Delving More Than Skin Deep
There are several schools of thought on cancer. One claims it’s a basic knowledge problem. A lot of things can be done, but we still don’t have a complete understanding of the process. Vincent Hearing, Ph.D., Deputy Chief of CCR’s Laboratory of Cell Biology, belongs to this school. He has spent 42 years at the bench characterizing a single cell type called a melanocyte. For him, this groundwork is necessary in order to target the abnormal melanocytes that often result in the deadly skin cancer—melanoma.
Melanocytes are specialized skin cells that are embedded between fibroblasts in the dermis and keratinocytes in the epidermis. They are the only type of cell in the body that can produce light-absorbing, high-molecular-weight biopolymers called melanins. Two major types of melanin molecules give skin its color: brown-black eumelanins and yellow-red pheomelanins. The capacity of melanocytes to produce mixtures of these light-absorbing pigments is genetically determined, but the amounts of pigments made and their ratios to one another can change over time in response to physiological requirements and/or to environmental influences.
Hearing began his research career studying pigmentary disorders, and his interest has never waned. His sustained attention to the molecular mechanisms that underlie pigmentary changes in the skin has produced a much clearer picture of the biochemistry and regulation of melanins, their role in the photoprotection of human skin, and their usefulness in targeting pigmentary diseases and melanoma.
After much study, Hearing and his research team have helped to paint the molecular picture of what normal epidermal melanocytes do: They produce, package, and move light-absorbing pigments called melanins to keratinocytes which then distribute them towards the skin’s surface. Melanocytes package melanins in membrane-bound organelles called melanosomes. The maturation of melanosomes depends upon the proper trafficking of structural and enzymatic components, the synthesis of melanin, followed by their transport to the melanocyte’s dendritic extensions. Once there, melanosomes are transferred to adjacent skin cells called keratinocytes. When all goes well, keratinocytes form vesicles to engulf and internalize the donated, pigment-rich melanosomes, and transport machineries, including a special protein called dynein, then move them to the vicinity of the keratinocyte’s nucleus.
“This magnificent biochemistry shields the underlying skin cell’s DNA from damage caused by ultraviolet radiation (UV). And all this action is usually accelerated automatically in skin cells in response to UV exposure,” explains Hearing.
Meet the Proteins: Builders and Modulators
Over the years, Hearing and his colleagues have drilled down to the molecular level to identify just how melanins—which are actually synthesized from the amino acid tyrosine via the action of a critical enzyme tyrosinase (TYR)—are produced and distributed to protect human skin from UV radiation. One by one, the team has identified key players in the process. Hidenori Watabe, Ph.D., who worked in Hearing’s lab and is now at Haruhino Dermatological Clinic in Kawasaki, Japan, and Julio Valencia, M.D., currently a Staff Scientist in Hearing’s lab, followed the movement of two major melanosomal proteins, TYR and PMEL17, showing that they first interact with additional proteins named TYRP1, DCT, and MART1 to regulate the structure of melanosomes and to modify the types of melanin produced, while PMEL17 acts later in melanogenesis, building the structural fibrils essential to the maturation and movement of mature melanosomes.
Other members of the Hearing lab characterized the proteins that move these organelles out to keratinocytes in an orderly manner. Still others identified several signaling proteins that respond to environmental factors by modulating pigment production. They showed how agents like melanocyte stimulating hormone (MSH), agouti signal protein (ASP), dickkopf1 (DKK1), and neuregulin 1 (NRG1) regulate the expression and function of pigmentation genes and modulate their responses to UV radiation.
Melanin Modulators of Interest
One interesting finding from Yuji Yamaguchi, Ph.D., formerly of the Hearing lab and now at Abbott Pharmaceuticals in Japan, explains why the skin on a person’s palms and soles are always thicker and lighter in color than on the rest of the body. He showed how a modulator protein called DKK1 is expressed at high levels in dermal fibroblasts derived from the lightly pigmented skin of an individual’s palms and soles, but is expressed at much lower levels in fibroblasts derived from skin on the body’s trunk. The high levels of DKK1 produced by fibroblasts in the palms and soles both reduce pigment there and thicken the skin in those areas.
Wonseon Choi, Ph.D., a Research Fellow in Hearing’s lab, produced another interesting finding. She identified the important role of NRG1 to modulate skin pigmentation. She demonstrated how this protein, when it is secreted by fibroblasts, regulates ethnic differences in constitutive skin color. NRG1 does this by regulating melanocyte differentiation and by altering the amount of melanins produced.
