

September 13, 2006
The Honorable Michael O. Leavitt
Secretary
Department of Health and Human Services
200 Independence Avenue SW
Washington, D.C. 20201
Dear Secretary Leavitt:
The National Committee on Vital and Health Statistics (NCVHS) appreciates your continued support for the Consolidated Health Informatics Initiative (CHI), as evidenced by its widespread inclusion in many Federal solicitations. This recommendation letter continues the role that NCVHS has played in the CHI Council acceptance process. In this role, NCVHS provides an open forum for review of the CHI standards recommendations and provides an independent assessment of these recommendations.
Enclosed are the CHI recommendations on the Allergy domain. The NCVHS concurs with these recommendations. The NCVHS recommends approval of this CHI Standard by the Secretary, and formal government adoption.
We are pleased to continue our role in the CHI recommendation process.
Sincerely,
/s/
Simon Cohn, M.D., M.P.H.
Chairman, National Committee on Vital and Health Statistics
Cc: HHS Data Council Co-Chairs
Enclosure
Index
Summary
Standards Adoption Recommendation:
Health Level Seven® (HL7®) Version 2.4+
And Associated Vocabularies
SCOPE
The standards will be used to set requirements for “exchanging” allergy data across the federal health enterprise, using allergy information exchange requirements and several related allergy vocabulary standards for Allergen Code, Allergen Group, Allergy Type, Allergy Severity and Allergen Reaction.
RECOMMENDATION
1. Health Level Seven® (HL7®), Version 2.4 and higher: Information Exchange Segment/Reference Information Model
2. Unique Ingredient Identifier (UNII): Allergen Code, Mnemonic, Description, for food, drug, biologic and environmental substances. Derived from the Food and Drug Administration Substance Registration System (FDA SRS) and Environmental Protection Agency Substance Registry System (EPA SRS). Supporting standards include FDA’s Structured Product Labeling (SPL), RxNorm, Unified Medical Language System® (UMLS®), United States Adopted Names (USAN) and International Nonproprietary Names (INN), Language of Foods Thesaurus (LanguaL), Food Allergen Labeling and Consumer Protection Act (FALCPA), Veteran’s Administration (VA) Non Drug Allergen List.
3. RxNorm BN: Allergen Code, Mnemonic, Description for branded drugs instances. Supporting standards include Medical Subject Headings (MeSH), FDA’s SPL, National Drug Code (NDC), Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT®), and National Drug File Reference Terminology (NDF-RT™).
4. National Drug File Reference Terminology (NDF-RT™): Allergen group/drug classes (chemical structure). Supporting standards include MeSH.
5. Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT®): Allergy Type, Allergy Severity and Allergy Reaction
OWNERSHIP
Health Level Seven® (HL7®)
holds the copyright,
www.hl7.org
UNII - Food and Drug Administration (FDA) develops and maintains the public domain FDA SRS, assigns the UNII, and provides chemical information about the allergens, including such items as chemical structure, chemical formula, molecular weight, Chemical Abstract Service (CAS) number, preferred term (as well as synonyms). The Agency for Healthcare Research and Quality (AHRQ) supports, both conceptually and financially, the development of the UNII to improve patient safety and quality of care.
The Environmental Protection Agency (EPA) develops and maintains the public domain EPA SRS and provides access to toxicological and health information about environmental allergens. The FDA and EPA are developing an integration strategy so that the public and other users can access the data held by both systems. The integration process will allow UNIIs to be made available through both the FDA SRS and EPA SRS.
RxNorm - The National Library of Medicine (NLM) has primary responsibility for the RxNorm terminology. As steward, NLM works in close collaboration with other governmental agencies (e.g. the VA, and the FDA), with the private sector (e.g. First Databank, Micromedex, Multum, Medispan) and other nations (e.g. UK and Australia). AHRQ supports, both conceptually and financially, the development of RxNorm to improve patient safety and quality of care. In addition, the FDA Structured Product Label (SPL) will link the Rx Norm Brand Name to the UNII code.
NDF-RT™ - The Department of Veterans Affairs developed and maintains the NDF-RT™ through the NDF-RT™ Board. This Board currently includes subject matter experts from the VA, but will soon be expanding its scope to also include subject matter experts from other selected federal agencies.
SNOMED® - SNOMED CT® is a copyrighted work of the College of American Pathologists (CAP). www.snomed.org
APPROVALS AND ACCREDITATIONS
HL7® is an ANSI-accredited Standards Developing Organization. This standard has been approved by full organizational ballot voting.
UNII will be part of both the FDA SRS and EPA SRS, which are both public domain systems.
RxNorm is a public domain system
NDF-RT™ is a public domain system
SNOMED CT® Healthcare Terminology Structure is American National Standards Institute (ANSI) approved. The CAP is an ANSI Standards Development Organization.
ACQUISITION AND COST
HL7® Standards are available from HL7®. HL7® asserts and retains copyright in all works contributed by members and non-members relating to all versions of the Health Level Seven® standards and related materials, unless other arrangements are specifically agreed upon in writing. No use restrictions are applied.
HL7® sells hard and computer readable forms of the various standard versions, cost from $50 - $500 depending on specific standard and member status.
UNII is freely available electronically from the FDA and EPA without a licensing agreement.
RxNorm is freely available through the NLM.
NDF-RT™ - May be obtained from the VA. It is available from the National Cancer Institute Enterprise Vocabulary Service (EVS) at http://nciterms.nci.nih.gov/NCIBrowser/Connect.do. It is also being incorporated into the UMLS®.
SNOMED CT® is available through the National Library of Medicine (NLM). The CAP and the NLM entered into an agreement to provide SNOMED CT® core content (English and Spanish language editions) via the UMLS ® at no charge to those who execute a license agreement. This agreement is for healthcare applications and uses within the US and any application of use of SNOMED CT® by any US government facility or office, whether permanent or temporary, wherever located.
Health care entities can also choose to license SNOMED CT® as a stand-alone terminology directly from SNOMED® International at (http://www.snomed.org)
Target Vocabulary Domain
| Common name used to describe the clinical/medical domain or messaging standard requirement that has been examined. Allergy Information |
| Describe the specific purpose/primary use of this standard in the federal health care sector (100 words or less) The standards, as identified in the following section of this document, will be used to set the requirements for exchanging allergy data, across the federal health community to provide the federal health community with a key component needed to enable federal agencies to build interoperable health data systems health vocabulary. It is anticipated that use of these standards will enable the federal health enterprise to exchange allergy health information across the wide range of federal activities helping to decrease medication errors, identify food and drug incompatibilities and provide consistent allergy data across the federal health community. |
Sub-domains Identify/dissect the domain into sub-domains, if any. For each, indicate if standards recommendations are or are not included in the scope of this recommendation.
| Domain/Sub-domain |
In-Scope (Y/N) |
| Allergy |
Y |
| Adverse Reactions |
N |
Information Exchange Requirements (IERs) Using the table at appendix A, list the IERs involved when using this vocabulary.
| Care Management Information |
| Case Management Information |
| Clinical Guidelines |
| Customer Demographic Data |
| Customer Healthcare Data |
| Customer Risk Factors |
| Population Member Health Data |
| Population Risk Reduction Plan |
| Tailored Education Information |
Team Members Team members’ names and agency names with phone numbers.
