Embryonic Stem Cells
by Junying Yu* and James A. Thomson**
Human embryonic stem (ES) cells capture the imagination because they are immortal and have an almost unlimited developmental potential (Fig. 1.1: How hESCs are derived). After many months of growth in culture dishes, these remarkable cells maintain the ability to form cells ranging from muscle to nerve to blood—potentially any cell type that makes up the body. The proliferative and developmental potential of human ES cells promises an essentially unlimited supply of specific cell types for basic research and for transplantation therapies for diseases ranging from heart disease to Parkinson's disease to leukemia. Here we discuss the origin and properties of human ES cells, their implications for basic research and human medicine, and recent research progress since August 2001, when President George W. Bush allowed federal funding of this research for the first time. A previous report discussed progress prior to June 17, 2001 (/info/scireport/.) 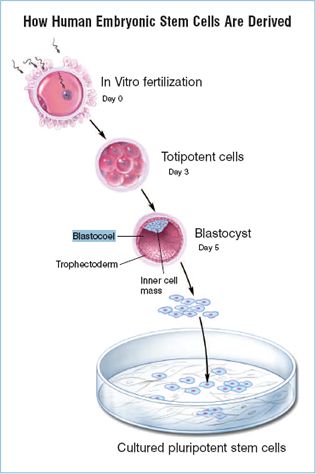
Figure 1.1. How Human Embryonic Stem Cells are Derived.
(© 2006 Terese Winslow)
What Are Embryonic Stem Cells?
Embryonic stem cells are derived from embryos at a developmental stage before the time that implantation would normally occur in the uterus. Fertilization normally occurs in the oviduct, and during the next few days, a series of cleavage divisions occur as the embryo travels down the oviduct and into the uterus. Each of the cells (blastomeres) of these cleavage-stage embryos are undifferentiated, i.e. they do not look or act like the specialized cells of the adult, and the blastomeres are not yet committed to becoming any particular type of differentiated cell. Indeed, each of these blastomeres has the potential to give rise to any cell of the body. The first differentiation event in humans occurs at approximately five days of development, when an outer layer of cells committed to becoming part of the placenta (the trophectoderm) separates from the inner cell mass (ICM). The ICM cells have the potential to generate any cell type of the body, but after implantation, they are quickly depleted as they differentiate to other cell types with more limited developmental potential. However, if the ICM is removed from its normal embryonic environment and cultured under appropriate conditions, the ICM-derived cells can continue to proliferate and replicate themselves indefinitely and still maintain the developmental potential to form any cell type of the body (quot;pluripotencyquot;; see Fig. 1.2: Characteristics of ESCs). These pluripotent, ICM-derived cells are ES cells. 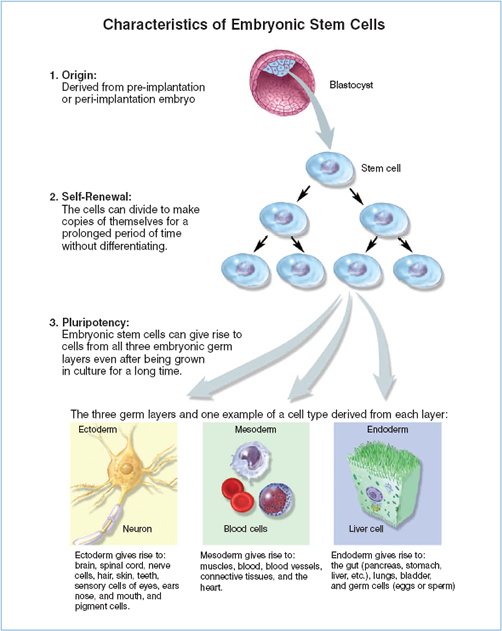
Figure 1.2.Characteristics of Embryonic Stem Cells.