“Constitutive skin pigmentation is strongly associated with the incidence of skin cancers among ethnic groups. The rates of basal and squamous cell carcinomas and melanomas in Caucasians are more than 70- and 30-fold greater, respectively, than in African-Americans, and the rates of these cancers in Asians, Hispanics, and other ethnic groups with intermediate levels of skin pigmentation fall within these two extremes,” said Hearing, who believes that the mechanisms underlying the different cancer risks of these ethnic populations are due at least in part to the UV-absorbing and chemical properties of the different types of melanins.
Realizing that manipulating melanin modulators—either DKK1 and/or NRG1—may have clinical utility, the Hearing team and NCI have filed patents for bioactive peptides to target them as possible therapies to correct hypo- or hyper-pigmented conditions or to modulate the production of melanin. Manipulating pigmentation and studying its effects is of even greater importance to cancer research because hypo-pigmentation is a common characteristic of very aggressive melanomas.
Beware of Traffic Jams
Realizing that their analysis of melanogenesis at the molecular level would not be complete without addressing the trafficking of melanosomal components—the Hearing team took a close look at pigmented and unpigmented melanocytic cells. (See Melanosomal Trafficking figure below.) They learned that in pigmented cells, the early movement of melanosomes involves the endoplasmic reticulum, while later transport to the dendritic extensions depends upon a protein called dyenin, and final movement to the periphery requires the interaction of well-positioned spectrin and actin filaments. When all these components are in the right place at the right time, working together, pigmentation occurs. Interestingly, they found that this orderly process does not occur efficiently in unpigmented melanocytic cells. Spectrin is missing in the plasma membrane, so interactions with actin do not occur, and pigment trafficking breaks down.

Movement of melanosomes in unpigmented (top) and in pigmented (bottom) melanoma cells. Vesicles containing melanosomal proteins bud from endoplasmic reticulum (1), and are then moved forward by dynein (2) and spectrin (3). At the transGolgi network (4), delivery to the plasma membrane region begins. New vesicles are internalized (5) and directed to melanosomes (6). Kinesins promote their transport to the cell periphery via microtubules (7) and actin filaments enable their secretion (8). Adapted from H. Watabe et al., J. Invest. Dermatol. 2008,128:162-174. (Image: V. Hearing, CCR)
A Breakthrough Discovery
Because melanosomal proteins are complex, transient, and always in motion—even when all is functioning correctly—identifying and correcting malfunctioning components is like trying to hit a moving target. Fortunately, Thierry Passeron, M.D., Ph.D., a former Postdoctoral Fellow in Hearing’s lab and now a researcher in the Department of Dermatology at the University Hospital in Nice, France, succeeded in doing just that. In a series of salient experiments performed while he was in the Hearing lab, he discovered a gene coding for a transcription factor called SOX9 that functions upstream in regulating the melanogenic pathway, so it regulates both DCT and TYR and other key melanosome-linked genes.
Gene expression of SOX9, which occurs in both neonatal and in adult human skin, increases pigmentation in response to UVB exposure and acts counter to a pigmentation inhibitor called ASP. Passeron noticed that the loss of SOX9 expression was a consistent marker of skin cancer malignancy. As melanoma progressed in aggressiveness, SOX9 expression decreased; and as the disease turned metastatic, SOX9 expression vanished completely.
So Passeron wondered if SOX9 expression could be reversed. Could expression of SOX9 be restored in melanoma cell lines? And if so, would tumorigenicity be inhibited? The answer was a resounding, “yes.” Passeron restored SOX9 function in melanoma cells by treating them with a substance found naturally in the body called prostaglandin D2 (PGD2).

Vincent Hearing, Ph.D., and Sergio Coelho (Photo: R. Baer)
While confirming this result in human ex vivo models, Passeron and his collaborators uncovered a possible explanation for its mechanism of action. When SOX9 expression ceased, over-expression of a melanoma antigen called PRAME occurs, which inhibits the retinoic acid receptor, making the melanoma cells insensitive to retinoic acid therapy (RA). This helped explain why RA, an effective therapy for many other cancer types, often proves ineffective for melanoma. They found that RA sensitivity returned when SOX9 expression was restored and that treatment with either PGD2 or PGD2 plus retinoic acid inhibited the proliferation of melanoma cells by 50 percent and 75 percent, respectively.
