Alternate Methods for Preparing Pluripotent Stem Cells
James F. Battey, Jr., MD, PhD; Laura K. Cole, PhD; and Charles A. Goldthwaite, Jr., PhD.
The Clinical Application of Pluripotent Cells: The Promise and the Challenges
Stem cells are distinguished from other cells by two characteristics: (1) they can divide to produce copies of themselves (self-renewal) under appropriate conditions and (2) they are pluripotent, or able to differentiate into any of the three germ layers: the endoderm (which forms the lungs, gastrointestinal tract, and interior lining of the stomach), mesoderm (which forms the bones, muscles, blood, and urogenital tract), and ectoderm (which forms the epidermal tissues and nervous system). Pluripotent cells, which can differentiate into any mature cell type, are distinct from multipotent cells (such as hematopoietic, or blood-forming, cells) that can differ into a limited number of mature cell types. Because of their pluripotency and capacity for self-renewal, stem cells hold great potential to renew tissues that have been damaged by conditions such as type 1 diabetes, Parkinson's disease, heart attacks, and spinal cord injury. Although techniques to transplant multipotent or pluripotent cells are being developed for many specific applications, some procedures are sufficiently mature to be established options for care. For example, human hematopoietic cells from the umbilical cord and bone marrow are currently being used to treat patients with disorders that require replacement of cells made by the bone marrow, including Fanconi's anemia and chemotherapy-induced bone marrow failure after cancer treatment.
However, differentiation is influenced by numerous factors, and investigators are just beginning to understand the fundamental properties of human pluripotent cells. Researchers are gradually learning how to direct these cells to differentiate into specialized cell types and to use them for research, drug discovery, and transplantation therapy (see Figure 8.1). However, before stem cell derivatives are suitable for clinical application, scientists require a more complete understanding of the molecular mechanisms that drive pluripotent cells into differentiated cells. Scientists will need to pilot experimental transplantation therapies in animal model systems to assess the safety and long-term stable functioning of transplanted cells. In particular, they must be certain that any transplanted cells do not continue to self-renew in an unregulated fashion after transplantation, which may result in a teratoma, or stem cell tumor. In addition, scientists must ascertain that cells transplanted into a patient are not recognized as foreign by the patient's immune system and rejected.
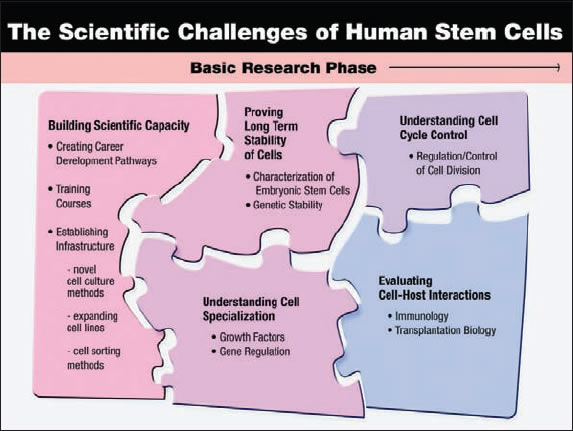
Figure 8.1. The Scientific Challenge of Human Stem Cells
The state of the science currently lies in the development of fundamental knowledge of the properties of human pluripotent cells. The scientific capacity needs to be built, an understanding of the molecular mechanisms that drive cell specialization needs to be advanced, the nature and regulation of interaction between host and transplanted cells needs to be explored and understood, cell division needs to be understood and regulated, and the long-term stability of the function in transplanted cells needs to be established.
Stem cells derived from an early-stage human blastocyst (an embryo fertilized in vitro and grown approximately five days in culture) have the capacity to renew indefinitely, and can theoretically provide an unlimited supply of cells. It is also possible to derive stem cells from non-embryonic tissues, including amniotic fluid, placenta, umbilical cord, brain, gut, bone marrow, and liver. These stem cells are sometimes called "adult" stem cells, and they are typically rare in the tissue of origin. For example, blood-forming (hematopoietic) stem cell experts estimate that only 1 in 2000 to fewer than 1 in 10,000 cells found in the bone marrow is actually a stem cell.1 Because so-called "adult" stem cells include cells from the placenta and other early stages of development, they are more correctly termed "non-embryonic stem cells." Non-embryonic stem cells are more limited in their capacity to self renew in the laboratory, making it more difficult to generate a large number of stem cells for a specific experimental or therapeutic application. Under normal conditions, non-embryonic stem cells serve as a repair pool for the body, so they typically differentiate only into the cell types found in the organ of origin. Moreover, there is little compelling evidence for trans-differentiation, whereby a stem cell from one organ differentiates into a mature cell type of a different organ. New discoveries may overcome these limitations of stem cells derived from non-embryonic sources, and research directed toward this goal is currently underway in a number of laboratories.
