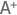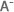NTP panel reviews outcomes of women treated for cancer while pregnant
By Robin Mackar

The peer review meeting ran smoothly under the capable leadership of Spong, right. Birnbaum, left, and Bucher, center, listened closely to the discussions. (Photo courtesy of Steve McCaw)
A stellar peer review panel convened by the National Toxicology Program (NTP) provided input and invaluable medical, pediatric, pharmaceutical, and other expertise on the draft NTP Monograph (http://ntp.niehs.nih.gov/?objectid=C7E8A38B-0A6E-1052-1ED9E587C8A857B3) on Developmental Effects and Pregnancy Outcomes Associated with Cancer Chemotherapy Use During Pregnancy.
“We know very little about pregnancy outcomes of women being treated for cancer. This is an extremely important topic that hasn’t been systematically looked at before,” said NIEHS/NTP Director Linda Birnbaum, Ph.D., as she welcomed the nine-member panel of external experts to the Oct. 1-2 meeting at NIEHS.
Under efficient chairmanship of Catherine Spong, M.D., associate director for Extramural Research at the Eunice Kennedy Shriver National Institute of Child Health and Human Development, part of the National Institutes of Health, and a board-certified obstetrician and gynecologist, the panel was able to have in-depth discussions and concur by unanimous vote on each of the NTP’s five main findings.
Main findings
One of the first findings, presented by the lead scientist for the evaluation, Kembra Howdeshell, Ph.D., of the NTP Office of Health Assessment and Translation (OHAT), (http://ntp.niehs.nih.gov/?objectid=497C419D-E834-6B35-8AF15D389859AF07) focused on congenital malformations.
Both NTP and the reviewers agreed that the evidence showed that chemotherapy for treatment of cancer in the first trimester represents a higher apparent risk of major malformations than treatment in the second or third trimesters only. This finding confirmed current medical opinion and predictions, based on the timing of fetal organ development during pregnancy. However, the lead reviewers for each of the findings presented by Howdeshell acknowledged the imprecision in the data available to NTP, and called on the NTP to point out the limitations.
“I sympathize with those of you working on this topic. As someone who works in this area, I realize we all report things differently and our terminology is always changing. You can’t make the data better — you can simply point out limitations,” said Michael Greene, M.D., chief of obstetrics at Massachusetts General Hospital.
The panel also heard, and then voted on, additional health outcome findings, including the effects of chemotherapy on the risk of spontaneous abortion and stillbirth; pregnancy complications, such as reductions in amniotic fluid and fetal growth restriction; newborn weight and health; and growth development of the children. They again drew attention to data limitations and noted that some adverse effects, such as effects on the reproductive system and other organs, may not be apparent until later in life.
Research and outreach needs
The need for long-term follow-up studies of the gestationally exposed offspring was emphasized, as was the need for more easily accessible national registries for physicians to document cases. “There are so many research needs crying out for attention in this area,” Greene emphasized. “For one, we need to get our colleagues to do a better job of developing quality case reports.”
Other panelists, such as John Mulvihill, M.D., from the University of Oklahoma pediatrics department, a respected researcher on the topic of pregnancy outcomes following treatment with chemotherapy during pregnancy, suggested getting woman advocates, who have been successfully treated for cancer during pregnancy, to tell their stories. Panel member Janine Polifka, Ph.D., a developmental biologist in the department of pediatrics at the University of Washington, emphasized creating more awareness around resources already available for decision-making, such as OTIS, the Organization of Teratology Information Specialists, (http://www.otispregnancy.org/) a nonprofit organization that provides information to patients and health care professionals about exposures during pregnancy and lactation.
Mulvihill called on the NTP to make clear, bottom line messages in its conclusions. After one of Howdeshell’s presentations, he commented, “You are now the international expert on this topic. You will benefit the world with bottom line messages that can be understood by all. Be clear and spread your message widely.”
Howdeshell, who has been working with her OHAT colleagues, including Vickie Walker and Mike Shelby, Ph.D., who recently retired, for nearly two years on this topic, gave a heartfelt thanks to all the panel members for their invaluable input. “This has been an incredible panel whose expert advice will surely make this document a state-of–the-science resource for both patients and physicians.”
In offering his thanks and appreciation to the chair, his staff, and the reviewers, NTP Associate Director John Bucher, Ph.D., joked that it was the first time that all the research needs identified by a panel did not fall on the NTP alone. The final NTP monograph should be available by summer 2013.
(Robin Mackar is the news director in the NIEHS Office of Communications and Public Liaison.)

Howdeshell showcased what she had learned over the past two years, as she analyzed more than 430 published studies, on 52 cancer drugs, that reported pregnancy outcomes of female cancer patients treated with the drugs during pregnancy. (Photo courtesy of Steve McCaw)

Mulvihill, a developmental geneticist who has authored many papers related to pregnancy outcomes of women receiving treatment with chemotherapy for cancer during pregnancy, referred to the NTP monograph as a landmark publication. (Photo courtesy of Steve McCaw)

Greene, chief of obstetrics at Massachusetts General Hospital, offered his perspective on the interpretation of published studies on pregnancy outcomes. (Photo courtesy of Steve McCaw)

Panel member Polifka, center, suggested evaluating the reported data on pregnancy outcomes, to identify patterns of malformations possibly linked to a cancer drug or combination therapy, following exposure during the first trimester of pregnancy. Panel member Tina Rizack, M.D., right, from The Warren Alpert Medical School of Brown University, and OHAT staff member Abee Boyles, Ph.D., listened to the discussion. (Photo courtesy of Steve McCaw)
"Designing the next generation ..." - previous story ![]()
![]() next story - "Fellows and scientists stand ..."
next story - "Fellows and scientists stand ..."
November 2012 Cover Page