ACR Appropriateness Criteria®
Clinical Condition: Multiple Gestations
Variant 1: Patients with high index of suspicion for multiple gestations—assisted reproductive techniques, large dates for pregnancy, and elevated maternal serum alpha-fetoprotein.
| Radiologic Procedure |
Rating |
Comments |
RRL* |
| Ultrasound (US) pregnant uterus transabdominal or transvaginal |
9 |
|
O |
| Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate |
*Relative Radiation Level |
Variant 2: Patients with low index of suspicion for multiple gestations—all pregnancies or family history of twins.
| Radiologic Procedure |
Rating |
Comments |
RRL* |
| Ultrasound (US) pregnant uterus transabdominal or transvaginal |
9 |
|
O |
| Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate |
*Relative Radiation Level |
Variant 3: Initial ultrasound has diagnosed twins on the same scan.
| Radiologic Procedure |
Rating |
Comments |
RRL* |
| On the Same US Exam |
| Ultrasound (US) pregnant uterus transabdominal or transvaginal (determine chorionicity and amnionicity) |
9 |
|
O |
| US pregnant uterus transabdominal or transvaginal (detailed anatomic survey) |
9 |
Fetal anomalies are more frequent in twins than in singletons. |
O |
| US pregnant uterus transabdominal or transvaginal (assess amniotic fluid) |
9 |
Frequency of follow-up examinations is usually based on chorionicity and size concordance. See the narrative below. |
O |
| US pregnant uterus transabdominal or transvaginal (assess twin sizes and discordancy) |
9 |
Frequency of follow-up examinations is usually based on chorionicity and size concordance. See the narrative below. |
O |
| US pregnant uterus transabdominal or transvaginal (assess cervix) |
9 |
Frequency of follow-up examinations is usually based on chorionicity and size concordance. See the narrative below. |
O |
| US pregnant uterus transabdominal or transvaginal (assess nuchal translucency for each twin) |
9 |
Not routine for many centers but can be performed at 11-14 weeks if the patient desires first-trimester screening. The scan may be less optimal for fetuses farther away from the transducer. |
O |
| US pregnant uterus transabdominal or transvaginal (umbilical artery Doppler for each twin) |
3 |
Frequency of follow-up examinations is usually based on chorionicity and size concordance. See the narrative below. |
O |
| Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate |
*Relative Radiation Level |
Variant 4: Parameters to measure for twin discordance.
| Radiologic Procedure |
Rating |
Comments |
RRL* |
| Measurement Parameter |
| Ultrasound (US) pregnant uterus transabdominal or transvaginal (abdominal circumference) |
9 |
Same table to be used as for singletons. |
O |
| US pregnant uterus transabdominal or transvaginal (weight) |
9 |
Same table to be used as for singletons. Weight based upon a standard regression equation using measurements of at least three parameters. |
O |
| US pregnant uterus transabdominal or transvaginal (biparietal diameter) |
9 |
Same table to be used as for singletons. |
O |
| US pregnant uterus transabdominal or transvaginal (head circumference) |
9 |
Same table to be used as for singletons. |
O |
| US pregnant uterus transabdominal or transvaginal (femur) |
9 |
Same table to be used as for singletons. |
O |
| US pregnant uterus transabdominal or transvaginal (head/abdomen circumference ratio) |
6 |
Same table to be used as for singletons. |
O |
| US pregnant uterus transabdominal or transvaginal (femur/abdomen circumference ratio) |
3 |
Same table to be used as for singletons. |
O |
| Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate |
*Relative Radiation Level |
Summary of Literature Review
Multiple gestations are high-risk compared with singleton pregnancies. Dichorionic twin pregnancies must also be diamniotic, and they have the lowest risk (i.e., a 10% risk) that one or both fetuses will not survive beyond the neonatal period. When twins share one placenta (i.e., monochorionic-diamniotic twinning) the risk increases to 25%. This increased mortality is due to complications related to blood vessel communications between the cardiovascular circulations of the individual twins. These conditions include twin-twin transfusion syndrome (TTTS), twin embolization syndrome, and acardius, or twin-reversed arterial perfusion sequence. When twins also share the same compartment—monochorionic-monoamniotic twinning—the loss rate jumps to 50%, with the incremental mortality attributable to cord entanglement accidents.