| Name |
Agency/Department |
| Marcia Insley (Colead) |
Department of Veterans Affairs, Veterans Health Administration |
| Lenora Barnes (Colead) |
Department of Veterans Affairs, Veterans Health Administration |
| Siew Lam |
Department of Veterans Affairs, Veterans Health Administration |
| Pradnya Warnekar |
Department of Veterans Affairs, Veterans Health Administration |
| Mike J. Lincoln |
Department of Veterans Affairs, Veterans Health Administration |
| Roger Sigley |
Department of Veterans Affairs, Veterans Health Administration |
| Randy Levin |
Department of Health and Human Services, Food and Drug Administration |
| William A. Hess |
Department of Health and Human Services, Food and Drug Administration |
| Elizabeth C. Smith |
Department of Health and Human Services, Food and Drug Administration |
| Stefano Luccioli |
Department of Health and Human Services, Food and Drug Administration |
| William Pierce |
Department of Health and Human Services, Food and Drug Administration |
| Cheryl Ford |
Department of Health and Human Services, Centers for Medicare and Medicaid |
| Don Reese |
Department of Health and Human Services, Centers for Medicare and Medicaid |
| Fola Parrish |
Department of Defense, OASD(HA)/TRICARE Management Activity |
| Nancy Orvis |
Department of Defense, OASD(HA)/TRICARE Management Activity |
| Mark Charles |
Department of Defense, OASD(HA)/TRICARE Management Activity |
| Ronald Nosek |
Department of Defense, OASD(HA)/TRICARE Management Activity |
| Mathew Garber |
Department of Defense, OASD(HA)/TRICARE Management Activity |
| Michael Datena |
Department of Defense, OASD(HA)/TRICARE Management Activity |
| John Kilbourne |
Department of Health and Human Services, National Library of Medicine |
| John Harman |
Environmental Protection Agency |
Work Period Dates work began/ended.
| Start |
End |
| May 27, 2005 |
April 30, 2006 |
Recommendation Identify the solution recommended.
| Health Level Seven® (HL7®), Version 2.4 and higher: Information Exchange Segment |
| UNII: Allergen Code, Mnemonic, Description RxNorm, Drug Brand Name (BN): Allergen Code, Mnemonic, Description |
| NDF-RT™: Allergen Group, Drug Classes (Chemical Structure) |
| SNOMED CT®: Allergy Type, Allergy Severity, Allergy Reaction |
Ownership Structure Describe who “owns” the standard, how it is managed and controlled.
| HL7® |
| UNII The UNII is linked to drug products as part of the FDA's Structured Product Labeling (SPL). SPL (including the UNII) will be made available in the DailyMed®, a web-based distribution of the SPL maintained by NLM. In addition, FDA UNII codes will be linked to RxNorm clinical drug codes. This will allow the brand name clinical drug to be captured and reported via a UNII code. The UNII was adopted in May 2004 by the Consolidated Health Informatics initiative as a part of its Medication Domain after being recommended to the DHHS Secretary by the National Committee on Vital and Health Statistics. The FDA SRS uses chemical structure, names, and descriptions to generate the UNII. The primary means for defining a substance is by its molecular structure represented on a two-dimensional plane. When a molecular structure is not available, the UNII is defined by a distinct name, and where necessary, an identifying description. Substances are registered in the FDA SRS following detailed business rules for processing the information. The standard is maintained by the FDA Data Standards Council and is monitored by the FDA SRS Board consisting of experts from the FDA and USP. The FDA SRS includes substances used in, or intended to be used in drugs, biologics, devices, cosmetics and foods. Drug-related substances include both active and inactive ingredients used in drug products, including those for veterinary purposes. FDA is aggressively working on dramatically increasing the number of UNII codes, through a Cooperative Research and Development Agreement (CRADA) with the United States Pharmacopeia. FDA’s goal is to load all 8,000 drug-related substances that currently appear in the USP Dictionary of USAN and International Drug Names by September 2006. The FDA has (1) UNII codes available for 100% of the active ingredients that are in approved and currently marketed prescription human drugs, and these UNII codes are now being incorporated into Structured Product Labeling (SPL), (2) a total of approximately 6,000 drug-related substances in the FDA SRS, and of these, approximately 3,000 have UNIIs, (3) publicly released 972 UNII codes in March 2005, which at that point in time represented approximately 80% of the active ingredients that were in approved and marketed prescription drug products, and (4) these 972 UNII codes that were released in March 2005 have appeared in the National Cancer Institute’s publicly accessible Terminology Browser at http://nciterms.nci.nih.gov/NCIBrowser/Dictionary.do since August 2005; they have been, and currently are, accessible through both an on-line web-based query and through a public API. Biologic substances include both active and inactive ingredients used in biologics, such as blood products, therapeutic products, vaccines, cellular and gene therapy products, allergenic products, tissues, and certain devices (e.g., enzymes in stabilized solutions). Although not technically always regulated by FDA as a biologic substance, botanical substances (also sometimes known as ‘herbals’) will be available in the FDA SRS. Device substances include certain components of some devices (e.g. silicon for implants, and chemical reagents for glucose test kits). Cosmetic substances are components of cosmetic products, such as flavors, fragrances, colorants, vitamins, plant- and animal-derived ingredients, and polymers. Since allergenic extracts are regulated by FDA, they will be included in the FDA SRS. Similarly, since there are many drugs, biologics, foods, and device components that can result in an allergic response, and since the FDA regulates these substances, they will be included in the FDA SRS. Therefore, since there are so many substances that can cause an allergic response already in the FDA SRS, it makes sense to expand the scope of the FDA SRS to include other substances that are capable of an allergic response. Food substances are specific foods or components of food, regardless of whether the food is in conventional food form or a dietary supplement, such as vitamins, minerals, herbs, or other similar nutritional substances. While the SRS will not serve as a classification system, the description of a substance may sometimes be at a high enough level where classification is readily understood. An example of this is the FALCPA (Food Allergen Labeling and Consumer Protection Act) which identifies eight (8) major food allergen categories that will be entered in the FDA SRS. FDA plans to import substances into the FDA SRS from several sources, including but not limited to, the European Agency for the Evaluation of Medicinal Products (EMEA), Environmental Protection Agency Substance Registry System (EPA SRS), Food Allergen Labeling and Consumer Protection Act (FALCPA), International Nonproprietary Names (INN,) LanguaL (Language of Foods), VA Non Drug Allergen List, National Cancer Institute (NCI), National Library of Medicine (NLM), Therapeutic Goods Administration, Australia (TGA), United States Adopted Names (USAN), and United States Pharmacopeia (USP). Environment Protection Agency Substance Registry System (EPA SRS) The EPA SRS is that agency’s authoritative resource for basic information about chemicals, biological organisms and other substances of interest to the Environmental Protection Agency (EPA) and its state and tribal partners. It relies on standardized fields to ensure that each substance is uniquely identified across all of the Agency’s programs. As chemicals and biological organisms a primary focus of the EPA, it is fundamental that they are properly cataloged and identified. EPA created the EPA SRS to provide a central place for identifying substances that are