(© 2006 Terese Winslow)
The derivation of mouse ES cells was first reported in 1981,1,2 but it was not until 1998 that derivation of human ES cell lines was first reported.3 Why did it take such a long time to extend the mouse results to humans? Human ES cell lines are derived from embryos produced by in vitro fertilization (IVF), a process in which oocytes and sperm are placed together to allow fertilization to take place in a culture dish. Clinics use this method to treat certain types of infertility, and sometimes, during the course of these treatments, IVF embryos are produced that are no longer needed by the couples for producing children. Currently, there are nearly 400,000 IVF-produced embryos in frozen storage in the United States alone,4 most of which will be used to treat infertility, but some of which (~2.8%) are destined to be discarded. IVF-produced embryos that would otherwise have been discarded were the sources of the human ES cell lines derived prior to President Bush's policy decision of August 2001. These human ES cell lines are now currently eligible for federal funding. Although attempts to derive human ES cells were made as early as the 1980s, culture media for human embryos produced by IVF were suboptimal. Thus, it was difficult to culture single-cell fertilized embryos long enough to obtain healthy blastocysts for the derivation of ES cell lines. Also, species-specific differences between mice and humans meant that experience with mouse ES cells was not completely applicable to the derivation of human ES cells. In the 1990s, ES cell lines from two non-human primates, the rhesus monkey5 and the common marmoset,6 were derived, and these offered closer models for the derivation of human ES cells. Experience with non-human primate ES cell lines and improvements in culture medium for human IVF-produced embryos led rapidly to the derivation of human ES cell lines in 1998.3
Because ES cells can proliferate without limit and can contribute to any cell type, human ES cells offer an unprecedented access to tissues from the human body. They will support basic research on the differentiation and function of human tissues and provide material for testing that may improve the safety and efficacy of human drugs (Figure 1.3: Promise of SC Research).7,8 For example, new drugs are not generally tested on human heart cells because no human heart cell lines exist. Instead, researchers rely on animal models. Because of important species-specific differences between animal and human hearts, however, drugs that are toxic to the human heart have occasionally entered clinical trials, sometimes resulting in death. Human ES cell-derived heart cells may be extremely valuable in identifying such drugs before they are used in clinical trials, thereby accelerating the drug discovery process and leading to safer and more effective treatments.9–11 Such testing will not be limited to heart cells, but to any type of human cell that is difficult to obtain by other sources. 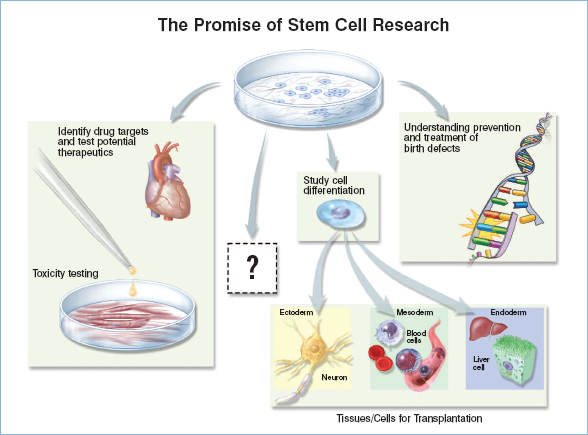
Figure 1.3: The Promise of Stem Cell Research.
(© 2006 Terese Winslow)
Human ES cells also have the potential to provide an unlimited amount of tissue for transplantation therapies to treat a wide range of degenerative diseases. Some important human diseases are caused by the death or dysfunction of one or a few cell types, e.g., insulin-producing cells in diabetes or dopaminergic neurons in Parkinson's disease. The replacement of these cells could offer a lifelong treatment for these disorders. However, there are a number of challenges to develop human ES cell-based transplantation therapies, and many years of basic research will be required before such therapies can be used to treat patients. Indeed, basic research enabled by human ES cells is likely to impact human health in ways unrelated to transplantation medicine. This impact is likely to begin well before the widespread use of ES cells in transplantation and ultimately could have a more profound long-term effect on human medicine. Since August 2001, improvements in culture of human ES cells, coupled with recent insights into the nature of pluripotency, genetic manipulation of human ES cells, and differentiation, have expanded the possibilities for these unique cells.
Culture of ES Cells
Mouse ES cells and human ES cells were both originally derived and grown on a layer of mouse fibroblasts (called quot;feeder cellsquot;) in the presence of bovine serum. However, the factors that sustain the growth of these two cell types appear to be distinct. The addition of the cytokine, leukemia inhibitory factor (LIF), to serum-containing medium allows mouse ES cells to proliferate in the absence of feeder cells. LIF modulates mouse ES cells through the activation of STAT3 (signal transducers and activators of transcription) protein. In serum-free culture, however, LIF alone is insufficient to prevent mouse ES cells from differentiating into neural cells. Recently, Ying et al. reported that the combination of bone morphogenetic proteins (BMPs) and LIF is sufficient to support the self-renewal of mouse ES cells.12 The effects of BMPs on mouse ES cells involve induction of inhibitor of differentiation (Id) proteins, and inhibition of extracellular receptor kinase (ERK) and p38 mitogen-activated protein kinases (MAPK).12,13 However, LIF in the presence of serum is not sufficient to promote the self-renewal of human ES cells,3 and the LIF/STAT3 pathway appears to be inactive in undifferentiated human ES cells.14,15 Also, the addition of BMPs to human ES cells in conditions that would otherwise support ES cells leads to the rapid differentiation of human ES cells.16,17
Several groups have attempted to define growth factors that sustain human ES cells and have attempted to identify culture conditions that reduce the exposure of human ES cells to non human animal products. One important growth factor, bFGF, allows the use of a serum replacement to sustain human ES cells in the presence of fibroblasts, and this medium allowed the clonal growth of human ES cells.18 A quot;feeder-freequot; human ES cell culture system has been developed, in which human ES cells are grown on a protein matrix (mouse Matrigel or Laminin) in a bFGF-containing medium that is previously quot;conditionedquot; by co-culture with fibroblasts.19 Although this culture system eliminates direct contact of human ES cells with the fibroblasts, it does not remove the potential for mouse pathogens being introduced into the culture via the fibroblasts. Several different sources of human feeder cells have been found to support the culture of human ES cells, thus removing the possibility of pathogen transfer from mice to humans.20–23 However, the possibility of pathogen transfer from human to human in these culture systems still remains. More work is still needed to develop a culture system that eliminates the use of fibroblasts entirely, which would also decrease much of the variability associated with the current culture of human ES cells. Sato et al. reported that activation of the Wnt pathway by 6-bromoindirubin3'-oxime (BIO) promotes the self-renewal of ES cells in the presence of bFGF, Matrigel, and a proprietary serum replacement product.24 Amit et al. reported that bFGF, TGFβ, and LIF could support some human ES cell lines in the absence of feeders.25 Although there are some questions about how well these new culture conditions will work for different human ES cell lines, there is now reason to believe that defined culture conditions for human ES cells, which reduce the potential for contamination by pathogens, will soon be achieved*.