The Role of Cultured Cells in Understanding The Differentiation Process
Cultures of human pluripotent, self-renewing cells enable researchers to understand the molecular mechanisms that regulate differentiation (see Figure 8.2), including epigenetic changes (traits that may be inherited that do not arise from changes in the DNA sequence) in the chromatin structure, developmental changes in gene expression, exposure to growth factors, and interactions between adjacent cells. Understanding these basic mechanisms may enable future scientists to mobilize and differentiate endogenous populations of pluripotent cells to replace a cell type ravaged by injury or disease. Alternatively, scientists may some day be able to coax human pluripotent cells grown in the laboratory to become a specific type of specialized cell, which physicians could subsequently transplant into a patient to replace cells damaged by these same disease processes.
Scientists are gradually learning to direct the differentiation of pluripotent cell cultures into a specific type of cell, which can then be used as cellular models of human disease for drug discovery or toxicity studies. While it is not possible to predict the myriad ways that a basic understanding of stem cell differentiation may lead to new approaches for treating patients with cellular degenerative diseases, some avenues can be theorized. For example, in the case of Huntington's disease, a fatal neurodegenerative disorder, one could imagine that pluripotent cells derived from an embryo that carries Huntington's disease and differentiated into neurons in culture could be used to test drugs to delay or prevent degeneration.
Despite the incredible growth in knowledge that has occurred in stem cell research within the last couple of decades, investigators are just beginning to unravel the process of differentiation. Human pluripotent cell lines are an essential tool to understand this process and to facilitate the ultimate use of these cells in the clinic. To provide background on this fundamental topic, this article reviews the various potential sources and approaches that have been used to generate human pluripotent and multipotent cell lines, both of embryonic and non-embryonic origin.
Establishing Human Pluripotent Stem Cell Lines from Embryonic or Fetal Tissues
Currently, at least six embryonic sources have been used to establish human pluripotent stem cell lines. All approaches involve isolation of viable cells during an early phase of development, followed by growth of these cells in appropriate culture medium. The various sources of these initial cell populations are discussed in brief below. It should be noted that the manipulation and use of embryonic tissues has raised a number of ethical issues.2,3 This article focuses on the scientific and technical issues associated with creating pluripotent cells, with the understanding that some of these techniques are currently subject to debates that extend beyond discussions of their scientific merits.
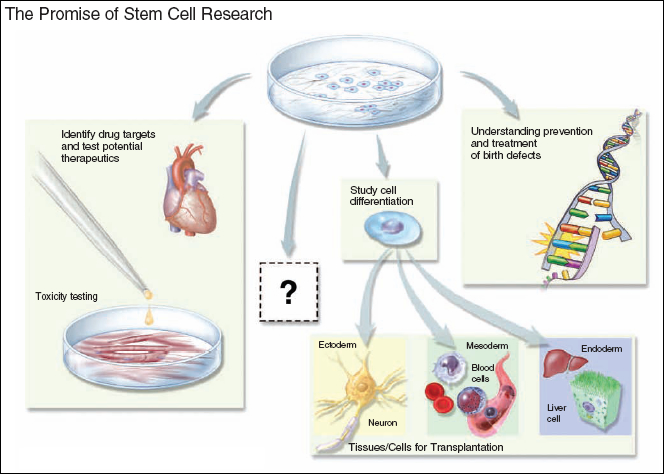
Figure 8.2. The Promise of Stem Cell Research
Stem cell research provides a useful tool for unraveling the molecular mechanisms that determine the differentiation fate of a pluripotent cell and for understanding the gene expression properties and epigenetic modifications essential to maintain the pluripotent state. In the future, this knowledge may be used to generate cells for transplantation therapies, whereby a specific cell population compromised by disease is replaced with new, functional cells. Differentiated derivatives of human pluripotent cells may also prove to be useful as models for understanding the biology of disease and developing new drugs, particularly when there is no animal model for the disease being studied. The greatest promise of stem cell research may lie in an area not yet imagined.
© 2008 Terese Winslow
Traditional Human Embryonic Stem Cell (hESC) Line Generation
Drawing upon twenty years of communal expertise with mouse ES cells,4 and on human inner cell mass culture conditions developed by Ariff Bongso and colleagues,5 James Thomson and colleagues at the University of Wisconsin generated the first hESC lines in 1998 using tissue from embryos fertilized in vitro.6 This method uses embryos generated for in vitro fertilization (IVF) that are no longer needed for reproductive purposes. During IVF, medical professionals usually produce more embryos than a couple attempting to start a family may need. Spare embryos are typically stored in a freezer to support possible future attempts for additional children if desired. It is estimated that there are approximately 400,000 such spare embryos worldwide.6 If these embryos are never used by the couple, they either remain in storage or are discarded as medical waste. Alternatively, these embryos can potentially be used to generate a hESC line.
