200 ml KSR | Life Technologies | 10828-028 |
| 5 ml L-glutamine | Life Technologies | 25030-081 |
| 10 ml MEM NEAA | Life Technologies | 11140-050 |
| 1 ml 2-mercaptoethanol |
Life Technologies | 21985-023 |
Sterile filter, then add FGF2 to 6 ng/ml. Use within 2 weeks.
Fibroblast media
| 900 ml DMEM | Life Technologies | 11965-118 |
| 100 ml FBS | Life Technologies | 26140-095 |