Once a set of defined culture conditions is established for the derivation and culture of human ES cells, challenges to improve the medium will still remain. For example, the cloning efficiency of human ES cells—the ability of a single human ES cell to proliferate and become a colony—is very low (typically less than 1%) compared to that of mouse ES cells. Another difficulty is the potential for accumulation of genetic and epigenetic changes over prolonged periods of culture. For example, karyotypic changes have been observed in several human ES cell lines after prolonged culture, and the rate at which these changes dominate a culture may depend on the culture method.26,27 The status of imprinted (epigenetically modified) genes and the stability of imprinting in various culture conditions remain completely unstudied in human ES cells**. The status of imprinted genes can clearly change with culture conditions in other cell types.28,29 These changes present potential problems if human ES cells are to be used in cell replacement therapy, and optimizing medium to reduce the rate at which genetic and epigenetic changes accumulate in culture represents a long-term endeavor. The ideal human ES cell medium, then, (a) would be cost-effective and easy to use so that many more investigators can use human ES cells as a research tool; (b) would be composed entirely of defined components not of animal origin; (c) would allow cell growth at clonal densities; and (d) would minimize the rate at which genetic and epigenetic changes accumulate in culture. Such a medium will be a challenge to develop and will most likely be achieved through a series of incremental improvements over a period of years.
Among all the newly derived human ES cell lines, twelve lines have gained the most attention. In March 2004, a South Korean group reported the first derivation of a human ES cell line (SCNT-hES-1) using the technique of somatic cell nuclear transfer (SCNT). Human somatic nuclei were transferred into human oocytes (nuclear transfer), which previously had been stripped of their own genetic material, and the resultant nuclear transfer products were cultured in vitro to the blastocyst stage for ES cell derivation.30*** Because the ES cells derived through nuclear transfer contain the same genetic material as that of the nuclear donor, the intent of the procedure is that the differentiated derivatives would not be rejected by the donor's immune system if used in transplantation therapy. More recently, the same group reported the derivation of eleven more human SCNT-ES cell lines*** with markedly improved efficiency (16.8 oocytes/line vs. 242 oocytes/line in their previous report).31*** However, given the abnormalities frequently observed in cloned animals, and the costs involved, it is not clear how useful this procedure will be in clinical applications. Also, for some autoimmune diseases, such as type I diabetes, merely providing genetically-matched tissue will be insufficient to prevent immune rejection.
Additionally, new human ES cell lines were established from embryos with genetic disorders, which were detected during the practice of preimplantation genetic diagnosis (PGD). These new cell lines may provide an excellent in vitro model for studies on the effects that the genetic mutations have on cell proliferation and differentiation.32
* Editor's note: Papers published since this writing report defined culture conditions for human embryonic stem cells. See Ludwig et al., Nat. Biotech 24: 185–187, 2006; and Lu et al., PNAS 103:5688–5693, 2006.08.14.
** Editor's note: Papers published since the time this chapter was written address this: see Maitra et al., Nature Genetics 37, 1099–1103, 2005; and Rugg-Gunn et al., Nature Genetics 37:585–587, 2005.
*** Editor's note: Both papers referenced in 30 and 31 were later retracted: see Science 20 Jan 2006; Vol. 311. No. 5759, p. 335.
