Rock Talk Archives for June 2011
Application Tips from the Regional Seminar
Last week I was in Weston, Florida participating in our second and final NIH Regional Seminar of this year. Our seminars draw approximately 500 individuals who, with the help of approximately 35 NIH and HHS staff members, learn how to apply for and manage NIH grants. This year we began tweeting live from the seminars our favorite tips, and some of you followed along. For those of you who missed the tweets, here are some of my favorites.
- RFAs usually don’t come out until we have a NIH budget. So pay attention to NIH guide.
- Subscribe to the NIH Guide. Best way to find out about programs and policies. You’ll get it every Friday. http://t.co/NYzQvyf
- Success rates are at 20% but pay lines at an institute may be lower. Understand the difference.
- Thinking about applying for a R21? Think about applying for a R01 instead if you are ready. R01 has higher success rate.
- Some ICs have paylines for early stage investigators and assistance for new investigators at first renewal stage See IC funding strategies.
- Office of Laboratory Animal Welfare has many outreach efforts. See http://t.co/6g1wsop
- Loan repayment is available to help pay back advanced educational debt in biomedical sciences. www.lrp.nih.gov
Like what you see and want more? Find me on Twitter @RockTalking. You don’t have to join to read my tweets.
Help Reduce Administrative Burden and Costs
This is your chance to have input on an important issue that greatly impacts the research community each and every day. Today, on behalf of the A-21 Task Force that reports to the Research Business Models Working Group, we released a request for information regarding revisions to the current OMB Circular A-21. This document, the Cost Principles for Educational Institutions, guides universities in determining what can and cannot be charged to federal grants and contracts. We are requesting information on opportunities for revisions that promise to reduce administrative burden or costs associated with compliance requirements of this circular and other requirements associated with federal support of research.
Representatives from multiple federal agencies, the Office of Management and Budget, and the Office of Science and Technology Policy will review the potential revisions. Several suggested areas that have potential to reduce burden have been identified for consideration within the request for information. You can see the list in the notice. Improvements to additional areas will also be considered. We are interested in hearing about A-21 issues that impact institutions, but also are particularly interested in how A-21 impacts the conduct of research by the scientist in his or her lab.
If you would like to submit a potential revision or comment, please use this website. Responses will be accepted through July 28, 2011, and a summary of the responses will be available by September 2011. More information is available in the NIH Guide for Grants and Contracts. If you have questions about this request for information, send us an e-mail.
Who Are We?
As part of our effort to characterize the community that is supported by NIH, we looked at the degrees held by NIH-supported principal investigators (PIs) and whether the composition of that population has changed over the years. As you can see below, ~70% of PIs on NIH research project grants hold a PhD, and this proportion has remained steady for the past 25 years. Most of the remaining PIs have either MD or MD/PhD degrees, with a small percentage unknown or holding other advanced degrees (for example, DVM, DDS, and DMD). The percentage of MDs has declined over the years from ~20% to around ~17%, while the percentage of PIs with a MD/PhD degree has increased from ~8.5% to almost 11% in the same timeframe.
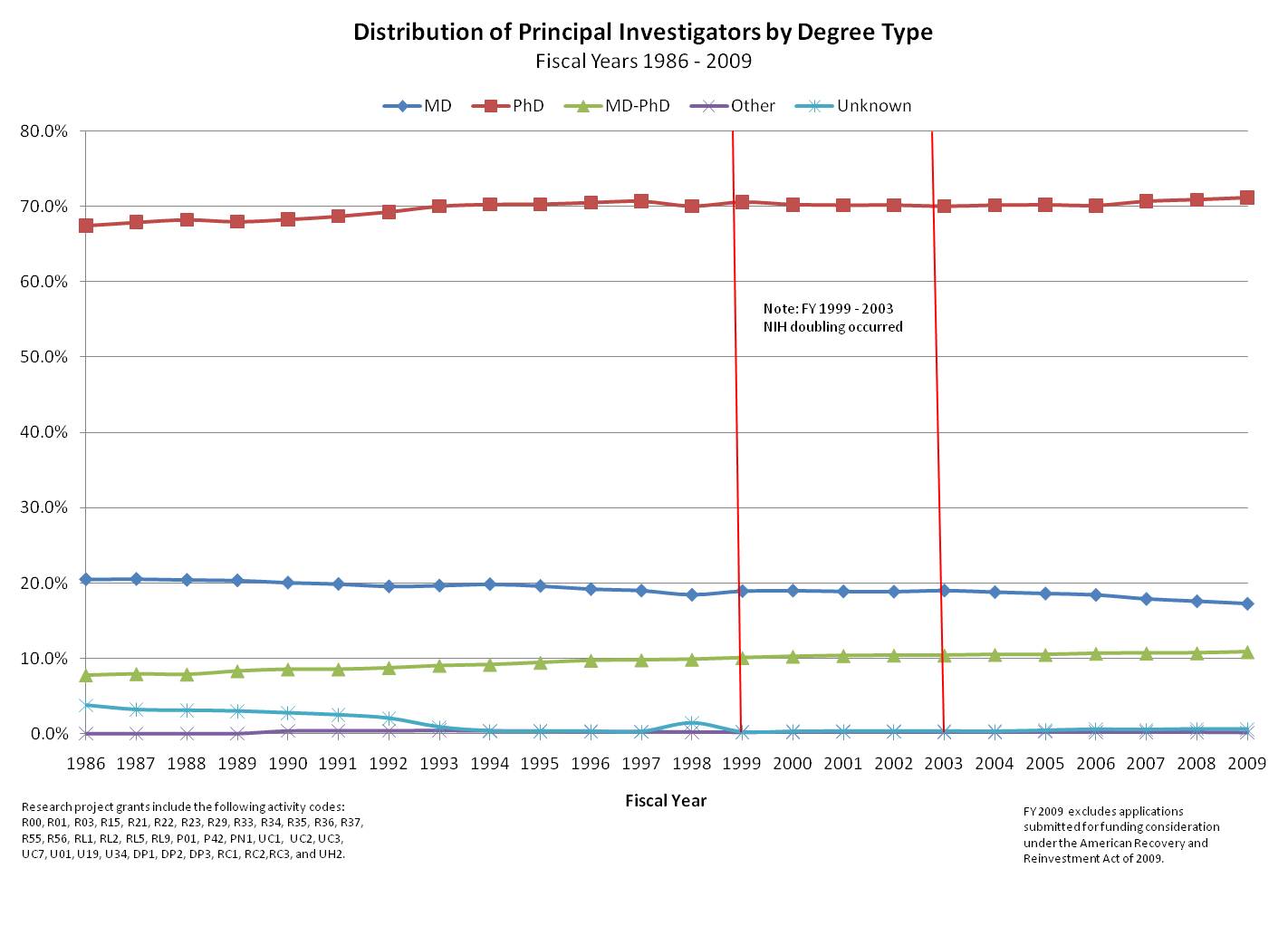 The total number of PIs has increased in this timeframe, and the range of NIH training and career development awards has evolved (see details of specific programs). Notably, we established several new career development awards for clinicians pursuing patient-oriented research in the late 1990s. It seems, however, that the system is remarkably stable. It will be interesting to see if these proportions remain stable over the course of the next few years as science continues to advance and translational research receives emphasis through the National Center for Translational Sciences (NCATS). Meanwhile, we’ll be taking a look at these data and others in our quest to model the future of the biomedical workforce. We’re going to be busy!