The major sources of morbidity and mortality common to all twin gestations are prematurity and intrauterine growth restriction, which may affect one or both fetuses. There may be an earlier onset to placental postmaturity complications. There is also an increased incidence of congenital anomalies among all twins, although anatomic malformations occur four to five times more frequently in monozygotic than in dizygotic twins. The overall risk for at least one of a monochorionic/monoamniotic twin pair having a structural congenital cardiac anomaly is eight times that of a monochorionic/diamniotic twin pair. In addition, if one of a pair of monochorionic twins is affected, the risk of the other twin for a cardiac anomaly is also higher. All categories of perinatal morbidity and mortality among twins occur with even greater frequency in higher-order multiple gestations.
Predicting or identifying pregnancy complications is an important obstetric goal. It allows the perinatal team to plan antenatal management, necessary interventions, and timing of delivery. To date, in spite of the increasing incidence of multifetal pregnancies related to assisted reproduction, there is a paucity of medical literature regarding imaging schedules for diagnosis and follow-up in this population. The appropriateness criteria presented here have been revised to address diagnosing a multiple gestation in the first trimester, and to scan for detailed anatomic evaluation and comparative growth at 18-20 weeks. The roles of the monitoring of fetal well-being, nuchal translucency screening (NTS), and the timing of follow-up growth scans are discussed. Triplet and higher-order multiple gestations are not specifically addressed, but they should be treated as very-high-risk pregnancies.
Initial Ultrasound Examination
Determination of multiple gestations in the first trimester is important for several reasons. Monochorionic twins and triplets are at a higher risk for twin-twin transfusion, fetal growth restriction, congenital anomalies, vasa previa, velamentous insertion of the umbilical cord, and fetal death. Because of the increased morbidity and mortality directly related to problems resulting from chorionicity, establishment of amnionicity and chorionicity is essential in implementing an antepartum management strategy. Because of the high risk of perinatal loss in higher order multiple pregnancies, ultrasound (US) guided reduction may be offered to reduce the number of fetuses with the hope of optimizing the outcome for the remaining fetuses. Chorionicity and amnionicity must be determined prior to reduction because the presence of vascular anastomoses in monochorionic gestations increases the risk of injury or damage to the remaining fetuses.
The first trimester is considered the most optimal time to diagnose chorionicity with US, as the number of gestational sacs equals the number of chorions. Sonographic assignment of monochorionicity during the first trimester had higher sensitivity, specificity, and positive and negative predictive values than assignment during the second trimester. As with twins, the sonographic determination of chorionicity can be applied to higher order gestations with a high degree of accuracy.
It has been suggested that amnionicity can be predicted indirectly by counting the number of yolk sacs; however, if the amnions are not evident by US due to the early gestational age or only a single yolk sac is identified, a follow-up US to directly identify the amnion(s) is useful. Chorionicity can be accurately predicted in the early to mid first trimester by counting the number of gestational sacs. Features that may be helpful for determining chorionicity in the very late first through third trimester include number of placentas, fetal gender, the lambda or twin peak sign, and dividing membrane thickness. Several studies support the finding of a thick dividing intertwin membrane and the identification of the "lambda" or "twin peak" sign during the 11-14 week scan as the best sign to determine chorionicity. While it is practical to perform the initial scan at the time of second trimester biochemical screening, earlier recognition of pregnancies that will require closer antenatal monitoring and carrying higher potential risks may be desired so that women with high risk pregnancies may be afforded opportunity to access appropriate antenatal input prior to routine anomaly scanning and allow more accurate diagnosis of amnionicity. One study found that performing the US at 7-9 weeks gestation showed a very high agreement with 11-14 week scan in the diagnosis of chorionicity and amnionicity. In monochorionic pregnancies, however, the diagnosis of amnionicity was less accurate owing to normal later sonographic demonstration of the amniotic membrane. In these cases, a later scan at 11-14 weeks can be obtained when the diagnosis can be made with greater confidence.