To generate a hESC line, scientists begin with a donated blastocyst-stage embryo, at approximately five days after IVF (see Figure 8.3a). The blastocyst consists of approximately 150–200 cells that form a hollow sphere of cells, the outer layer of which is called the trophectoderm. During normal development, the trophoblast becomes the placenta and umbilical cord. At one pole of this hollow sphere, 30–50 cells form a cluster that is called the inner cell mass (ICM), which would give rise to the developing fetus. ICM cells are pluripotent, possessing the capacity to become any of the several hundred specialized cell types found in a developed human, with the exception of the placenta and umbilical cord.
Scientists remove the ICM from the donated blastocyst and place these cells into a specialized culture medium. In approximately one in five attempts, a hESC line begins to grow. Stem cells grown in such a manner can then be directed to differentiate into various lineages, including neural precursor cells,8 cardiomyocytes,9 and hematopoietic (blood forming) precursor cells.10
However, hESC lines are extremely difficult to grow in culture; the cells require highly specialized growth media that contain essential ingredients that are difficult to standardize. Yet the culture conditions are critical to maintain the cells' self-renewing and pluripotent properties. Culture requires the support of mouse or human cells, either directly as a "feeder" cell layer6,11,12 or indirectly as a source of conditioned medium in feeder-free culture systems.13 The feeder cells secrete important nutrients and otherwise support stem cell growth, but are treated so they cannot divide. Although the complete role of these feeder cells is not known, they promote stem cell growth, including detoxifying the culture medium and secreting proteins that participate in cell growth.14 hESC lines used to produce human cells for transplantation therapies may need to be propagated on a human feeder cell layer to reduce the risk of contamination by murine viruses or other proteins that may cause rejection. Thus, hESC lines often grow only under highly specific culture conditions, and the identification of ideal growth conditions presents a challenge regardless of the source of the hESCs.
Furthermore, human ES cell cultures must be expanded using an exacting protocol to avoid cell death and to control spontaneous differentiation. Since a limited number of laboratories in the United States are growing these cells, there is a shortage of people well-versed in the art and science of successful hESC culture. In the short term, challenges of working with these cells include developing robust culture conditions and protocols, understanding the molecular mechanisms that direct differentiation into specific cell types, and developing the infrastructure to advance this scientific opportunity. Once these challenges have been met, scientists will need to conduct transplantation studies in animal models (rodent and non-human primates) to demonstrate safety, effectiveness, and long-term benefit before stem cell therapies may enter clinical trials.
hESC Lines from Human Primordial Germ Cells
A second method for generating human pluripotent stem cell lines was published in 1998 by John Gearhart and coworkers at The Johns Hopkins Medical School.15
These researchers isolated specialized cells known as primordial germ cells (PGCs) from a 5–7-week-old embryo and placed these cells into culture (see Figure 8.3b). PGCs are destined to become either oocytes or sperm cells, depending on the sex of the developing embryo. The resulting cell lines are called embryonic germ cell lines, and they share many properties with ES cells. As with ES cells, however, PGCs present challenges with sustained growth in culture.16,17 Spontaneous differentiation, which hinders the isolation of pure clonal lines, is a particular issue. Therefore, the clinical application of these cells requires a more complete understanding of their derivation and maintenance in vitro.
hESC Lines from Dead Embryos
Embryos that stop dividing after being fertilized in vitro are not preferentially selected for implantation in a woman undergoing fertility treatment. These embryos are typically either frozen for future use or discarded as medical waste. In 2006, scientists at the University of Newcastle, United Kingdom, generated hESC lines from IVF embryos that had stopped dividing.18 These scientists used similar methods as described under "Traditional hESC Line Generation" except that their source material was so-called "dead" IVF embryos (see Figure 8.3c). The human stem cells created using this technique behaved like pluripotent stem cells, including producing proteins critical for "stemness" and being able to produce cells from all three germ layers. It has been proposed that an IVF embryo can be considered dead when it ceases to divide.19 If one accepts this definition, such an embryo that "dies" from natural causes presumably cannot develop into a human being, thereby providing a source to derive human ES cells without destroying a living embryo.