The total number of PIs has increased in this timeframe, and the range of NIH training and career development awards has evolved (see details of specific programs). Notably, we established several new career development awards for clinicians pursuing patient-oriented research in the late 1990s. It seems, however, that the system is remarkably stable. It will be interesting to see if these proportions remain stable over the course of the next few years as science continues to advance and translational research receives emphasis through the National Center for Translational Sciences (NCATS). Meanwhile, we’ll be taking a look at these data and others in our quest to model the future of the biomedical workforce. We’re going to be busy!
Clinical Center to Open Doors to Grantees?
 Last week at the NIH Advisory Committee to the Director meeting, John Gallin, head of the NIH Clinical Center, and I presented the early progress of a committee developed to examine how we can promote the Clinical Center as a national resource by extending its capabilities to you, the extramural community. The NIH Clinical Center is the nation’s largest hospital devoted entirely to clinical research. Currently used for intramural research by 18 of NIH’s institutes and centers, the Clinical Center has a wide variety of world-class or unique research services and resources that we think the extramural community will also find valuable. We are currently determining how we can provide both services and training and how to support collaborations between intramural and extramural investigators to take advantage of the broad range of resources the Clinical Center can provide.
Last week at the NIH Advisory Committee to the Director meeting, John Gallin, head of the NIH Clinical Center, and I presented the early progress of a committee developed to examine how we can promote the Clinical Center as a national resource by extending its capabilities to you, the extramural community. The NIH Clinical Center is the nation’s largest hospital devoted entirely to clinical research. Currently used for intramural research by 18 of NIH’s institutes and centers, the Clinical Center has a wide variety of world-class or unique research services and resources that we think the extramural community will also find valuable. We are currently determining how we can provide both services and training and how to support collaborations between intramural and extramural investigators to take advantage of the broad range of resources the Clinical Center can provide.
We started with the low hanging fruit and are drafting a program that would provide access to the pharmaceutical development facility for our grantees and contract recipients. We recognize there could well be other resources such as the metabolic unit and the PET program where collaborations with extramural investigators could be established. We are also looking at ways to use and possibly expand the current Bench-to-Bedside program to promote collaboration between extramural basic scientists and intramural clinical investigators, with the goal of understanding important diseases and translating basic science into new therapeutics.
We are excited to provide this opportunity to you. But we want to make sure that if we build it, you will come, so look for further opportunities to provide input on your interest in accessing the NIH Clinical Center resources. I’ll keep you posted on our progress.
Kicking Off the Biomedical Research Workgroup
As I mentioned previously, the Future of the Biomedical Research Workforce Working Group has been formed to advise the NIH Director on how to support a sustainable biomedical infrastructure. I’ve also shared some of the data we prepared for them with you in previous posts. The working group met for the first time last month, and we are excited to get started. We’ve posted the charge to the working group and the roster of its members on the Web.
A significant amount of our work will be focused on developing a model for a sustainable and diverse U.S. biomedical research workforce. To do this we have constituted a modeling subcommittee that will work specifically on the parameters of the model. The roster of the subcommittee is also posted on the Web.
Just to give you a taste of some of the previous work done in this area that our committee will be considering, take a look at the National Academies of Sciences study on “Research Training in the Biomedical, Behavioral and Clinical Sciences.”
I will keep you updated on the activities of the working group and look forward to getting your input as we progress.