Because first-trimester US for pregnancy is not universally routine, the early diagnosis of multiple gestation via US relies on maintaining a high index of suspicion in patients with uterine size greater than expected for dates, a history of assisted reproduction, and elevation of maternal serum alpha-fetoprotein. Detection rates for multiple gestation in the first trimester approach 99.3%. In the RADIUS trial, 129 multifetal pregnancies were studied. Those screened with US were consistently diagnosed earlier than those not screened. In spite of this, no statistically significant fetal benefits were demonstrated in those who were screened in this trial. During the initial scan it is also important to evaluate the amount of amniotic fluid for each twin, to image the cervix to check for changes of shortening or dilatation, and to assess the size of each twin and the degree of discordance, if any, between them. Intertwin disparity in size has been shown to be predictive to the development of TTTS. Although there are a few studies with contradicting results, one study showed that twins with discordant crown-rump length (CRL) had a poor outcome and therefore early discordance of CRL >10% should probably heighten fetal surveillance. In the absence of reliable predictors of TTTS, serial surveillance of monochorionic pregnancies starting in early second trimester may be prudent. Some investigators suggest US every two weeks to monitor growth, perform US Doppler and check amniotic fluid volume.
The prevalence of congenital anomalies is higher in twin gestations than in singletons, further underscoring the importance of detailed US scans in all patients carrying multiple gestations. Fetal echocardiography should be considered in monochorionic gestations. Risk assessment for aneuploidy using maternal serum biochemistry is limited. It is not possible to accurately assess the contribution of each fetus; therefore, one cannot distinguish which twin is affected with an abnormal result. In addition, levels from the normal twin can mask abnormal levels in the affected twin. Because it is fetus specific, nuchal translucency may be the best option for aneuploidy screening in multiple gestations. The feasibility of NTS in twins has been found to be comparable with that in singletons. However, it is important to keep in mind that the quality of NTS measurements in a pregnancy with multiple fetuses compared to a singleton pregnancy may be less optimal especially in fetuses located farther from the abdominal wall.
Follow-up Ultrasound Examination and Antepartum Surveillance
Tests for evaluating fetal well-being include sonographic assessment of fetal growth and amniotic fluid volume, umbilical artery Doppler (UAD), nonstress test (NST), and biophysical profile (BPP). NST and UAD are considered more predictive of fetal well-being than the other two because of the inaccuracy of amniotic fluid volume estimation in multiple gestations.
Estimation of fetal weight in twin gestations is essential for management and the selection of optimal timing of delivery. Evaluation for growth restriction, weight discordance, and TTTS relies on accurate estimation of fetal weight. The formulas for estimated fetal weight (EFW) currently in use are based on singleton pregnancies, and data on the accuracy of these formulas for twin pregnancies are sparse and contradictory. A group of researchers found the accuracy of sonographically determined EFW to be lower for twin gestations than for singleton gestation. Therefore, EFW based on US measurements should be treated with caution.
One study showed that growth restriction confers worse perinatal outcome in twins when compared with singletons. Some investigators suggest that serial US scans to assess fetal weight and growth should be done every 3-4 weeks from 18-20 weeks until delivery. Others advocate follow-up beginning at 28 weeks since fetal growth in twin pregnancies mirrors singleton growth until 28 to 32 weeks. There are others that advocate the use of twin growth charts in the third trimester. Fetal growth in twin and multifetal pregnancies after 30 weeks of gestation lags behind the growth of singleton pregnancies. Therefore, 2- to 4-week intervals for serial US scans to monitor interval growth in the third trimester are recommended. Even closer surveillance may be indicated if there is a monochorionic or monoamniotic twin pair as part of the multifetal pregnancy, particularly if there is discordance in fetal sizes or amniotic fluid volumes.