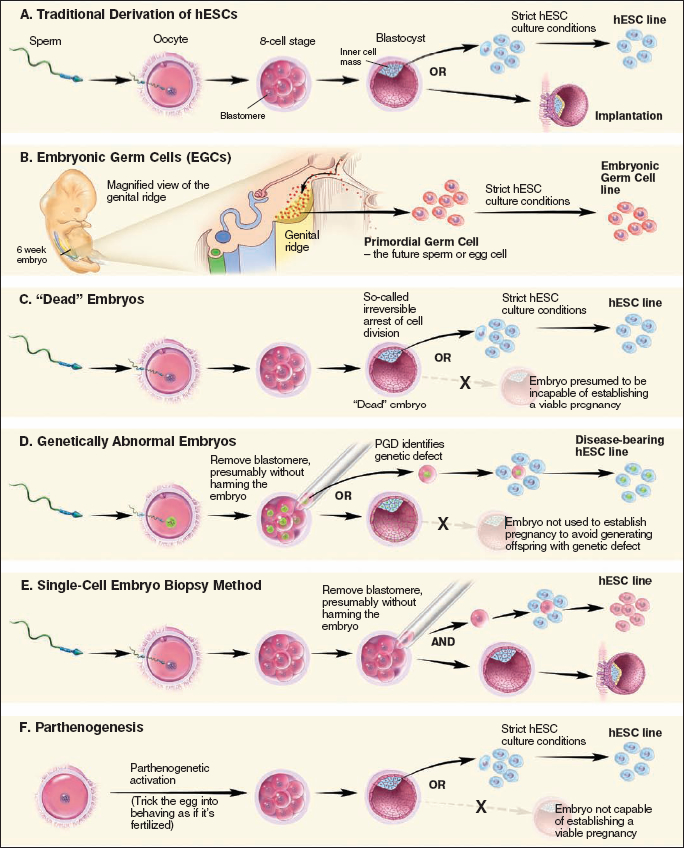
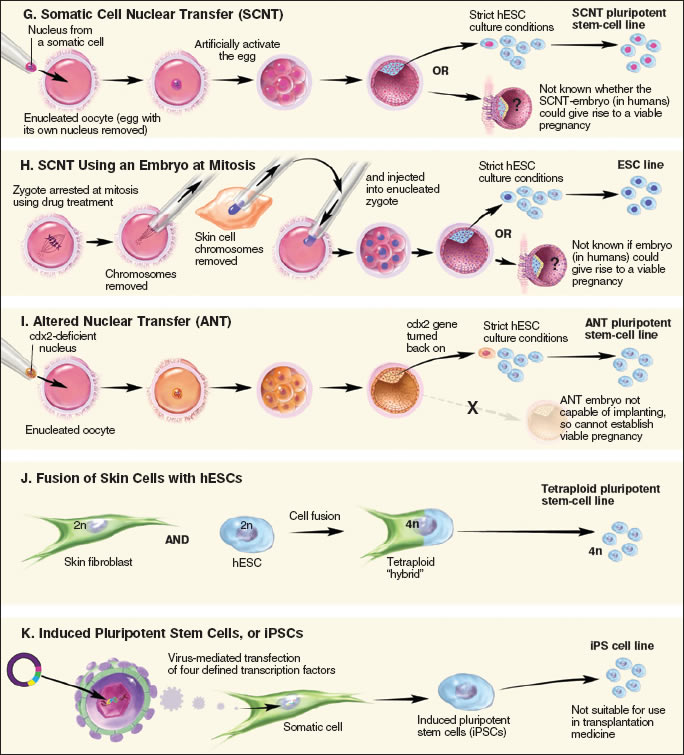
Figure 8.3. Alternative Methods for Preparing Pluripotent Stem Cells
© 2008 Terese Winslow
hESC Lines from Genetically Abnormal Embryos
Couples who have learned that they carry a genetic disorder sometimes use pre-implantation genetic diagnosis (PGD) and IVF to have a child that does not carry the disorder. PGD requires scientists to remove one cell from a very early IVF human embryo and test it for diseases known to be carried by the hopeful couple. Normally, embryos identified with genetic disorders are discarded as medical waste. However, Dr.Yuri Verlinsky and colleagues have capitalized on these embryos as a way to further our understanding of the diseases they carry (see Figure 8.3d) by deriving hESC lines from them.20 These stem cell lines can then be used to help scientists understand genetically-based disorders such as muscular dystrophy, Huntington's disease, thalessemia, Fanconi's anemia, Marfan syndrome, adrenoleukodystrophy, and neurofibromatosis.
hESC Lines from Single Cell Embryo Biopsy
In 2006, Dr. Robert Lanza and colleagues demonstrated that it is possible to remove a single cell from a pre-implantation mouse embryo and generate a mouse ES cell line.21 This work was based upon their experience with cleavage-stage mouse embryos. Later that same year, Dr. Lanza's laboratory reported that it had successfully established hESC lines (see Figure 8.3e) from single cells taken from pre-implantation human embryos.22 The human stem cells created using this technique behaved like pluripotent stem cells, including making proteins critical for "stemness" and producing cells from all three germ layers. Proponents of this technique suggest that since it requires only one embryonic cell, the remaining cells may yet be implanted in the womb and develop into a human being. Therefore, scientists could potentially derive human embryonic stem cells without having to destroy an embryo. However, ethical considerations make it uncertain whether scientists will ever test if the cells remaining after removal of a single cell can develop into a human being, at least in embryos that are not at risk for carrying a genetic disorder. Moreover, it is unclear whether the single cell used to generate a pluripotent stem cell line has the capacity to become a human being.