tracked or regulated at the Agency and to catalog basic information about them. It contains records for more than 95,000 chemicals, biological organisms, viruses and other substances. The significant work required to maintain the currency of the data, including the CAS names and numbers, benefits the CHI work. Each record contains standardized agency identifiers, as required by two data standards developed between EPA and its state and tribal partners. States and tribes now are adopting these identifiers internally. For chemicals, these standardized identifiers include the Chemical Abstracts Service (CAS) Number, the molecular weight, and the EPA preferred name. For biological organisms, standardized identifiers include the Taxonomic Serial Number (TSN) and the taxonomic name. In addition, each record includes synonyms used in the Agency and identifies the statutes, regulations and databases that use those synonyms. Importantly for the CHI work, there are links to health and safety data developed internally at EPA or externally by states, other federal agencies, or international organizations. Although there can be many synonyms for the same substance, SRS makes it possible to determine which EPA program is tracking or regulating a substance and the name used by that program. SRS therefore is a one-stop resource that enables EPA staff, states, tribes, industry, and the public to discover where to find Agency data about a substance. |
RxNorm The lack of interoperability among the terminologies used in the proprietary pharmaceutical knowledge bases was the primary motivation for RxNorm’s development. A user wishing to use one pharmaceutical knowledge base system for pricing and inventory control, for example, and a different system for interaction checking finds it difficult to merge the two systems into a larger environment. This issue was discussed at HL7 meetings for several years, with representatives from each of the pharmaceutical knowledge base providers as active participants. It became apparent that interoperability at the clinical drug level might be an achievable goal, if a standard clinical drug nomenclature was developed and the terminologies in the various proprietary systems were mapped to it. The NLM initiated the development of the public domain RxNorm vocabulary to achieve this goal – and simultaneously to eliminate the problem of undetected synonymy among proprietary clinical drug names within the UMLS® Metathesaurus. RxNorm has a robust information model that also supports ordering of medications. The VA, the FDA, the NCI, and the DOD are building use of RxNorm into their system development plans. The NLM continues to add new clinical drugs and links to additional drug terminologies and to refine the model in response to feedback. RxNorm is currently released on a monthly basis, and the May 2006 release had 31432 current clinical drugs. RxNorm links to more complete information in the VA NDF-RT™, such as drug classes, as well as to other terminologies available from commercial drug database vendors in the USA. RxNorm is being continuously developed and upgraded in conjunction with these various sources as well as with the FDA SPL data, and so represents the best potential for a comprehensive superset of all available drugs and an interlingua between various commercial and governmental drug sources. |
| NDF-RT™: Drug Classification (Chemical Structure) The National Drug File Reference Terminology (NDF-RT™) classification scheme has already been endorsed as a CHI Adopted standard for Physiological Effect and Mechanism of Action. NDF-RT™ chemical structure classification is being recommended under the allergy domain for chemical structure classification. NDF-RT™ contains the chemical structures, and makes links to drug ingredients, but the chemical structure concepts come MeSH® stored in the UMLS®. This classification scheme will assist in identifying a class of drug that a patient may be allergic to even if a specific drug is not known. If a specific drug is known, a chemical classification standard may assist in identifying a wider potential group of drug allergens. In addition, the FDA UNII codes are being included in NDF-RT™ as properties of the various drug ingredients. As part of the FDA Structured Product Label (SPL) initiative, UNII codes will be transmitted to NLM’s DailyMed® as part of the SPL and will then be loaded into NDF-RT™ through the “New Drug Transaction”. Thus the percentage of ingredients with UNII will rise progressively. |
| SNOMED® The College of American Pathologists (CAP) is holder of the copyright, trademark and patent rights in SNOMED®. The CAP owns the copyright in all editions of SNOMED®, including the copyright in any allowable adaptations, the trademarks SNOMED® and SNOMED CT®, and any and all patent rights in SNOMED®. Within the governance structure of the CAP, the SNOMED® International Authority has the direct responsibility for terminology-related activities. It establishes strategic direction for the CAP’s clinical terminology activities, advises management, monitors division performance, and provides connections to the broader outside world. The SNOMED® International Authority protects the purpose of SNOMED® for clinical care and prevents drift of its purpose through its constitution, decision-making criteria, and the expertise of voting members. The SNOMED® International Editorial Board is responsible for the scientific direction, editorial processes, and scientific validity of the terminology. The Editorial Board, composed of voting members and organizational liaisons, recommends guidelines for external input and field-testing. It also oversees the quality assurance process. The Editorial Board consists of both clinical content experts and medical informatics experts, with equal representation from the UK’s National Health Service. In addition, liaisons from numerous associations reflect the vision of an integrated clinical vocabulary useful for dentistry, nursing, veterinary medicine, radiology, ophthalmology, public health, and other clinical specialties, and that is compatible with standards such as HL7® and DICOM®. Participation of liaisons ensures scientific input from a range of clinical specialties and government agencies. Chaired by the SNOMED® Scientific Director, this group provides scientific direction for and supports the work of a multidisciplinary team of modelers and data administrators. |
Summary Basis for Recommendation Summarize the team’s basis for making the recommendation (300 words or less).
| The approach of this workgroup was as follows:
|
Summary of Recommendations
| Allergy Domain |
Recommendation |
Status |
| Information Exchange Segment |
HL7® 2.x and above |
|
| Allergen Name, Mnemonic, Description for Brand Name Drugs |
RxNorm BN |
|
| Allergen Name, Mnemonic, Description, |
UNII code from the FDA SRS / EPA SRS |
Conditional as noted below and components summarized on Allergy Schematic |
| Allergen Group, Drug Classes (Chemical structure) |
NDF-RT™ |
Conditional as noted below |
| Allergy Type, Allergy Severity and Allergy Reaction |
SNOMED CT® |
With recommendation for SNOMED®/HL7® collaboration and SNOMED CT® allergy subset consideration |
Conditional Recommendation If this is a conditional recommendation, describe conditions upon which the recommendation is predicated.
| The Work Group recommends the adoption of the proposed allergy standards. For implementation purposes, the following conditions must be met:
The workgroup identified gaps and areas of needed improvement in the standard that would improve utility, these can be found in the “Gaps” section, Part III. |
Approvals & Accreditations
Indicate the status of various accreditations and approvals for HL7 and SNOMED®:
|
Approvals |
Yes/Approved |
Applied |
Not Approved |
| Full SDO Ballot |
Y |
|
|
| ANSI |
Y |
|
|
Options Considered Inventory solution options considered and summarize the basis for not recommending the alternative(s). SNOMED® must be specifically discussed.