The necessary parameters to measure or calculate in assessing the likelihood of intrauterine growth restriction include weight and abdominal circumference. Measurements of biparietal diameter, head circumference, and femur length are all indicated, but ratios of head or femur to abdominal circumference are probably not needed. There is conflicting evidence about whether the same measurement tables developed for singleton pregnancies are indicated for twins, rather than tables specifically generated for twins. Twin pregnancies are at greater risk of intrauterine growth restriction, which may affect one or both fetuses, and there is concern that growth tables for twins, which do show smaller measurements than singletons in the third trimester, may be incorporating tendencies toward growth restriction within their normal values. It is important to remember that twins can be concordantly growth-restricted. If both twins are small for dates on follow-up sonograms, protocols for monitoring fetal well-being are indicated, just as they would be in significantly discordant twins.
One study showed a positive predictive value of 85% for birthweight discordance (BWD) (20% or more) where growth restriction using EFW was first documented at 20-24 weeks gestation. This predictive value decreased with increasing gestational age, which supports screening at 20-24 weeks gestation, 28 weeks at the latest. If growth discordance (>20% to 30%, calculated as a percentage of the larger twin's weight) or growth restriction (<10%) is discovered, US is recommended every 2 weeks.
Discordant fetal growth is seen in a significant proportion of twin pregnancies. Detection is important because discordance is associated with perinatal complications and with higher risk of fetal structural or chromosomal abnormalities. Significant discordance often influences decision-making regarding timing and mode of delivery. Although there is conflicting evidence in the literature as to the accuracy of US in predicting the delivery of twins with discordant birthweight. A group of authors found that US prediction of BWD within 15 days of delivery is accurate enough for routine clinical use. Discordance in EFW is the most widely used method of identifying discordant growth. Some authors suggest that discordance should be defined as mild if weight estimates for the twins are 15% different, moderate if 20% different, and severe if 25% different or greater. The incidence of infant death is 25%, congenital anomalies 38%, and small for gestational age 32% in premature infants with BWD ≥30%. For mild discordance, scans for growth at 3-week intervals with use of UAD analysis are probably indicated. For moderate discordance, scans for growth at 2-3-week intervals should be considered, and UAD, BPP, and/or NST are indicated. When discordance is severe, growth scans at 2-week intervals are preferred, with BPP and/or NST necessary and UAD also indicated. If both twins have fallen below the 10th percentile for gestational age relative to menstrual dates and/or dating by the initial sonogram, that should also be taken as an indication for increased surveillance of growth and fetal health.
Approximately 10% to 20% of monochorionic twin pregnancies may be associated with TTTS, a type of twin discordance. In the past, the diagnosis was made when US demonstrated a 20% weight discordance. More recently, it was found that around 20 weeks gestational age, if there is polyhydramnios of the recipient twin (with the largest fluid pocket of 8-10 cm) and there is oligohydramnios in the donor twin (with largest pocket ≤2 cm), then the diagnosis can be made. In addition to the evaluation of amniotic fluid volume, bladder volume, and hydrops in each twin, Doppler findings may be used to assess these pregnancies complicated by intertwin vascular connections within the placenta or between cord insertion sites. While arterio-arterial anastomoses are most common, it is the number and location of arteriovenous anastomoses that are most important in TTTS. Other Doppler findings include absent or reversed end-diastolic flow within the umbilical artery, pulsatile umbilical vein flow, and absent or reversed end-diastolic flow within the ductus venosus. UAD generally is not a rapidly fluctuating or deteriorating parameter, but rather a long-term predictor of the status of the uteroplacental circulation. As such, it has prognostic significance for the likelihood of growth restriction and perinatal morbidity and mortality, and it may change weekly if abnormal. The values and patterns of change in vascular resistance are the same as for singletons. Doppler assessment of umbilical venous blood flow and umbilical artery systolic/diastolic ratios is considered useful in predicting and confirming concordant and discordant growth in twins. Absent end-diastolic flow is associated with low birth weight, growth restriction, and perinatal mortality in triplet and quadruplet pregnancies.