Parthenogenesis is the creation of an embryo without fertilizing the egg with a sperm, thus omitting the sperm's genetic contributions. To achieve this feat, scientists "trick" the egg into believing it is fertilized, so that it will begin to divide and form a blastocyst (see Figure 8.3f). In 2007, Dr. E.S. Revazova and colleagues reported that they successfully used parthenogenesis to derive hESCs.23 These stem cell lines, derived and grown using a human feeder cell layer, retained the genetic information of the egg donor and demonstrated characteristics of pluripotency. This technique may lead to the ability to generate tissue-matched cells for transplantation to treat women who are willing to provide their own egg cells.24 It also offers an alternate method for deriving tissue-matched hESCs that does not require destruction of a fertilized embryo.
Human Stem Cell Lines Whose Potency is Currently Being Determined: Amniotic Fluid Cells
Amniotic fluid surrounding the developing fetus contains cells shed by the fetus and is regularly collected from pregnant women during amniocentesis. In 2003, researchers identified a subset of cells in amniotic fluid that express Oct-4, a marker for pluripotent human stem cells that is expressed in ES cells and embryonic germ cells.25 Since then, investigators have shown that amniotic fluid stem cells can differentiate into cells of all three embryonic germ layers and that these cells do not form tumors in vivo.26,27
For example, Anthony Atala and colleagues at the Wake Forest University have recently generated non-embryonic stem cell lines from cells found in human and rat amniotic fluid.27 They named these cells amniotic fluid-derived stem cells (AFS). Experiments demonstrate that AFS can produce cells that originate from each of the three embryonic germ layers, and the self-renewing cells maintained the normal number of chromosomes after a prolonged period in culture. However, undifferentiated AFS did not produce all of the proteins expected of pluripotent cells, and they were not capable of forming a teratoma. The scientists developed in vitro conditions that enabled AFS to produce nerve cells, liver cells, and bone-forming cells. AFS-derived human nerve cells could make proteins typical of specialized nerve cells and were able to integrate into a mouse brain and survive for at least two months. Cultured AFS-derived human liver cells secreted urea and made proteins characteristic of normal human liver cells. Cultured AFS-derived human bone cells made proteins expected of human bone cells and formed bone in mice when seeded onto scaffolds and implanted under the mouse's skin. Although scientists do not yet know how many different cell types AFS can generate, AFS may one day allow researchers to establish a bank of cells for transplantation into humans.
Strategies to "Reprogram" Non-Pluripotent Cells Become Pluripotent Cells
An alternative to searching for an existing population of stem cells is to create a new one from a population of non-pluripotent cells. This strategy, which may or may not involve the creation of an embryo, is known as "reprogramming." This section will summarize reprogramming approaches, including several recent breakthroughs in the field..
Reprogramming through Somatic Cell Nuclear Transfer (SCNT)
In SCNT (see Figure 8.3g), human oocytes (eggs) are collected from a volunteer donor who has taken drugs that stimulate the production of more than one oocyte during the menstrual cycle. Scientists then remove the nucleus from the donated oocyte and replace it with the nucleus from a somatic cell, a differentiated adult cell from elsewhere in the body. The oocyte with the newly-transferred nucleus is then stimulated to develop. The oocyte may develop only if the transplanted nucleus is returned to the pluripotent state by factors present in the oocyte cytoplasm. This alteration in the state of the mature nucleus is called nuclear reprogramming. When development progresses to the blastocyst stage, the ICM is removed and placed into culture in an attempt to establish a pluripotent stem cell line. To date, the technique has been successfully demonstrated in two primates: macaque monkeys28 and humans.29
However, successful SCNT creates an embryo-like entity, thereby raising the ethical issues that confront the use of spare IVF embryos. However, pluripotent cell lines created by embryos generated by SCNT offer several advantages over ES cells. First, the nuclear genes of such a pluripotent cell line will be identical to the genes in the donor nucleus. If the nucleus comes from a cell that carries a mutation underlying a human genetic disease such as Huntington's disease, then all cells derived from the pluripotent cell line will carry this mutation. In this case, the SCNT procedure would enable the development of cellular models of human genetic disease that can inform our understanding of the biology of disease and facilitate development of drugs to slow or halt disease progression. Alternatively, if the cell providing the donor nucleus comes from a specific patient, all cells derived from the resulting pluripotent cell line will be genetically matched to the patient with respect to the nuclear genome. If these cells were used in transplantation therapy, the likelihood that the patient's immune system would recognize the transplanted cells as foreign and initiate tissue rejection would be reduced. However, because mitochondria also contain DNA, the donor oocyte will be the source of the mitochondrial genome, which is likely to carry mitochondrial gene differences from the patient which may still lead to tissue rejection.