| Allergy Information Exchange/Models/Initiatives: HL7® |
| Terminologies: Allergen Type: |
| Allergy Severity: |
| Allergy Reaction: |
| Allergen Code/Mnemonic/Description: Drug: Food: Environmental: Non Drug/Other: |
| Class of Allergen Drug Classification: Food Classification: |
Current Deployment
HL7®
| Summarize the degree of market penetration today; i.e., where is this solution installed today? HL7® is used in many places as the messaging standard for health care data. Furthermore, HL7® has a great deal of support in the user community and 1999 membership records indicate over 1,600 total members, approximately 739 vendors, 652 healthcare providers, 104 consultants, and 111 general interest/payer agencies. HL7® standards are also widely implemented, though complete usage statistics are not available. In a survey of 153 chief information officers in 1998, 80% used HL7® within their institutions, and 13.5% were planning to implement HL7® in the future. In hospitals with over 400 beds, more than 95% use HL7®. As an example, one vendor has installed 856 HL7® standard interfaces as of mid 1996. It is the proposed message standard for the Claims Attachment transaction of the Administration Simplification section of the Health Insurance Portability and Accountability Act (HIPAA). Anecdotal information indicates that the major vendors of medical software, including Cerner, Misys (Sunquest), McKesson, Siemens (SMS), Eclipsys, AGFA, Logicare, MRS, Tamtron, IDX (Extend and CareCast), and 3M, support HL7®. The most common use of HL7® is probably admission/discharge/transfer (ADT) interfaces, followed closely by laboratory results, orders, and then pharmacy. HL7® is also used by many federal agencies including VHA, DoD and CDC, hence federal implementation time and cost is minimized. The widespread and long-standing use of HL7® leads to the team conclusion that this is a strong recommendation. What number or percentage of federal agencies have adopted the standard? Many federal agencies, several of which are represented within the CHI group, have adopted HL7 for messaging. Is the standard used in other countries? Yes, Argentina, Australia, Canada, China, Czech Republic, Finland, Germany, India, Japan, Korea, Lithuania, The Netherlands, New Zealand, Southern Africa, Switzerland, Taiwan, Turkey and the United Kingdom are also part of HL7® initiatives. Are there other relevant indicators of market acceptance? Yes, this standard is so widely accepted and used across the healthcare industry; see the market penetration section for vendor and federal agency use. |
SNOMED®
| Summarize the degree of market penetration today; i.e., where is this solution installed today? On July 1, 2003, an agreement with the College of American Pathologists (CAP) and HHS was announced that made SNOMED Clinical Terms (SNOMED CT®) available to U.S. users at no cost through the National Library of Medicine's® Unified Medical Language System® (UMLS®). Produced by the College of American Pathologists (CAP), SNOMED CT® (Systematized Nomenclature of Medicine--Clinical Terms) was formed by the convergence of SNOMED RT® and the United Kingdom's Clinical Terms Version 3 (formerly known as the Read Codes). With terms for more than 344,000 concepts, SNOMED CT® is the most comprehensive clinical terminology available. It is being implemented throughout the National Health Service in the United Kingdom. The National Library of Medicine (NLM), a component of the National
Institutes of Health (NIH), Department of Health and Human Services, has issued
a 5-year, $32.4 million contract to the CAP for a perpetual
license
for the core SNOMED CT® (in Spanish and English) and
ongoing updates. NLM is paying the annual update fees. Funding for the one-time
payment for the perpetual license was provided by: NLM distributes SNOMED CT®within the UMLS® Metathesaurus under the terms of a revised UMLS® license agreement, which includes additional language concerning SNOMED CT® . U.S. licensees will be able to use SNOMED® (as distributed by NLM) in the U.S. without charge and without signing a separate license agreement with the CAP. Non-U.S. UMLS® users will continue to require a separate license agreement with the CAP for production uses of SNOMED CT®. Current UMLS® users will have to sign the revised license agreement before receiving SNOMED CT® within the UMLS®. With the release of the 2004AA version of the UMLS®, the Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT®), produced by the CAP, becomes available for free U.S. use under a license agreement concluded last year. Users must register via the Web for a free UMLS® license before downloading the Metathesaurus or requesting a copy on DVD. What number or percentage of relevant vendors has adopted the standard? The state of incorporation into vendor systems varies and is largely dependent on the vendor’s development cycle. Following is a representative list of the vendors who have licensed SNOMED CT®, it should be noted that license does not equate to adoption.
What number or percentage of healthcare institutions has adopted the standard? More than 50 commercial healthcare software developers have incorporated SNOMED CT® into their systems. Two examples of the extent of support for SNOMED CT® are Kaiser Permanente and the National Health Service (NHS) of the United Kingdom. Kaiser Permanente, who provides health care coverage to 3% of the U.S. population, has actively participated in the development of SNOMED CT® and is actively rolling out SNOMED CT®-compatible solutions throughout its organization. Kaiser is using SNOMED CT® within domain-specific standard documentation templates for use throughout the organization. Also, as of April 1, 2003, the NHS, representing a population of 56 million covered lives, officially stated that: “Subject to successful development and testing of implementability, after April 1, 2003 any computerized information system being developed to support any clinical information system, such as EPRs and EHRs, should use the NHS preferred clinical terminology, SNOMED® Clinical Terms.” Other examples of health care institutions that have adopted SNOMED CT® are summarized as follows: The University of Nebraska Medical Center is using SNOMED CT® in the development of problem lists which are then mapped to ICD-9; Cedars Sinai Medical Center used SNOMED CT® in its web-based order entry system which processed 700,000 orders for over 8,000 patients between October 2002 and January 2003; HCA is implementing SNOMED CT® within its laboratory network, consisting of over 200 sites in both the US and Canada, for lab test results and diagnosis; University of Tennessee used SNOMED CT® in the lab to improve patient safety by detecting cases for which follow-up intervention did not occur despite abnormal Pap tests; Barnes Jewish Christian Health Care is using SNOMED CT® within its perioperative and surgery suites for medical transcription. What number or percentage of federal agencies have adopted the standard? Versions of SNOMED CT® are currently used by: the Centers for Disease Control and Prevention (CDC), Department of Defense (DoD), Indian Health Services (IHS) and the Department of Veterans Affairs (VA) in specific applications. As SNOMED CT® was first released in January, 2002, most of the government applications for which SNOMED CT® has been licensed are in evaluation or developmental stages.
Is the standard used in other countries? As of April, 2003, the CAP has licensed users of SNOMED CT® in 31 countries. Earlier editions of SNOMED® have been licensed in over 40 countries. Following are the countries in which SNOMED CT® has been licensed:
As previously noted, the UK’s National Health Service has officially stated that any computerized information system being developed to support any clinical information system, should use the NHS preferred clinical terminology, SNOMED® Clinical Terms. In Australia, where the use of electronic health cares systems to support general practice is relatively advanced, a “Coding Jury” had been established to select a single coding system to support GP clinical systems. Currently, the GP Vocabulary Project is underway, and is designed to assist in the building and support of a standard general practice interface terminology suitable for the management of information collected during the clinical encounter. Phase 2 of this project will include the mapping of a subset of the GP Vocabulary to SNOMED CT®. Are there other relevant indicators of market acceptance? Market share information provided by CAP indicates that 79% of computerized patient record systems and 85% of laboratory systems vendors have made licensing commitment. Following are other relevant indicators of SNOMED’s® market acceptance:
|
UNII