To translate this discovery into clinical benefit as quickly as possible, Passeron, Hearing, and NCI have patented this noncytotoxic PGD2 approach to targeting and restoring SOX9 activity and thereby restoring melanoma’s sensitivity to RA treatment. Passerson is testing this and other SOX9 gene reactivators in his laboratory. This is an important contribution, because treatment options for aggressive melanoma are limited.
Finding Protein Biomarkers
In an attempt to find better melanoma therapies, the Hearing team is also tackling another very sinister behavior of aggressive melanomas. As melanocytes turn cancerous and become increasingly malignant, they tend to stop differentiating, and so they express fewer differentiation antigens. This allows them to evade detection by the body’s immune system. So Hearing and his team are searching for preserved melanocyte-specific biomarkers, ones that are expressed at an earlier-stage and continue to be expressed by less-differentiated melanocytes and melanoma cells. These markers will be useful for detecting melanoma earlier and may even serve as targets for antibody-based therapies. Toward this end, Hearing’s group is developing techniques needed to purify earlier stages of melanosomes and to characterize them in terms of new markers within the melanosome proteome.
The search for new biomarkers is important because the markers currently used to detect melanoma lack either specificity in the case ofthe S100 protein, or sensitivity in the case of the PMEL17 protein. One possible biomarker being investigated by the Hearing lab is a melanosome-specific proteolytically released protein called GPNMB that appears in the melanosomes of nonmetastatic melanoma and is enriched and secreted from mature melanosomes.
Understanding UVA and UVB Exposures
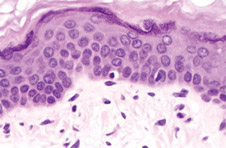
Melanocytes comprise from 1 to 2 percent of the cells in the basal layer of the epidermis. They are pigment producing factories that provide a surface tan to protect the human body from UV-induced DNA damage to deeper layers of the skin. (Image: Duke University, Department of Pathology)
To determine the effects of UVA and UVB exposures on human skin, the Hearing team participated in collaborative clinical studies with the U.S. Food and Drug Administration (FDA). They discovered that the two component wavelengths of UV, UVA and UVB, produce different effects on the skin. Yoshinori Miyamura, Ph.D., now at the University California-Davis, and Sergio Coelho, M.S., a Research Biologist in Hearing’s lab, noted that, after both types of UV exposure, melanins are distributed in all epidermal layers, but the pigment granules are larger after UVB exposure, and are only intermediate in size after UVA exposure. Also, while both wavelengths induced visible tans, the UVA-induced tans allowed more DNA damage to occur, while the UVB-induced tans provide a modest photoprotection against a subsequent UV exposure.
“We found that the areas that had been irradiated with UVB only or with UVA+UVB actually synthesized new melanin, which provided some protection against the subsequent UV challenge, but the UVA-only tans did not synthesize new melanin and provided no photoprotective benefit. This is an important consideration given that UVA-rich lamps are now frequently used in the indoor tanning industry to promote tanning with the implied potential benefit of reducing DNA damage and increasing protection against subsequent UV exposure,” said Hearing.
Another important factor identified in this study was the highly variable rate of DNA damage removal and/or repair among individuals even within the same ethnic group. Some individuals were highly efficient at repairing DNA lesions and no damage was evident one week after UV exposure; other individuals were inefficient in this process and repaired less than 50 percent of the initial UV damage within one week. This research will help cancer researchers to better understand the factors responsible for photocarcinogenesis, and the data also will inform the FDA as it regulates UV lamps.
Armed with Antibodies and 3-D Skin Models
Future research will include studies by Coelho, who is optimizing antibodies to eumelanins and pheomelanins. These antibodies will enable Hearing and his colleagues to assess the types and distributions of melanins in future projects. In addition, Hearing is working with 3-dimensional artificial, but physiologically relevant, skin models. These models will permit the Hearing team to regulate precisely the type, amounts, and distribution of melanins in the skin and then challenge the system with different wavelengths of UV to more critically assess the roles of different types of melanins in photoprotection.
Of course, Hearing and his colleagues will then have to validate all of the activities seen in these model systems. They will have to demonstrate that the same changes occur in human skin and see whether there are the same consequences. With validation complete, Hearing will yet again contribute valuable insights to skin cancer research.
“While we already have drilled down more than skin deep, much remains unknown about relationships between DNA damage repair and the different types, quantities, and distribution of melanins in the skin. Ongoing studies within our laboratory will continue to clarify these and new questions as they arise,” said Hearing.
To learn more about Dr. Hearing’s research, please visit his CCR Web site at http://ccr.cancer.gov/staff/ staff.asp?Name=hearing.