A technique reported in 2007 by Dr. Kevin Eggan and colleagues at Harvard University may expand scientists' options when trying to "reprogram" an adult cell's DNA30. Previously, successful SCNT relied upon the use of an unfertilized egg. Now, the Harvard scientists have demonstrated that by using a drug to stop cell division in a fertilized mouse egg (zygote) during mitosis, they can successfully reprogram an adult mouse skin cell by taking advantage of the "reprogramming factors" that are active in the zygote at mitosis. They removed the chromosomes from the single-celled zygote's nucleus and replaced them with the adult donor cell's chromosomes (see Figure 8.3h). The active reprogramming factors present in the zygote turned genes on and off in the adult donor chromosomes, to make them behave like the chromosomes of a normally fertilized zygote. After the zygote was stimulated to divide, the cloned mouse embryo developed to the blastocyst stage, and the scientists were able to harvest embryonic stem cells from the resulting blastocyst. When the scientists applied their new method to abnormal mouse zygotes, they succeeded at reprogramming adult mouse skin cells and harvesting stem cells. If this technique can be repeated with abnormal human zygotes created in excess after IVF procedures, scientists could use them for research instead of discarding them as medical waste.
Reprogramming Through Altered Nuclear Transfer (ANT)
Altered nuclear transfer is a variation on standard SCNT that proposes to create patient-specific stem cells without destroying an embryo. In ANT, scientists turn off a gene needed for implantation in the uterus (Cdx2) in the patient cell nucleus before it is transferred into the donor egg (see Figure 8.3i). In 2006, Dr. Rudolph Jaenisch and colleagues at MIT demonstrated that ANT can be carried out in mice.31 Mouse ANT entities whose Cdx2 gene is switched off are unable to implant in the uterus and do not survive to birth. Although ANT has been used to create viable stem cell lines capable of producing almost all cell types, the authors point out that this technique must still be tested with monkey and human embryos. Moreover, the manipulation needed to control Cdx2 expression introduces another logistical hurdle that may complicate the use of ANT to derive embryonic stem cells. Proponents of ANT, such as William Hurlbut of the Stanford University Medical Center, suggest that the entity created by ANT is not a true embryo because it cannot implant in the uterus.32, 33 However, the technique is highly controversial, and its ethical implications remain a source of current debate.4,32
Reprogramming Through Cell Fusion
In 2005, Kevin Eggan and colleagues at Harvard University reported that they had fused cultured adult human skin cells with hESCs (see Figure 8.3j).36 The resulting "hybrid" cells featured many characteristics of hESCs, including a similar manner of growth and division and the manufacture of proteins typically produced by hESCs. Some factor(s) within the hESCs enabled them to "reprogram" the adult skin cells to behave as hESCs. However, these cells raised a significant technical barrier to clinical use. Because fused cells are tetraploid (they contain four copies of the cellular DNA rather than the normal two copies), scientists would need to develop a method to remove the extra DNA without eliminating their hESC-like properties. The fusion method serves as a useful model system for studying how stem cells "reprogram" adult cells to have properties of pluripotent cells. However, if the reprogramming technique could be carried out without the fusion strategy, a powerful avenue for creating patient-specific stem cells without using human eggs could be developed.
Induced Pluripotent Stem Cells (iPSCs): Reprogramming Adult Somatic Cells to Become Pluripotent Stem Cells
In 2007, two independent research groups published manuscripts that described successful genetic reprogramming of human adult somatic cells into pluripotent human stem cells.34,35 Although some technical limitations remain, this strategy suggests a promising new avenue for generating pluripotent cell lines that can inform drug development, models of disease, and ultimately, transplantation medicine. These experiments, which are discussed below, were breakthroughs because they used adult somatic cells to create pluripotent stem cells that featured hallmarks of ES cells.
In 2006, Shinya Yamanaka and colleagues at Kyoto University reported that they could use a retroviral expression vector to introduce four important stem cell factors into adult mouse cells and reprogram them to behave like ES cells (see Figure 8.3k).37 They called the reprogrammed cells "iPSCs," for induced pluripotent stem cells. However, iPSCs produced using the original technique failed to produce sperm and egg cells when injected into an early mouse blastocyst and did not make certain critical DNA changes. These researchers then modified the technique to select for iPSCs that can produce sperm and eggs,38 results that have since been reproduced by Rudolph Jaenisch and colleagues at the Massachusetts Institute of Technology (MIT).39
In addition, the MIT scientists determined that iPSCs DNA is modified in a manner similar to ES cells, and important stem cell genes are expressed at similar levels. They also demonstrated that iPSCs injected into an early mouse blastocyst can produce all cell types within the developing embryo, and such embryos can complete gestation and are born alive.
Once these research advances were made in mice, they suggested that similar techniques might be used to reprogram adult human cells. In 2007, Yamanaka and coworkers reported that introducing the same four genetic factors that reprogrammed the mouse cells into adult human dermal fibroblasts reprogrammed the cells into human iPSCs.35 These iPSCs were similar to human ES cells in numerous ways, including morphology, proliferative capacity, expression of cell surface antigens, and gene expression. Moreover, the cells could differentiate into cell types from the three embryonic germ layers both in vitro and in teratoma assays. Concurrent with the Yamanaka report, James Thomson and coworkers at the University of Wisconsin published a separate manuscript that detailed the creation of human iPSCs through somatic cell reprogramming using four genetic factors (two of which were in common with the Yamanaka report).34 The cells generated by the Thomson group met all defining criteria for ES cells, with the exception that they were not derived from embryos.