| UNII is distributed by FDA and EPA without restriction. Summarize the degree of market penetration today; i.e., where is this solution installed today? UNII is part of the CHI medication domain for active ingredient. As such, it is incorporated into FDA’s Structured Product Labeling (SPL) initiative, which will soon be used worldwide. In addition, FDA SRS has already made its substance names and their Unique Ingredient Identifier (UNIIs) available to the following: Department of Veterans Affairs (NDF-RT™), the National Library of Medicine® (UMLS®), the National Cancer Institute (NCI EVS), Environmental Protection Agency (EPA SRS) and the United States Pharmacopeia (USP Dictionary of USAN and International Drug Names). Finally, each of these organizations, as well as the European Medicines Agency and Australian Therapeutics Goods Administration, has expressed a high degree of interest in loading their substance data into the FDA SRS. Currently, available UNIIs are accessible for public access and download through the NCI Terminology Browser/NCI_Thesaurus (see http://nciterms.nci.nih.gov/NCIBrowser/Dictionary.do). UNIIs will also be available as part of the new labeling format will be integrated into FDA's other e-Health efforts through a variety of ongoing initiatives. As prescription information is updated in this new format it will be used to provide medication information for DailyMed® – an interagency online health information clearinghouse, sponsored by the National Library of Medicine, which is maintaining the most up-to-date medication information free to consumers, healthcare professionals, and healthcare information providers. The DailyMed® is making up-to-date information about FDA-regulated products widely available on the Internet at no cost. (http://dailymed.nlm.nih.gov) FDA has scheduled a second release of UNIIs by the end of July 2006, and that the dataset will include 100% of the active ingredients of approved and marketed prescription drug products, as well as many inactive ingredients; it may also include the initial portion of the USP work, as well as color additives, 760 botanical substances, vaccine substances, and allergenic food substances. As noted, UNIIs for all active ingredients and many inactive ingredients are being integrated into SPL, and SPL is being made available through DailyMed® at NLM. Therefore, UNIIs are available by accessing DailyMed®. Presently, there are only a few SPL submissions in DailyMed®, but that number is expected to exponentially increase due to a federal regulation that went into affect in October 2005. The regulation requires that for approved and marketed prescription drugs, (1) all labeling that is included in annual reports shall be submitted in SPL, and (2) all labeling for newly approved drugs shall be submitted in SPL. This means that by December 2006, all approved and marketed prescription drugs shall have SPL available (with UNIIs) through DailyMed®. What number of or percentage of relevant vendors have adopted the standard? 100% of pharmaceutical companies marketing prescription drug products in the United States and Structured Product Labeling vendors must use the UNII standard. Most likely, a large percentage of healthcare decision support software vendors will seriously consider, if not overtly adopt, the UNIIs that enable healthcare decision support will already be embedded in SPL. What number or percentage of healthcare institutions have adopted the standard? None to date, but that is expected to rapidly change. What number or percentage of federal agencies have adopted the standard? 100% of federal agencies have adopted the UNII as their standard for active ingredient through the White House’s Consolidated Health Informatics initiative. Many federal agencies are in the process of implementing the UNII as their standard for active ingredient. Since the UNII plans to also include inactive ingredients, it will soon have much greater utility, and federal agencies have begun to recognize that eventuality. Is the standard used in other countries? None to date, but that is expected to rapidly change. The EMEA, Japan, Health Canada, and TGA are interested in adopting the UNII as their standard, and since the USP will be associating a UNII with each INN, and this adoption would make sense for these and other foreign drug regulatory bodies. Are there other relevant indicators of market acceptance? There are no additional relevant indicators of market acceptance. |
RxNorm
The need to recommend RxNorm in addition to UNII codes stems from potential situations where data (historical data) may not be coded to the ingredient level. This is identified as a data need within DOD, where allergy information is captured at Brand Name and RxNorm (BN) can be assigned and exchanged. RxNorm is distributed by without restriction. Summarize the degree of market penetration today; i.e., where is this solution installed today? RxNorm is distributed by the NLM without restriction. A listing of current users is not kept. What number of or percentage of relevant vendors have adopted the standard? RxNorm is distributed by the NLM without restriction. A listing of current users who are vendors is not kept. What number or percentage of healthcare institutions have adopted the standard? RxNorm is distributed by the NLM without restriction. A listing of current users who are healthcare institutions is not kept. What number or percentage of federal agencies have adopted the standard? RxNorm is distributed via by the NLM without restriction. A listing of current users who are federal agencies is not kept. Is the standard used in other countries? RxNorm is distributed via by the NLM without restriction. A listing of current users in other countries is not kept. Are there other relevant indicators of market acceptance? There are no additional relevant indicators of market acceptance. |
NDF-RTTM
| Drug classification schemes developed to date meet specific decision support needs that vary across institutions. Content requirements placed on legacy systems and their supporting knowledge bases differ vastly depending on the use cases that the organization has to support. As a result there are structural classes where drugs are grouped together based on their chemistry or there are functional classes where drugs are grouped together based on their mechanism of action or therapeutic intent or physiologic effect. In the allergy domain, there are two reasons why drugs would need to be grouped. First, to prevent ordering of drugs similar to the ones that the patient is allergic to, and second to simplify patient allergy documentation when it is difficult for a patient to recall the specific drug that (s)he is allergic to. Allergy to any drug is due to the presence of a specific chemical entity in the drug. For example, if a patient is allergic to a sulfonamide containing antimicrobials, then it is very likely that (s)he is also allergic to one of the thiazide diuretics that are used to lower blood pressure. This illustrates that even though the two drugs have completely different therapeutic uses, they are likely to cause the same allergic reaction in the patient. This is because of similarity in chemical structures between the two classes of drugs. Thus, grouping drugs based on chemistry for patient allergy documentation is critical. NDF-RTTM is a reference terminology for medications developed by the VHA. It is based on the VHA’s current production system called VA NDF, but is intended to help separate the specific legacy use cases in VA for drug interactions and checking from the definitional classifications of drug ingredients. As a result, NDF-RT™ supports VA legacy codes (beta lactam antibiotics, opioid analgesics) that support existing VistA use cases. It also supports, in a parallel fashion, a logically decomposed set of attributes for all ingredients like mechanism_of_action, physiologic_effect, chemical_structure, and diseases_treated, etc. The data in NDF-RTTM is modeled using various types of relationships to represent the semantics between information at different levels in its hierarchy. NDF-RTTM is also linked with various external national standards like RxNorm, FDA UNII, MeSH® and HL7. NDF-RTTM classes drugs in a complex web of semantics. Two of the classification schemes (Mechanism of action and Physiological effects) have been adopted as CHI standards as part of CHI Medications sub domain. NDF-RTTM’s active ingredients hierarchy was initialized from MeSH® and is synchronized with the same at regular intervals. Concepts in this hierarchy are differentiated from the other concepts by a Ingredient_Kind designation. The following information for Ingredient_Kind concepts is stored in NDF-RT™.
Medical Subject Headings (MeSH®) Benefits- MeSH® offers several advantages as a drug classification scheme for allergy domain. It offers an unrestricted and open license, a publicly accessible change control board, and a wide acceptance and implementation in information systems. Another important advantage of MeSH® is the multihierarchical arrangement of concepts. This provides the ability to support different use cases of legacy systems. For example, ampicillin is categorized as a penicillin, beta lactam, 2-ring heterocyclic compound and a bridged ring heterocyclic compound. While the association of ampicillin as a penicillin is useful in allergy checking, the relationship to beta lactams group could support drug resistance checking. NDF-RT™ is distributed by without restriction. Summarize the degree of market penetration today; i.e., where is this solution installed today? None to date What number of or percentage of relevant vendors have adopted the standard? None to date What number or percentage of healthcare institutions have adopted the standard? None to date What number or percentage of federal agencies have adopted the standard? 100% of federal agencies have adopted the NDF-RT™ as their standard mechanism of action and physiological effect through the White House’s Consolidated Health Informatics initiative. Is the standard used in other countries? None to date Are there other relevant indicators of market acceptance? There are no additional relevant indicators of market acceptance. |
Provide all information gathered in the course of making the recommendation that may assist with adoption of the standard in the federal health care sector. This information will support the work of an implementation team.
Existing Need & Use Environment
Measure the need for this standard and the extent of existing exchange among federal users. Provide information regarding federal departments and agencies use or non-use of this health information in paper or electronic form, summarize their primary reason for using the information, and indicate if they exchange the information internally or externally with other federal or non-federal entities.