On each indicated follow-up sonogram, it remains equally important to continue to measure twins for development of new or progressive discordance, and to evaluate the cervix and each twin's amniotic fluid. Transvaginal sonography has been shown to be able to accurately assess cervical length and predict spontaneous delivery before 34 weeks as early as the 23rd week. A large multicenter study of cervical length in twin pregnancies determined that a cervical length of <25 mm at 24 weeks gestation was the best predictor of delivery before 32 weeks of gestation and was significantly more common in the 24th and 28th weeks of gestation in twin gestations compared with singletons. Cervical length can be predictive of preterm delivery in TTTS. One study found that in twin gestations, a cervical length that decreases by 20% over two measurements is a significant predictor of very preterm birth, even in the setting of a normal cervical length. They advocate taking serial cervical length measurements starting at <24 weeks. As mentioned previously, oligohydramnios in one sac may be a sign of TTTS if the other twin is presenting with polyhydramnios. Oligohydramnios in one or both sacs may indicate uteroplacental insufficiency necessitating further testing to screen for fetal well-being.
Weekly surveillance for all multifetal pregnancies has not been validated in prospective studies. Surveillance with an NST or BPP for pregnancies complicated by abnormal fluid volumes, pregnancy-induced hypertension, fetal anomalies, growth abnormalities, monoamnionicity, or other standard obstetric indications is as reliable in multiple gestations as in singleton gestations.
The most effective fetal surveillance system for multiple gestations is currently not known. The gestational age at which testing should be initiated is still not established. It is also still unclear whether testing should be performed once or more than once per week or whether there is a need to test normally growing dichorionic twins. There are no current studies that prove that routine antepartum surveillance provides objective benefit in the absence of other high-risk conditions. At present, antepartum fetal testing in multiple gestations is recommended in all situations in which surveillance would ordinarily be performed in a singleton pregnancy (including in utero growth restriction). In the most recent practice bulletin of the American College of Obstetricians and Gynecologists, the recommendation based on consensus and expert opinion was that the management of discordant growth restriction or death of one fetus in a high-order multiple gestation should be individualized, taking into consideration the welfare of the other fetuses.
Summary
- The evaluation of multiple gestations is a challenging and important task. The intensity of the obstetrical management of such pregnancies must be titrated to the degree of risk present in each individual case.
- The number of fetuses present, their chorionic and amniotic status, and risk factors such as growth restriction of one or more fetuses, amniotic fluid alterations, or presence of fetal anomalies must all be taken into account. These parameters will all affect the frequency of growth assessment, the intensity of surveillance for fetal well-being, and the institution of pharmacological and other medical therapeutic interventions.
- US imaging, together with its associated techniques for monitoring fetal compensation or distress, serves as the mainstay for evaluating the complexities of each multifetal pregnancy, helping the obstetrician chart a course toward a successful outcome.
Relative Radiation Level Designations
| Relative Radiation Level* |
Adult Effective Dose Estimate Range |
Pediatric Effective Dose Estimate Range |
| O |
0 mSv |
0 mSv |
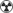 |
<0.1 mSv |
<0.03 mSv |
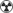 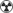 |
0.1-1 mSv |
0.03-0.3 mSv |
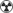   |
1-10 mSv |
0.3-3 mSv |
    |
10-30 mSv |
3-10 mSv |
     |
30-100 mSv |
10-30 mSv |
| *RRL assignments for some of the examinations cannot be made, because the actual patient doses in these procedures vary as a function of a number of factors (e.g., region of the body exposed to ionizing radiation, the imaging guidance that is used). The RRLs for these examinations are designated as NS (not specified). |