These breakthroughs have spurred interest in the field of iPSCs research. In early 2008, investigators at the Massachusetts General Hospital40 and the University of California, Los Angeles41 reported generating reprogrammed cells. As scientists explore the mechanisms that govern reprogramming, it is anticipated that more reports will be forthcoming in this emerging area. Although these reprogramming methods require the use of a virus, non-viral strategies may also be possible in the future. In any case, these approaches have created powerful new tools to enable the "dedifferentation" of cells that scientists had previously believed to be terminally differentiated.42,43
Although further study is warranted to determine if iPS and ES cells differ in clinically significant ways, these breakthrough reports suggest that reprogramming is a promising strategy for future clinical applications. Induced pluripotent cells offer the obvious advantage that they are not derived from embryonic tissues, thereby circumventing the ethical issues that surround use of these materials. Successful reprogramming of adult somatic cells could also lead to the development of stem cell lines from patients who suffer from genetically-based diseases, such as Huntington's Disease, spinal muscular atrophy, muscular dystrophy, and thalessemia. These lines would be invaluable research tools to understand the mechanisms of these diseases and to test potential drug treatments. Additionally, reprogrammed cells could potentially be used to repair damaged tissues; patient-specific cell lines could greatly reduce the concerns of immune rejection that are prevalent with many transplantation strategies.
However, several technical hurdles must be overcome before iPSCs can be used in humans. For example, in preliminary experiments with mice, the virus used to introduce the stem cell factors sometimes caused cancers.37 The viral vectors used in these experiments will have to be selected carefully and tested fully to verify that they do not integrate into the genome, thereby harboring the potential to introduce genetic mutations at their site of insertion. This represents a significant concern that must be addressed before the technique can lead to useful treatments for humans. However, this strategy identifies a method for creating pluripotent stem cells that, together with studies of other types of pluripotent stem cells, will help researchers learn how to reprogram cells to repair damaged tissues in the human body.
Other Sources of Pluripotent And/or Multipotent Cells
Stem cell research is a rapidly evolving field, and researchers continue to isolate new pluripotent cells and create additional cell lines. This section briefly reviews other sources of pluripotent cells and the implications that their discovery may have on future research.
Epiblast Cells. While rodent and human ES cells are pluripotent, they maintain their respective pluripotencies through different molecular signaling pathways. It is not known why these differences exist. Recently, several research groups have reported the generation of stable, pluripotent cell lines from mouse and rat epiblast, a tissue of the post-implantation embryo that ultimately generates the embryo proper.44,45 These cells are distinct from mouse ES cells in terms of the signals that control their differentiation. However, the cells share patterns of gene expression and signaling responses with human ES cells. The establishment of epiblast cell lines can therefore provide insight into the distinctions between pluripotent cells from different species and illuminate ways that pluripotent cells pursue distinct fates during early development.
Existing Adult Stem Cells. As has been discussed in other chapters, numerous types of precursor cells have been isolated in adult tissues.46 Although these cells tend to be relatively rare and are dispersed throughout the tissues, they hold great potential for clinical application and tissue engineering. For example, tissues created using stem cells harvested from an adult patient could theoretically be used clinically in that patient without engendering an immune response. Moreover, the use of adult stem cells avoids the ethical concerns associated with the use of ES cells. In addition, adult-derived stem cells do not spontaneously differentiate as do ES cells, thus eliminating the formation of teratomas often seen with implantation of ES cells. The potential of adult stem cells for regenerative medicine is great; it is likely that these various cells will find clinical application in the upcoming decades.
Conclusion: Pluripotent Cell Lines are Tools for Future Research
Although the recent advances in reprogramming of adult somatic cells has generated a wave of interest in the scientific community, these cell lines will not likely replace hESC lines as tools for research and discovery. Rather, both categories of cells will find unique uses in the study of stem cell biology and the development and evaluation of therapeutic strategies. Pluripotent cells offer a number of potential clinical applications, especially for diseases with a genetic basis. However, researchers are just beginning to unlock the many factors that govern the cells' growth and differentiation. As scientists make strides toward understanding how these cells can be manipulated, additional applications, approaches, and techniques will likely emerge. As such, pluripotent cells will play a pivotal role in future research into the biology of development and the treatment of disease.
References
- Domen J, Wagers A, Weissman IL. Bone marrow (hematopoietic) stem cells. Regenerative Medicine [/info/scireport/2006report. Accessed February 22, 2008.
- Rao M, Condic ML. Alternative sources of pluripotent stem cells: scientific solutions to an ethical dilemma. Stem Cells Dev. 2008;17:1-10.
- The President's Council on Bioethics. White paper: alternative sources of human pluripotent stem cells. http://www.bioethics.gov/reports/white_paper/index.html Accessed February 26, 2008.
- Downing GJ, Battey JF Jr. Technical assessment of the first 20 years of research using mouse embryonic stem cell lines. Stem Cells. 2004;22:1168-1180.