Column A: Agency or Department
Identity (name)
Column B: Use data in this domain
today? (Y or N)
Column C: Is use of data a core
mission requirement? (Y or N)
Column D: Exchange with others in
federal sector now? (Y or N)
Column E: Currently exchange paper or
electronic (P, E, B (both), N/A)
Column F: Name of paper/electronic
vocabulary, if any (name)
Column G: Basis/purposes for data use
(research, patient care, benefits, clinical trials, information steward)
| Department/Agency |
B |
C |
D |
E |
F |
G |
| Department of Veterans Affairs (VA) |
Y |
Y |
Y |
B |
|
Patient Care |
| Department of Defense (DOD |
Y |
Y |
Y |
B |
|
Patient Care |
| HHS Office of the Secretary |
|
|
|
|
|
|
| Administration for Children and Families (ACF) |
|
|
|
|
|
|
| Administration on Aging (AOA) |
|
|
|
|
|
|
| Agency for Healthcare Research and Quality (AHRQ) |
|
|
|
|
|
|
| Agency for Toxic Substances and Disease Registry (ATSDR) |
|
|
|
|
|
|
| Centers for Disease Control and Prevention (CDC) |
|
|
|
|
|
|
| Centers for Medicare and Medicaid Services (CMS) |
Y |
Y |
Y |
B |
|
Benefits |
| Food and Drug Administration (FDA) |
Y |
Y |
Y |
B |
SPL; DARRTS; AERS; SPOTS |
Regulation, Research |
| Health Resources and Services Administration (HRSA) |
|
|
|
|
|
|
| Indian Health Service (IHS) |
Y |
Y |
Y |
B |
|
Patient Care |
| National Institutes of Health (NIH) |
Y |
Y |
Y |
B |
|
Clinical Trials |
| Substance Abuse and Mental Health Services Administration (SAMHSA) |
|
|
|
|
|
|
| Social Security Administration (SSA) |
|
|
|
|
|
|
| Department of Agriculture (DOA) |
|
|
|
|
|
|
| Department of State (DOS) |
|
|
|
|
|
|
| US Agency for International Development (USAID) |
|
|
|
|
|
|
| Department of Justice (DOJ) |
|
|
|
|
|
|
| Department of Treasury (DOT) |
|
|
|
|
|
|
| Department of Education (DOE) |
|
|
|
|
|
|
| General Services Administration |
|
|
|
|
|
|
| Environmental Protection Agency |
N |
Y |
N |
|
|
Information Steward |
| Department of Housing & Urban Development (HUD) |
|
|
|
|
|
|
| Department of Transportation (DOT) |
|
|
|
|
|
|
| Department of Homeland Security (DHS) |
|
|
|
|
|
|
| Number of Terms Quantify the number of vocabulary terms, range of terms or other order
of magnitude. How often are terms updated? HL7® v2.x standards are issued every two to three years. HL7® V 3 vocabulary tables are updated three times per year. FDA SRS terms are added daily, and updated as necessary throughout each year. The terms can be reviewed several times a year as requested. EPA SRS The data in the SRS is periodically updated with new EPA regulations and program system lists. EPA and FDA are developing process to update each agency’s SRS’. RxNorm Monthly releases SNOMED CT® Semiannually (January 31st and July 31st) NDF-RT™ updated with MeSH® annually. |
| Range of Coverage Within the recommended vocabulary, what portions of the standard are complete and can be implemented now? (300 words or less) HL7® 2.x is currently available for information exchange. The HL7® v3 Allergy Reference Information Model is in available but not widely tested. Once the SPL data (including the UNII code portion) has been submitted by the FDA to NLM for distribution in the DailyMed®, that data will also be incorporated into RxNorm RxNorm maintains Brand Name information but no vocabulary can be complete. Drug vocabulary, in particular, is constantly changing, with new ingredients, new dosage forms, and new strengths. RxNorm contains all the clinical drugs contained in the other vocabularies, and has an update model. With the understanding as stated, it is ready for implementation in its coverage of prescription drugs. It remains incomplete for multi-ingredient OTC preparations (e.g., multivitamins) and contrast media. A new version of NDF-RT™, planned for 2006 or later, will be submitted to NLM and included in UMLS®. |
| Acquisition How are the data sets/codes acquired and use licensed? Standards are available from HL7®. HL7® asserts and retains copyright in all works contributed by members and non-members relating to all versions of the Health Level Seven® standards and related materials, unless other arrangements are specifically agreed upon in writing. No use restrictions are applied. Each recommended vocabulary standard is available as noted in the vocabulary standards section. |
| Cost What is the direct cost to obtain permission to use the data sets/codes? (licensure, acquisition, other external data sets required, training and education, updates and maintenance, etc.) HL7® sells hard and computer readable forms of the various standard versions, cost from $50 - $500 depending on specific standard and member status. Draft versions of standards are available to all from their website. No specific cost is associated with using the standards. Training is offered through HL7® and others are varying costs from several hundred to several thousand-dollars/per person. Consultation services are available at standard industry cost for training, update instillation and maintenance. FDA SRS is available free of charge through SPL and DailyMed®. It is then also made public through the NLM and NCI, and incorporated into the USP Dictionary of USAN and International Drug Names (a.k.a., ‘USAN’). RxNorm and SPL is available free of charge through the UMLS®. EPA SRS is available free of charge through EPA website. Enhanced capabilities are currently being redesigned to improved access to the EPA SRS. RxNorm is available free of charge through NLM UMLS® NDF-RT™ is available free of charge through NCI SNOMED CT® is available free of charge through NLM UMLS® |
| Systems Requirements Is the standard associated with or limited to a specific hardware or software technology or other protocol? No |
| Guidance What public domain and implementation and user guides, implementation tools or other assistance is available and are they approved by the SDO? HL7® is in widespread use and has many implementation guides and tools, some in the public domain and some accessible by authorized personnel or organizations. Please refer to www.hl7.org for more details. Full implementation conformance requirements for interoperability will need to be developed between exchanging partner built of the HL7 and additional vocabulary standards being recommended. Is a conformance standard specified? Are conformance tools available? A standard is not specified. Conformance tools are not available through the SDO, but private sector tools do exist. |
| Maintenance How do you coordinate inclusion and maintenance with the standards developer/owners? Voluntary upgrade to new versions of standards, generally by trading partner agreement. Messages are transmitted with version number and use of prior versions is generally supported for a period of time after introduction of a new one. What is the process for adding new capabilities or fixes? The process for adding new capabilities or fixes is addressed at the individual Standards Development Organization. What is the average time between versions? Various, but approximately annually. What methods or tools are used to expedite the standards development cycle? None. The methods or tools used to expedite the standards development cycle is addressed at the individual Standards Development Organization (SDOs). Some SDOs conduct standard development meetings up to three times yearly, as well as in the workgroups between meetings. The standards development is often a lengthy process. lengthy. How are local extensions, beyond the scope of the standard, supported if at all? For HL7, yes, but not encouraged (Z segment) |
| Customization Describe known implementations that have been achieved without user customization, if any. None. If user customization is needed or desirable, how is this achieved? (e.g, optional fields, interface engines, etc.) Free text fields are available bit not recommended. |
| Mapping Requirements Describe the extent to which user agencies will likely need to perform mapping from internal codes to this standard. Because implementation of the CHI adopted standards is prospective, user agencies will need to perform mapping from internal codes to the allergy standards only in new systems and systems undergoing major upgrades. Identify the tools available to user agencies to automate or otherwise simplify mapping from existing codes to this standard. The NCI has already begun mapping the FDA SRS to its own databases, and it is anticipated that USP and NLM will also do the same. United States Health Information Knowledgebase (USHIK) is designing a portal to provide access to all CHI Standards. NLM UMLS® provide information for terminology mapping. |