To date, more than 120 human ES cell lines have been established worldwide,33* 67 of which are included in the National Institutes of Health (NIH) Registry. As of this writing, 21 cell lines are currently available for distribution, all of which have been exposed to animal products during their derivation. Although it has been eight years since the initial derivation of human ES cells, it is an open question as to the extent that independent human ES cell lines differ from one another. At the very least, the limited number of cell lines cannot represent a reasonable sampling of the genetic diversity of different ethnic groups in the United States, and this has consequences for drug testing, as adverse reactions to drugs often reflect a complex genetic component. Once defined culture conditions are well established for human ES cells, there will be an even more compelling need to derive additional cell lines.
* Editor's note: One recent report now estimates 414 hESC lines, see Guhr et al., www.StemCells.com early online version for June 15, 2006: quot;Current State of Human Embryonic Stem Cell Research: An Overview of Cell Lines and their Usage in Experimental Work.quot;
Pluripotency of ES Cells
The ability of ES cells to develop into all cell types of the body has fascinated scientists for years, yet remarkably little is known about factors that make one cell pluripotent and another more restricted in its developmental potential. The transcription factor Oct4 has been used as a key marker for ES cells and for the pluripotent cells of the intact embryo, and its expression must be maintained at a critical level for ES cells to remain undifferentiated.34 The Oct4 protein itself, however, is insufficient to maintain ES cells in the undifferentiated state. Recently, two groups identified another transcription factor, Nanog, that is essential for the maintenance of the undifferentiated state of mouse ES cells.35,36 The expression of Nanog decreased rapidly as mouse ES cells differentiated, and when its expression level was maintained by a constitutive promoter, mouse ES cells could remain undifferentiated and proliferate in the absence of either LIF or BMP in serum-free medium.12 Nanog is also expressed in human ES cells, though at a much lower level compared to that of Oct4, and its function in human ES cells has yet to be examined.
By comparing gene expression patterns between different ES cell lines and between ES cells and other cell types such as adult stem cells and differentiated cells, genes that are enriched in the ES cells have been identified. Using this approach, Esg-1, an uncharacterized ES cell-specific gene, was found to be exclusively associated with pluripotency in the mouse.37 Sperger et al. identified 895 genes that are expressed at significantly higher levels in human ES cells and embryonic carcinoma cell lines, the malignant counterparts to ES cells.38 Sato et al. identified a set of 918 genes enriched in undifferentiated human ES cells compared with their differentiated counterparts; many of these genes were shared by mouse ES cells.39 Another group, however, found 92 genes, including Oct4 and Nanog, enriched in six different human ES cell lines, which showed limited overlap with those in mouse ES cell lines.40 Care must be taken to interpret these data, and the considerable differences in the results may arise from the cell lines used in the experiments, methods to prepare and maintain the cells, and the specific methods used to profile gene expression.
Genetic Manipulation of ES Cells
Since establishing human ES cells in 1998, scientists have developed genetic manipulation techniques to determine the function of particular genes, to direct the differentiation of human ES cells towards specific cell types, or to tag an ES cell derivative with a certain marker gene. Several approaches have been developed to introduce genetic elements randomly into the human ES cell genome, including electroporation, transfection by lipid-based reagents, and lentiviral vectors.41–44 However, homologous recombination, a method in which a specific gene inside the ES cells is modified with an artificially introduced DNA molecule, is an even more precise method of genetic engineering that can modify a gene in a defined way at a specific locus. While this technology is routinely used in mouse ES cells, it has recently been successfully developed in human ES cells (See chapter 4: Genetically Modified Stem Cells), thus opening new doors for using ES cells as vehicles for gene therapy and for creating in vitro models of human genetic disorders such as Lesch-Nyhan disease.45,46 Another method to test the function of a gene is to use RNA interference (RNAi) to decrease the expression of a gene of interest (see Figure 1.4: RNA interference). In RNAi, small pieces of double-stranded RNA (siRNA; small interfering RNA) are either chemically synthesized and introduced directly into cells, or expressed from DNA vectors. Once inside the cells, the siRNA can lead to the degradation of the messenger RNA (mRNA), which contains the exact sequence as that of the siRNA. mRNA is the product of DNA transcription and normally can be translated into proteins. RNAi can work efficiently in somatic cells, and there has been some progress in applying this technology to human ES cells.47–49 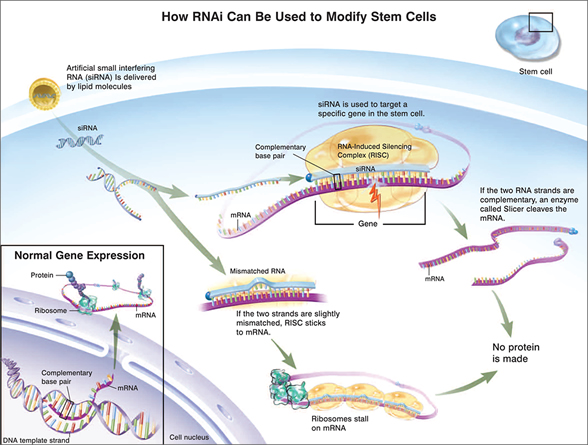
Figure 1.4. How RNAi Can Be Used To Modify Stem Cells.