- Bongso A, Chui-Yee F, Soon-Chye N, Ratnam S. Isolation and culture of inner cell mass cells from human blastocysts. Hum Reprod 1994; 9: 2110-2117.
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145-1147.
- Hoffman DI, Zellman GL, Fair CC, et al. Cryopreserved embryos in the United States and their availability for research. Fertil Steril. 2003;79:1063-1069.
- Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonicstem cells. Nat Biotechnol. 2001;19:1129-1133.
- He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32-39.
- Wang L, Li L, Menendez P, Cerdan C, Bhatia M. Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood. 2005;105:4598-4603.
- Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933-936.
- Richards M, Tan S, Fong CY, Biswas A, Chan WK, Bongso A. Comparative evaluation of various human feeders for prolonged undifferentiated growth of human embryonic stem cells. Stem Cells. 2003;21:546-556.
- Xu C, Inokuma MS, Denham J, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971-974.
- Lim JWE, Bodnar A. Proteome analysis of conditioned medium from mouse embryonic fibroblast feeder layers which support the growth of human embryonic stem cells. Proteomics. 2002;2:1187-1203.
- Shamblott MJ, Axelman J, Wang S, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998;95:13726-13731.
- Turnpenny L, Brickwood S, Spalluto CM, et al. Derivation of human embryonic germ cells: an alternative source of pluripotent stem cells. Stem Cells. 2003;21:598-609.
- Aflatoonian B, Moore H. Human primordial germ cells and embryonic germ cells, and their use in cell therapy. Curr Opin Biotechnol. 2005;16:530-535.
- Zhang X, Stojkovic P, Przyborski S, et al. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669-2676.
- Landry DW, Zucker HA. Embryonic death and the creation of human embryonic stem cells. J Clin Invest. 2004;114:1184-1186.
- Verlinsky Y, Strelchenko N, Kukharenko V, et al. Human embryonic stem cell lines with genetic disorders. Reprod Biomed Online. 2005;10:105-110.
- Chung Y, Klimanskaya I, Becker S, et al. Embryonic and extraembryonic stem cell lines derived from single mouse blastomeres. Nature. 2006;439:216-219.
- Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444:481-485.
- Revazova ES, Turovets NA, Kochetkova OD, et al. Patient-specific stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007;9:432-449.
- Revazova ES, Turovets NA, Kochetkova OD, et al. HLA homozygous stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007;Dec 19:epub ahead of print.
- Prusa AR, Marton E, Rosner M, Bernaschek G, M H. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Hum Reprod. 2003;18:1489-1493.
- Siegel N, Rosner M, Hanneder M, Valli A, Hengstschlager M. Stem cells in amniotic fluid as new tools to study human genetic diseases. Stem Cell Rev. 2007;3:256-264.
- De Coppi P, Bartsch G Jr, Siddiqui MM, et al. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100-106.
- Byrne JA, Pedersen DA, Clepper LL, et al. Producing primate embryonic stem cells by somatic cell nuclear transfer. Nature. 2007;450:497-502.
- French A, Adams C, Anderson L, Kitchen J, Hughes M, Wood S. Development of human cloned blastocysts following somatic cell nuclear transfer (SCNT) with adult fibroblasts. Stem Cells. 2008;Epub ahead of print.
- Egli, D, Rosains, J, Birkhoff, G, Eggan, K. Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature. 2007; 447: 679-685.
- Meissner A, Jaenisch R. Generation of nuclear transfer-derived pluripotent ES cells from cloned Cdx2-deficient blastocysts. Nature. 2006;439:212-215.
- Hurlbut WB. Ethics and embryonic stem cell research: altered nuclear transfer as a way forward. BioDrugs. 2007;21:79-83.
- Hurlbut WB. Altered nuclear transfer: a way forward for embryonic stem cell research. Stem Cell Rev. 2005;1:293-300.
- Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917-1920.
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:1-12.
- Cowan CA, Atienza J, Melton DA, Eggan K. Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science. 2005;309:1369-1373.
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676.
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313-317.
- Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318-324.
- Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141-146.
- Lowry WE, Richter L, Yachechko R, et al. Generation of human induced pluripotent stem cells from dermal fibroblasts. Proc Natl Acad Sci USA. 2008; Epub ahead of print.
- Jaenisch R, Young R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. Cell. 2008;132:567-582.
- Collas P. Dedifferentiation of cells: new approaches. Cytotherapy. 2007;9:236-244.
- Tesar PJ, Chenoweth JG, Brook FA, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature. 2007;448:196-199.
- Brons IGM, Smither LE, Trotter MWB, et al. Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature. 2007;448:191-195.
- Young HE, Duplaa C, Katz R, et al. Adult-derived stem cells and their potential for use in tissue repair and molecular medicine. J Cell Mol Med. 2005;9:753-769.
 Chapter7 | Table of Contents | Chapter9
Chapter7 | Table of Contents | Chapter9 