 |
 |
 |
 |
|
|
 |
 |
The purpose of the Clinical Research LRP is to recruit and retain highly qualified health professionals as clinical investigators.
Program Announcement
See Notice for Extramural Loan Repayment Program for Clinical Research (LRP-CR)
for program and policy guidance.
Program Eligibility
- Meet general eligibility requirements
of the LRPs outlined in the eligibility section of the site
- Have an M.D., Ph.D., Pharm.D., Psy.D., D.O., D.D.S., D.M.D., D.P.M., D.C., N.D., O.D., D.V.M., or equivalent doctoral degree from an
accredited institution
- Engage in qualified clinical research
Definitions
Clinical Research
Patient-oriented research conducted with human subjects, or research on the causes and consequences of disease in human populations involving material of human origin (such as tissue specimens and cognitive phenomena) for which an investigator or colleague directly interacts with human subjects in an outpatient or inpatient setting to clarify a problem in human physiology, pathophysiology or disease, epidemiologic or behavioral studies, outcomes or health services research, or developing new technologies, therapeutic interventions, or clinical trials.
Glossary of Terms
LRP Data
|
New Applications for Clinical Research LRP
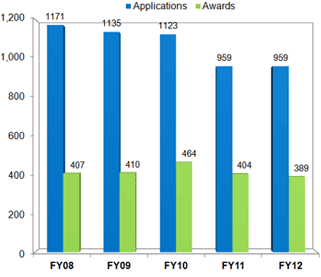
Source: NIH LRP
Data Accurate as of 10/01/12
|
|
Renewal Applications for Clinical Research LRP
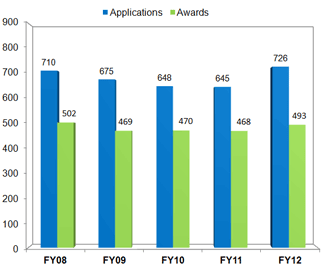
Source: NIH LRP
Data Accurate as of 10/01/12
|
Assistance
For further assistance, we encourage you to contact:
- Loan Repayment Programs (LRP) Information Center. Call 866-849-4047 or e-mail lrp@nih.gov
- LRP liaison
at the NIH Institute or Center (IC) most closely related to your research
- Your research supervisor or mentor
Date Last Updated: October 24, 2012
|
 |
 |
|