(© 2006 Terese Winslow)
The pluripotency of ES cells suggests possible widespread uses for these cells and their derivatives. The ES cell-derived cells can potentially be used to replace or restore tissues that have been damaged by disease or injury, such as diabetes, heart attacks, Parkinson's disease or spinal cord injury. The recent developments in these particular areas are discussed in detail in other chapters, and Table 1 summarizes recent publications in the differentiation of specific cell lineages.
The differentiation of ES cells also provides model systems to study early events in human development. Because of possible harm to the resulting child, it is not ethically acceptable to experimentally manipulate the postimplantation human embryo. Therefore, most of what is known about the mechanisms of early human embryology and human development, especially in the early postimplantation period, is based on histological sections of a limited number of human embryos and on analogy to the experimental embryology of the mouse. However, human and mouse embryos differ significantly, particularly in the formation, structure, and function of the fetal membranes and placenta, and the formation of an embryonic disc instead of an egg cylinder.50–52 For example, the mouse yolk sac is a well-vascularized, robust, extraembryonic organ throughout gestation that provides important nutrient exchange functions. In humans, the yolk sac also serves important early functions, including the initiation of hematopoiesis, but it becomes essentially a vestigial structure at later times or stages in gestation. Similarly, there are dramatic differences between mouse and human placentas, both in structure and function. Thus, mice can serve in a limited capacity as a model system for understanding the developmental events that support the initiation and maintenance of human pregnancy. Human ES cell lines thus provide an important new in vitro model that will improve our understanding of the differentiation of human tissues, and thus provide important insights into processes such as infertility, pregnancy loss, and birth defects.
Human ES cells are already contributing to the study of development. For example, it is now possible to direct human ES cells to differentiate efficiently to trophoblast, the outer layer of the placenta that mediates implantation and connects the conceptus to the uterus.17,53 Another use of human ES cells is for the study of germ cell development. Cells resembling both oocytes and sperm have been successfully derived from mouse ES cells in vitro.54–56 Recently, human ES cells have also been observed to differentiate into cells expressing genes characteristic of germ cells.57 Thus it may also be possible to derive oocytes and sperm from human ES cells, allowing the detailed study of human gametogenesis for the first time. Moreover, human ES cell studies are not limited to early differentiation, but are increasingly being used to understand the differentiation and functions of many human tissues, including neural, cardiac, vascular, pancreatic, hepatic, and bone (see Table 1). Moreover, transplantation of ES-derived cells has offered promising results in animal models.58–67
Although scientists have gained more insights into the biology of human ES cells since 2001, many key questions remain to be addressed before the full potential of these unique cells can be realized. It is surprising, for example, that mouse and human ES cells appear to be so different with respect to the molecules that mediate their self-renewal, and perhaps even in their developmental potentials. BMPs, for example, in combination with LIF, promote the self-renewal of mouse ES cells. But in conditions that would otherwise support undifferentiated proliferation, BMPs cause rapid differentiation of human ES cells. Also, human ES cells differentiate quite readily to trophoblast, whereas mouse ES cells do so poorly, if at all. One would expect that at some level, the basic molecular mechanisms that control pluripotency would be conserved, and indeed, human and mouse ES cells share the expression of many key genes. Yet we remain remarkably ignorant about the molecular mechanisms that control pluripotency, and the nature of this remarkable cellular state has become one of the central questions of developmental biology. Of course, the other great challenge will be to continue to unravel the factors that control the differentiation of human ES cells to specific lineages, so that ES cells can fulfill their tremendous promise in basic human biology, drug screening, and transplantation medicine.
Table 1. Publications on Differentiation of Human Embryonic Stem Cells since 2001
| Cell types |
Publications |
References |
| Neural |
8 |
61, 66, 68–73 |
| Cardiac |
6 |
9–11, 74–76 |
| Endothelial (Vascular) |
2 |
77, 78 |
| Hematopoietic (Blood) |
8 |
79–86 |
| Pancreatic (Islet-like) |
2 |
87, 88 |
| Hepatic (Liver) |
3 |
89–91 |
| Bone |
1 |
92 |
| Trophoblast |
2 |
17, 53 |
| Multilineages |
9 |
16, 57, 93–99 |
Acknowledgement
We thank Lynn Schmidt, Barbara Lewis, Sangyoon Han and Deborah J. Faupel for proofreading this report.
References
- Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156.
- Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci USA. 1981;78:7634–7638.
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147.
- Hoffman DI, Zellman GL, Fair CC, et al. Cryopreserved embryos in the United States and their availability for research. Fertil Steril. 2003;79:1063–1069.
