 |

By Patrick Zickler, NIDA NOTES Staff Writer
Nearly 23 percent of Americans 18 and older smoke cigarettes. Although this figure represents a substantial decrease since smoking rates were at their highest in 1965, most current smokers say they would like to quit. According to the Centers for Disease Control and Prevention, 71 percent of smokers interviewed in 2000 said they wanted to quit smoking, with 41 percent having tried to quit in the preceding year.
| Selegiline Helps Smokers Quit, Remain Abstinent Longer |
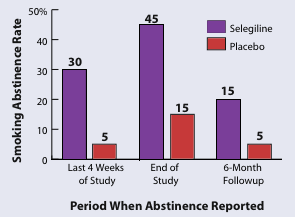 Smokers who received selegiline plus counseling were more likely to stop smoking and remain abstinent than smokers who received placebo and counseling. |
|
Many of those who still smoke are the hardest to treat, having failed to stop despite numerous attempts. Their efforts to quit are frustrated by nicotine's addictive effects, which result in large part from the drug's ability to trigger and sustain release of the pleasure-producing neurotransmitter dopamine in the brain. At Yale University in New Haven, Connecticut, NIDA-supported researchers have found that selegiline, a medication currently used by physicians primarily to delay the progression of symptoms in Parkinson's disease, can help smokers who want to quit but have been unsuccessful with other treatments.
"Our research group focused on difficult-to-treat smokers, who aren't responsive to nicotine replacement therapy or to bupropion," says Dr. Tony George of Yale University School of Medicine. "Many smokers who attempt to quit fail because of the powerful withdrawal symptoms smokers experience when they stop smoking. There is strong evidence that the symptoms of nicotine withdrawal are associated with sharp declines in dopamine levels, so we thought a medication that acts to boost dopamine levels might be of benefit." In Parkinson's disease, which involves massive loss of dopamine-producing cells, treatment with selegiline helps the brain retain its stores of dopamine longer by inhibiting the activity of monoamine oxidase-B, an enzyme that breaks down dopamine.
To evaluate the effect of selegiline in smoking cessation treatment, the researchers recruited 40 smokers (75 percent Caucasian, 15 men, 25 women, average age 49) who had unsuccessfully tried (at least 3 times and some as many as 20) to stop smoking and described themselves as highly motivated to quit. Over 8 weeks, all participants received weekly smoking cessation counseling that included motivational enhancement for the first 3 weeks of the study and work on relapse prevention strategies for the last 5 weeks. They took pills containing either placebo or 5 mg selegiline once a day for the first week and twice a day for the remaining 7 weeks. Twenty participants (8 men, 12 women) received selegiline and 20 (7 men, 13 women) received placebo. All participants were allowed to smoke during the first 2 weeks of the study, and a "quit date" was set for the first day of the third week.
At the end of the eighth week, 45 percent of the participants who received selegiline reported they had not smoked during the preceding week, compared with 15 percent of those receiving placebo. Measurement of carbon monoxide levels in the participants' exhaled breath verified their self-reports. The difference between the two treatment groups was even more pronounced when reports of 4-week abstinence were considered: Compared with 5 percent of the placebo group, 30 percent of those who received selegiline reported they had not smoked in the last 4 weeks of the study. Six weeks after the study ended, 20 percent of the selegiline group were still not smoking, compared with 5 percent of those who received placebo.
"In this study, selegiline appeared to substantially improve outcomes for smokers who have had a difficult time stopping," says Dr. Ivan Montoya of NIDA's Division of Treatment Research and Development. "The results, which are better than those typically achieved by smokers using nicotine replacement therapy to help them quit, offer strong confirmation that controlling the dopamine system could be an important approach to successful treatment of nicotine addiction, particularly for smokers with a history of unsuccessful quit attempts. Our next step is to confirm this in a much larger trial with several hundred smokers."
Source
George, T.P., et al. A preliminary placebo-controlled trial of selegiline hydrochloride for smoking cessation. Biological Psychiatry 53(2):136-143, 2003.
[Abstract]
Volume 18, Number 5 (December 2003)
|
 |
|