Compatibility
Identify the extent of off-the-shelf conformity with other standards and requirements:
| Conformity with other Standards |
Yes (100%) |
No (0%) |
Yes with exception |
| HIPAA Transaction and Code Set Standards |
X |
|
|
| HL7® 2.4 and higher |
X |
|
|
| Implementation Timeframe Estimate the number of months required to deploy this standard; identify unique considerations that will impact deployment schedules. Any estimate would differ by agency, due to the legacy systems currently in. In order to determine the compatibility of the standards, use cases need to be done, to look at all intended uses of allergy terminology being addressed. If some data sets/code sets are under development, what are the projected dates of completion/deployment? As of March 2006, the FDA has completed entering 100% of active ingredients for approved and marketed prescription drug products, 60% of over the counter and 99% of vitamins and minerals into the FDA SRS. It is anticipated that by October 2006, all vitamins and minerals will be added into the FDA SRS, as will the most common inactive ingredients that are in approved and marketed prescription drug products. By August 2006, it is anticipated that active ingredients for common food allergens and botanicals/herbals will be entered into the FDA SRS. By December 2008, it is anticipated that the FDA SRS will be loaded with
|
| Gaps Identify the gaps in data, vocabulary or interoperability. Any other required federal standards that should emerge will require a harmonization between HL7® and the vocabulary recommended standards identified. Availability of the UNII codes within the FDA SRS for all aspects (active ingredients, inactive ingredients, dyes) of drugs (prescription, OTC, herbals), biologics and food is progressing as noted in deployment schedule above. Since this is the basis of the Allergen recommendation, it is recommended that the FDA SRS UNII code availability be aggressively completed not only for all inactive ingredients that are in approved and marketed prescription drugs, but also for active and inactive ingredients that are in OTC drugs, cosmetics, foods, herbal products, and biologics. The furthest projected completion date at this point in time would be December 2008. A new version of NDF-RT™ will be submitted to NLM (planned for Summer 2006) to be included in UMLS® and will be made publicly available through a simple download mechanism. The Allergy Type codes and descriptions available in SNOMED should be reviewed and refined as necessary. It would be advantageous if all Allergy Reaction codes could hierarchically descend from a common concept. Current Allergy Reaction Codes as identified on the VA/DoD recommended allergen reaction list cross hierarchies, and so must be enumerated individually. A general approach to specifying and maintaining subsets drawn from SNOMED or other vocabularies has not been fully worked out. Such a process would indicate the organization maintaining the subset, the specific collection of concepts in the subset, the manner of distribution of such a subset and its updates, and other such metadata related to the subset. |
| Obstacles What obstacles, if any, have slowed penetration of this standard? (technical, financial, and/or cultural) To date, there has been no available vocabulary to support standardized Allergy content of the HL7 transaction, particularly as it is related to the allergen code. In addition, this is a complex but crucial areas for patient safety and medical errors. The immaturity of related standards to support this domain is being addressed by involved agencies including FDA and NLM. The VA and DOD as large healthcare providers have piloted efforts to share allergy data. The extent of this sharing is limited by the incomplete status of many of the allergen related vocabulary. To fill this gap, the VA and DOD have compiled allergen related vocabularies which are being pointed to SNOMED® codes and incorporated into the FDA SRS. The FDA is working to establish a complete set of Unique Ingredient Identifiers for drug, biologics, cosmetics, herbal products, food and non drug/food allergen. This FDA effort has already begun with the expected completion of all common allergen codes by December 06 and all ingredients by December 2008. With numerous systems currently deployed throughout the government, the cost to convert to a new version of HL7® is high. Furthering the difficulties are the legacy issues that still need to be addressed. While the team supports the use of HL7® messaging standards for allergy transactions, it notes that a large gap exists between the message standard and the ability to currently code allergen contents of the message. Version 2.x HL7® messages are currently implemented with a high degree of variability in content of the elements. Some of this difference relates to the use of local codes or non-standard use of publicly available codes and some involves subtle differences in the interpretation of the element’s meaning. The vocabulary standards recommended in this report attempt to address these obstacles. Version 3 of HL7® has a goal of increasing the ability to understand a received message by addressing these two broad issues through the use of an XML message structure and a Reference Information Model (RIM), though this has not been demonstrated. The acceptance of the message standard without standardization of code sets between users will not result in increased interoperability and a large gap will exist. The Allergy Work Group also encourages the continual developmental efforts to improve access tools to the recommended standards. |
Appendix A
Information Exchange Requirements (IERs)
| Information Exchange Requirement |
Description of IER |
| Beneficiary Financial / Demographic Data |
Beneficiary financial and demographic data used to support enrollment and eligibility into a Health Insurance Program. |
| Beneficiary Inquiry Information |
Information relating to the inquiries made by beneficiaries as they relate to their interaction with the health organization. |
| Beneficiary Tracking Information |
Information relating to the physical movement or potential movement of patients, beneficiaries, or active duty personnel due to changes in level of care or deployment, etc. |
| Body of Health Services Knowledge |
Federal, state, professional association, or local policies and guidance regarding health services or any other health care information accessible to health care providers through research, journals, medical texts, on-line health care data bases, consultations, and provider expertise. This may include: (1) utilization management standards that monitor health care services and resources used in the delivery of health care to a customer; (2) case management guidelines; (3) clinical protocols based on forensic requirements; (4) clinical pathway guidelines; (5) uniform patient placement criteria, which are used to determine the level of risk for a customer and the level of mental disorders (6) standards set by health care oversight bodies such as the Joint Commission for Accreditation of Health Care Organizations (JCAHO) and Health Plan Employer Data and Information Set (HEDIS); (7) credentialing criteria; (8) privacy act standards; (9) Freedom of Information Act guidelines; and (10) the estimated time needed to perform health care procedures and services. |
| Care Management Information |
Specific clinical information used to record and identify the stratification of Beneficiaries as they are assigned to varying levels of care. |
| Case Management Information |
Specific clinical information used to record and manage the occurrences of high-risk level assignments of patients in the health delivery organization.. |
| Clinical Guidelines |
Treatment, screening, and clinical management guidelines used by clinicians in the decision-making processes for providing care and treatment of the beneficiary/patient. |
| Cost Accounting Information |
All clinical and financial data collected for use in the calculation and assignment of costs in the health organization. |
| Customer Approved Care Plan |