- Thomson JA, Kalishman J, Golos TG, et al. Isolation of a primate embryonic stem cell line. Proc Natl Acad Sci USA. 1995;92:7844–7848.
- Thomson JA, Kalishman J, Golos TG, Durning M, Harris CP, Hearn JP. Pluripotent cell lines derived from common marmoset (Callithrix jacchus) blastocysts. Biol Reprod. 1996;55:254–259.
- Bremer S, Hartung T. The use of embryonic stem cells for regulatory developmental toxicity testing in vitro—the current status of test development. Curr Pharm Des. 2004;10:2733–2747.
- Rolletschek A, Blyszczuk P, Wobus AM. Embryonic stem cell-derived cardiac, neuronal and pancreatic cells as model systems to study toxicological effects. Toxicol Lett. 2004;149:361–369.
- He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–39.
- Mummery C, Ward-van Oostwaard D, Doevendans P, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740.
- Vanderlaan RD, Oudit GY, Backx PH. Electrophysiological profiling of cardiomyocytes in embryonic bodies derived from human embryonic stem cells. Circ Res. 2003;93:1–3.
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292.
- Qi X, Li TG, Hao J, et al. BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways. Proc Natl Acad Sci USA. 2004;101:6027–6032.
- Daheron L, Optiz SL, Zaehres H, et al. LIF/STAT3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells. 2004;22:770–778.
- Humphrey RK, Beattie GM, Lopez AD, et al. Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells. 2004;22:522–530.
- Pera MF, Andrade J, Houssami S, et al. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117:1269–1280.
- Xu RH, Chen X, Li DS, et al. BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat Biotechnol. 2002;20:1261–1264.
- Amit M, Carpenter MK, Inokuma MS, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974.
- Amit M, Margulets V, Segev H, et al. Human feeder layers for human embryonic stem cells. Biol Reprod. 2003;68:2150–2156.
- Lee JB, Lee JE, Park JH, et al. Establishment and maintenance of human embryonic stem cell lines on human feeder cells derived from uterine endometrium under serum-free condition. Biol Reprod. 2004.
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–936.
- Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546–556.
- Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor. Nat Med. 2004;10:55–63.
- Amit M, Shariki K, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845.
- Draper JS, Smith K, Gokhale P, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22:53–54.
- Inzunza J, Sahlen S, Holmberg K, et al. Comparative genomic hybridization and karyotyping of human embryonic stem cells reveals the occurrence of an isodicentric X chromosome after long-term cultivation. Mol Hum Reprod. 2004;10:461–466.
- Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62:1526–1535.
- Mann MR, Lee SS, Doherty AS, et al. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131:3727–3735.
- Hwang WS, Ryu YJ, Park JH, et al. Evidence of a pluripotent human embryonic stem cell line derived from a cloned blastocyst. Science. 2004;303:1669–1674.
- Hwang WS, Roh SI, Lee BC, et al. Patient-specific embryonic stem cells derived from human SCNT blastocysts. Science. Jun 17 2005;308(5729):1777–1783.
- Verlinsky Y, Strelchenko N, Kukharenko V, et al. Human embryonic stem cell lines with genetic disorders. Reprod Biomed Online. 2005;10:105–110.
- Stojkovic M, Lako M, Strachan T, Murdoch A. Derivation, growth, and applications of human embryonic stem cells. Reproduction. 2004;128:259–267.
- Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–376.
- Chambers I, Colby D, Robertson M, et al. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655.
- Mitsui K, Tokuzawa Y, Itoh H, et al. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642.
- Tanaka TS, Kunath T, Kimber WL, et al. Gene expression profiling of embryo-derived stem cells reveals genes associated with pluripotency and lineage specificity. Genome Res. 2002;12:1921–1928.
- Sperger JM, Chen X, Draper JS, et al. Gene expression patterns in human embryonic stem cells and human pluripotent germ cell tumors. Proc Natl Acad Sci USA. 2003;100:13350–13355.
- Sato N, Sanjuan IM, Heke M, Uchida M, Naef F, Brivanlou AH. Molecular signature of human embryonic stem cells and its comparison with the mouse. Dev Biol. 2003;260:404–413.
- Bhattacharya B, Miura T, Brandenberger R, et al. Gene expression in human embryonic stem cell lines: unique molecular signature. Blood. 2004;103:2956–2964.
- Eiges R, Schuldiner M, Drukker M, Yanuka O, Itskovitz-Eldor J, Benvenisty N. Establishment of human embryonic stem cell-transfected clones carrying a marker for undifferentiated cells. Curr Biol. 2001;11:514–518.
- Gropp M, Itsykson P, Singer O, et al. Stable genetic modification of human embryonic stem cells by lentiviral vectors. Mol Ther. 2003;7:281–287.
- Lakshmipathy U, Pelacho B, Sudo K, et al. Efficient transfection of embryonic and adult stem cells. Stem Cells. 2004;22:531–543.
- Ma Y, Ramezani A, Lewis R, Hawley RG, Thomson JA. High-level sustained transgene expression in human embryonic stem cells using lentiviral vectors. Stem Cells. 2003;21:111–117.
- Urbach A, Schuldiner M, Benvenisty N. Modeling for Lesch-Nyhan disease by gene targeting in human embryonic stem cells. Stem Cells. 2004;22:635–641.
- Zwaka TP, Thomson JA. Homologous recombination in human embryonic stem cells. Nat Biotechnol. 2003;21:319–321.
- Matin MM, Walsh JR, Gokhale PJ, et al. Specific knockdown of Oct4 and beta2-microglobulin expression by RNA interference in human embryonic stem cells and embryonic carcinoma cells. Stem Cells. 2004;22:659–668.
- Vallier L, Rugg-Gunn PJ, Bouhon IA, Andersson FK, Sadler AJ, Pedersen RA. Enhancing and diminishing gene function in human embryonic stem cells. Stem Cells. 2004;22:2–11.
- Velkey JM, O'Shea KS. Oct4 RNA interference induces trophectoderm differentiation in mouse embryonic stem cells. Genesis. 2003;37:18–24.
- Castellucci M, Scheper M, Scheffen I, Celona A, Kaufmann P. The development of the human placental villous tree. Anat Embryol (Berl). 1990;181:117–128.
- Luckett WP. The development of primordial and definitive amniotic cavities in early Rhesus monkey and human embryos. Am J Anat. 1975;144:149–167.
- Luckett WP. Origin and differentiation of the yolk sac and extraembryonic mesoderm in presomite human and rhesus monkey embryos. Am J Anat. 1978;152:59–97.
- Gerami-Naini B, Dovzhenko OV, Durning M, Wegner FH, Thomson JA, Golos TG. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology. 2004;145:1517–1524.
- Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154.
- Hubner K, Fuhrmann G, Christenson LK, et al. Derivation of oocytes from mouse embryonic stem cells. Science. 2003;300:1251–1256.
- Toyooka Y, Tsunekawa N, Akasu R, Noce T. Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci USA. 2003;100:11457–11462.
- Clark AT, Bodnar MS, Fox M, et al. Spontaneous differentiation of germ cells from human embryonic stem cells in vitro. Hum Mol Genet. 2004;13:727–739.
- Bjorklund LM, Sanchez-Pernaute R, Chung S, et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinsonian rat model. Proc Natl Acad Sci USA. 2002;99:2344–2349.
- Chiba S, Ikeda R, Kurokawa MS, et al. Anatomical and functional recovery by embryonic stem cell-derived neural tissue of a mouse model of brain damage. J Neurol Sci. 2004;219:107–117.
- Kim D, Gu Y, Ishii M, et al. In vivo functioning and transplantable mature pancreatic islet-like cell clusters differentiated from embryonic stem cells. Pancreas. 2003;27:e34-e41.
- Lee DH, Park S, Kim EY, et al. Enhancement of re-closure capacity by the intra-amniotic injection of human embryonic stem cells in surgically induced spinal open neural tube defects in chick embryos. Neurosci Lett. 2004;364:98–100.
- Marchetti S, Gimond C, Iljin K, et al. Endothelial cells genetically selected from differentiating mouse embryonic stem cells incorporate at sites of neovascularization in vivo. J Cell Sci. 2002;115:2075–2085.
- Min JY, Yang Y, Converso KL, et al. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol. 2002;92:288–296.
- Miyagi T, Takeno M, Nagafuchi H, Takahashi M, Suzuki M. Flk1+ cells derived from mouse embryonic stem cells reconstitute hematopoiesis in vivo in SCID mice. Exp Hematol. 2002;30:1444–1453.
- Nishimura F, Yoshikawa M, Kanda S, et al. Potential use of embryonic stem cells for the treatment of mouse parkinsonian models: improved behavior by transplantation of in vitro differentiated dopaminergic neurons from embryonic stem cells. Stem Cells. 2003;21:171–180.
- Park S, Kim EY, Ghil GS, et al. Genetically modified human embryonic stem cells relieve symptomatic motor behavior in a rat model of Parkinson's disease. Neurosci Lett. 2003;353:91–94.
- von Unge M, Dirckx JJ, Olivius NP. Embryonic stem cells enhance the healing of tympanic membrane perforations. Int J Pediatr Otorhinolaryngol. 2003;67:215–219.
- Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397.
- Park S, Lee KS, Lee YJ, et al. Generation of dopaminergic neurons in vitro from human embryonic stem cells treated with neurotrophic factors. Neurosci Lett. 2004;359:99–103.