The plan of care (or set of intervention options) mutually selected by the provider and the customer (or responsible person). |
| Customer Demographic Data |
Facts about the beneficiary population such as address, phone number, occupation, sex, age, race, mother's maiden name and SSN, father's name, and unit to which Service members are assigned |
| Customer Health Care Information |
All information about customer health data, customer care information, and customer demographic data, and customer insurance information. Selected information is provided to both external and internal customers contingent upon confidentiality restrictions. Information provided includes immunization certifications and reports, birth information, and customer medical and dental readiness status |
| Customer Risk Factors |
Factors in the environment or chemical, psychological, physiological, or genetic elements thought to predispose an individual to the development of a disease or injury. Includes occupational and lifestyle risk factors and risk of acquiring a disease due to travel to certain regions. |
| Encounter (Administrative) Data |
Administrative and Financial data that is collected on patients as they move through the healthcare continuum. This information is largely used for administrative and financial activities such as reporting and billing. |
| Improvement Strategy |
Approach for advancing or changing for the better the business rules or business functions of the health organization. Includes strategies for improving health organization employee performance (including training requirements), utilization management, workplace safety, and customer satisfaction. |
| Labor Productivity Information |
Financial and clinical (acuity, etc.) data used to calculate and measure labor productivity of the workforce supporting the health organization. |
| health organization Direction |
Goals, objectives, strategies, policies, plans, programs, and projects that control and direct health organization business function, including (1) direction derived from DoD policy and guidance and laws and regulations; and (2) health promotion programs. |
| Patient Satisfaction Information |
Survey data gathered from beneficiaries that receive services from providers that the health organization wishes to use to measure satisfaction. |
| Patient Schedule |
Scheduled procedure type, location, and date of service information related to scheduled interactions with the patient. |
| Population Member Health Data |
Facts about the current and historical health conditions of the members of an organization. (Individuals' health data are grouped by the employing organization, with the expectation that the organization's operations pose similar health risks to all the organization's members.) |
| Population Risk Reduction Plan |
Sets of actions proposed to an organization commander for his/her selection to reduce the effect of health risks on the organization's mission effectiveness and member health status. The proposed actions include: (1) resources required to carry out the actions, (2) expected mission impact, and (3) member's health status with and without the actions. |
| Provider Demographics |
Specific demographic information relating to both internal and external providers associated with the health organization including location, credentialing, services, ratings, etc. |
| Provider Metrics |
Key indicators that are used to measure performance of providers (internal and external) associated with the health organization. |
| Referral Information |
Specific clinical and financial information necessary to refer beneficiaries to the appropriate services and level of care. |
| Resource Availability |
The accessibility of all people, equipment, supplies, facilities, and automated systems needed to execute business activities. |
| Tailored Education Information |
Approved TRICARE program education information / materials customized for distribution to existing beneficiaries to provide information on their selected health plan. Can also include risk factors, diseases, individual health care instructions, and driving instructions. |
Appendix B
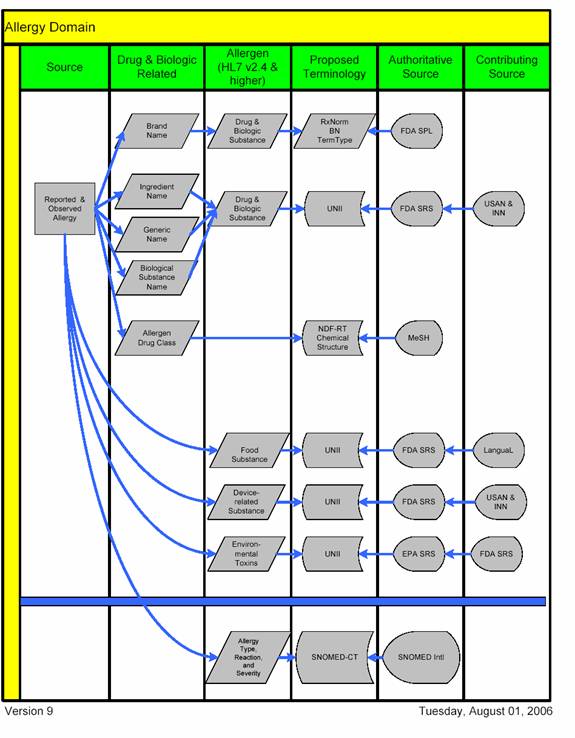
Allergy Recommendation Schematic Illustration
Appendix C
Glossary
| Acronym |
Description |
| ACF |
Administration for Children and Families |
| AERS |
Adverse Event Reporting System |
| AHFS |
American Hospital Formulary Service |
| AHRQ |
Agency for Healthcare Research and Quality |
| ANA |
American Nurses Association |
| ANSI |
American National Standards Institute |
| AOA |
Administration on Aging |
| ATC |
Anatomical Therapeutic Chemical Classification |
| ATSDR |
Agency for Toxic Substances and Disease Registry |
| AVMA |
American Veterinary Medical Association |
| CAP |
College of American Pathologists |
| CAS |
Chemical Abstract Service |
| CDC |
Centers for Disease Control and Prevention |
| CHDR |
Clinical Health Data Repository |
| CMS |
Centers for Medicare and Medicaid Services |
| CRADA |
Cooperative Research and Development Agreement |
| DARRTS |
Document Archiving, Reporting and Regulatory Tracking |
| DHS |
Department of Homeland Security |
| DOA |
Department of Agriculture |
| DOD |
Department of Defense |
| DOE |
Department of Education |
| DOJ |
Department of Justice |
| DOS |
Department of State |
| DOT |
Department of Treasury |
| DOT |
Department of Transportation |
| DTS |
Distributed Terminology System |
| EAFUS |
Everything Added to Foods in the United States |
| EMEA |
European Agency for the Evaluation of Medicinal Products |
| EVS |
Enterprise Vocabulary Services |
| EPA |
Environmental Protection Agency |
| EPA SRS |
Environmental Protection Agency Substance Registry System |
| FAET |
Federal Adverse Events Task Force |
| FALCPA |
Food Allergen Labeling and Consumer Protection Act |
| FDA |
Food and Drug Administration |
| FDA SRS |
Food and Drug Administration Substance Registration System |
| FDB |
First Data Bank |
| GCPR |
Government Computer-Based Patient Record |
| GSA |
General Services Administration |
| HEDIS |
Health Plan Employer Data and Information Set |
| HHS/DHSS |
Department of Health and Human Services |
| HIPAA |
Health Insurance Portability and Accountability Act |
| HL7® |
Health Level Seven® (HL7®) |
| HRSA |
Health Resources and Services Administration |
| HUD |
Department of Housing & Urban Development |
| IERs |
Information Exchange Requirements |
| IHS |
Indian Health Service |
| INN |
International Nonproprietary Names |
| IUIS |
International Union of International Societies |
| IUPAC |
International Union of Pure and Applied Chemistry |
| JCAHO |
Joint Commission for Accreditation of Health Care Organizations |
| LanguaL |
Langua aLimentaria; Language of Foods |
| MeSH |
Medical Subject Heading |
| NASA |
National Aeronautics and Space Administration |
| NCI |
National Cancer Institute |
| NCPDP |
National Council for Prescription Drug Programs |
| NDC |
National Drug Code |
| NDF-RT™ |
National Drug File Reference Terminology |
| NEHTA |
National E-Health Transition Authority, Australia |
| NIH |
National Institutes of Health |
| NLM |
National Library of Medicine |
| OASD(HA) |
Office of the Assistant Secretary of Defense for Health Affairs |
| RxNorm |
National Library of Medicine standardized nomenclature for clinical drugs |
| SAMHSA |
Substance Abuse and Mental Health Services Administration |
| SDOs |
Standards Development Organization |
| SNOMED® |
Systematized Nomenclature of Medicine |
| SNOMED CT® |
Systematized Nomenclature of Medicine Clinical Terms |
| SPL |
Structured Product Labeling |
| SPOTS |
Special Products On-Line Tracking System |
| SSA |
Social Security Administration |
| TGA |
Therapeutic Goods Administration (Australia) |
| TRICARE |
Health insurance program for military personnel and their families |
| UMLS® |
Unified Medical Language System |
| UNII |
Unique Ingredient Identifier |
| USAID |
United States Agency for International Development |
| USAN |
United States Adopted Names |
| USDA |
United States Department of Agriculture |
| USHIK |
United States Health Information Knowledgebase |
| USP |
United States Pharmacopeia |
| USP-NF |
United States Pharmacopeia-National Formulary |
| VA |
Department of Veterans Affairs |
| VHA |
Veterans Health Administration |
| WASPalm |
World Association of Societies of Pathology and Laboratory Medicine |