- Reubinoff BE, Itsykson P, Turetsky T, et al. Neural progenitors from human embryonic stem cells. Nat Biotechnol. 2001;19:1134–1140.
- Schuldiner M, Eiges R, Eden A, et al. Induced neuronal differentiation of human embryonic stem cells. Brain Res. 2001;913:201–205.
- Schulz TC, Palmarini GM, Noggle SA, Weiler DA, Mitalipova MM, Condie BG. Directed neuronal differentiation of human embryonic stem cells. BMC Neurosci. 2003;4:27.
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. in vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–1133.
- Kehat I, Amit M, Gepstein A, Huber I, Itskovitz-Eldor J, Gepstein L. Development of cardiomyocytes from human ES cells. Methods Enzymol. 2003;365:461–473.
- Satin J, Kehat I, Caspi O, et al. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol. 2004;559:479–496.
- Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–508.
- Gerecht-Nir S, Ziskind A, Cohen S, Itskovitz-Eldor J. Human embryonic stem cells as an in vitro model for human vascular development and the induction of vascular differentiation. Lab Invest. 2003;83:1811–1820.
- Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2002;99:4391–4396.
- Cerdan C, Rouleau A, Bhatia M. VEGF-A165 augments erythropoietic development from human embryonic stem cells. Blood. 2004;103:2504–2512.
- Chadwick K, Wang L, Li L, et al. Cytokines and BMP-4 promote hematopoietic differentiation of human embryonic stem cells. Blood. 2003;102:906–915.
- Kaufman DS, Hanson ET, Lewis RL, Auerbach R, Thomson JA. Hematopoietic colony-forming cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2001;98:10716–10721.
- Lu SJ, Li F, Vida L, Honig GR. CD34+CD38- hematopoietic precursors derived from human embryonic stem cells exhibit an embryonic gene expression pattern. Blood. 2004;103:4134–4141.
- Tian X, Kaufman DS. Hematopoietic development of human embryonic stem cells in culture. Methods Mol Biol. 2004;290:149–162.
- Vodyanik MA, Bork JA, Thomson JA, Slukvin II. Human embryonic stem cell-derived CD34+ cells: efficient production in the co-culture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2004.
- Wang L, Li L, Shojaei F, et al. Endothelial and hematopoietic cell fate of human embryonic stem cells originates from primitive endothelium with hemangioblastic properties. Immunity. 2004;21:31–41.
- Zhan X, Dravid G, Ye Z, et al. Functional antigen-presenting leucocytes derived from human embryonic stem cells in vitro. Lancet. 2004;364:163–171.
- Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–1697.
- Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells. 2004;22:265–274.
- Lavon N, Yanuka O, Benvenisty N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation. 2004;72:230–238.
- Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1–11.
- Shirahashi H, Wu J, Yamamoto N, et al. Differentiation of human and mouse embryonic stem cells along a hepatocyte lineage. Cell Transplant. 2004;13:197–211.
- Sottile V, Thomson JA, McWhir J. in vitro osteogenic differentiation of human ES cells. Cloning Stem Cells. 2003;5:149–155.
- Calhoun JD, Rao RR, Warrenfeltz S, et al. Transcriptional profiling of initial differentiation events in human embryonic stem cells. Biochem Biophys Res Commun. 2004;323:453–464.
- Conley BJ, Trounson AO, Mollard R. Human embryonic stem cells form embryoid bodies containing visceral endoderm-like derivatives. Fetal Diagn Ther. 2004;19:218–223.
- Dang SM, Gerecht-Nir S, Chen J, Itskovitz-Eldor J, Zandstra PW. Controlled, scalable embryonic stem cell differentiation culture. Stem Cells. 2004;22:275–282.
- Dang SM, Zandstra PW. Scalable production of embryonic stem cell-derived cells. Methods Mol Biol. 2004;290:353–364.
- Gertow K, Wolbank S, Rozell B, et al. Organized development from human embryonic stem cells after injection into immunodeficient mice. Stem Cells Dev. 2004;13:421–435.
- Goldstein RS, Drukker M, Reubinoff BE, Benvenisty N. Integration and differentiation of human embryonic stem cells transplanted to the chick embryo. Dev Dyn. 2002;225:80–86.
- Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–12746.
Notes:
* Genetics and Biotechnology Building, Madison, WI 53706, Email: jyu@primate.wisc.edu.
** John D. MacArthur Professor, Department of Anatomy, University of Wisconsin–Madison Medical School, The Genome Center of Wisconsin, and The Wisconsin National Primate Research Center, Madison, WI 53715, Email: thomson@primate.wisc.edu.
 Introduction | Table of Contents | Chapter 2 Introduction | Table of Contents | Chapter 2 
|
|