Rock Talk Archives for August 2011
Charting a Renewed Course for Managing Financial Conflicts of Interest
I’m very pleased to announce that the Department of Health and Human Services is issuing a final rule in the Federal Register revising the regulations on financial conflicts of interest of extramural investigators. The regulations, one of which applies to grants and cooperative agreements and the other to contracts, were first published in 1995, and with your input we have been working on revising them for the past several years.
The impetus for revising the regulations stemmed from many sources. We recognized that biomedical and behavioral research and the resulting interactions among government, research institutions, and the private sector have become increasing complex. While these interactions are often essential to the process of moving discoveries from the bench to the bedside, it can be challenging to manage the resulting financial relationships when federal funds are also involved so as to assure the public that they can trust in the objectivity of the research.
At the same time, there has been increased scrutiny of investigators’ financial relationships from Congress and the public, as well as the general principle of providing increased transparency and accountability in the use of all federal funds.
These factors highlighted the need to revisit the 1995 regulations. The process began with an Advance Notice of Proposed Rulemaking (ANPRM) in May 2009 asking the community whether the rule should be revised, and if so, how it might be strengthened. After considering these comments, we published a Notice of Proposed Rulemaking (NPRM) in May 2010 that proposed specific revisions to the regulations and again asked for your input.
The community provided numerous and very thoughtful comments. We considered them carefully when preparing the revised regulations. The same general framework of the 1995 regulation has been maintained:
- Institutions are responsible for implementing the regulations by their financial conflict of interest policy, evaluating their investigators’ significant financial interests, determining and managing financial conflicts of interest, and reporting financial conflicts of interest to the NIH.
- Investigators are responsible for complying with the institution’s policy, disclosing their significant financial interests, and complying with the institution’s management of any financial conflicts of interest.
- NIH’s role remains to provide guidance and oversight.
However, the revised regulations do institute a more rigorous approach to the management of investigator significant financial interests and resulting financial conflicts of interest to enhance the objectivity and integrity of the research process. These include changes to address investigator disclosure, institutional management of financial conflicts of interest, and federal oversight. In particular, the regulations:
- Require investigators to disclose to their institutions all of their significant financial interests related to their institutional responsibilities as opposed to only those that they see as related to Public Health Service (PHS)-supported research.
- Lower the monetary threshold for disclosure of significant financial interests, generally from $10,000 to $5,000.
- Require institutions to report to the PHS awarding component more comprehensively on identified financial conflicts of interest and how they are being managed.
- Require institutions to make certain information concerning identified financial conflicts of interest held by senior/key personnel accessible to the public.
- Require investigators to be trained on the regulations and their institution’s financial conflict of interest policy at designated times.
For more information on the major changes to the regulations, see the Financial Conflict of Interest website for a side-by-side comparison of the 1995 and 2011 regulations.
We recognize that it will require commitment and careful deliberation by both investigators and institutions to comply with these changes. Therefore, we have provided an implementation period of up to one year (see final rule for the exact language). Until your implementation is complete, you should continue to comply with the 1995 regulations. We are developing training materials for the community, including frequently asked questions and a tutorial. These will be posted on the Financial Conflict of Interest website in the coming weeks.
Looking back over the long road we’ve taken to get to this point, I am very grateful for the substantive and thoughtful comments you provided to both the ANPRM and the NPRM. They were invaluable in our efforts to draft regulations that provide greater transparency and accountability, are not overly burdensome, and that allow those so important relationships between NIH-funded researchers and industry to continue in a venue of integrity and objectivity.
Whew! And we’ll see you on the implementation side.
New NIH Study on Diversity
Let’s talk about the NIH sponsored study ![]() on review outcomes for R01 grant applications from different racial and ethnic groups and the accompanying commentary
on review outcomes for R01 grant applications from different racial and ethnic groups and the accompanying commentary ![]() that were published in Science this week. The paper, together with a previous NIH-commissioned study
that were published in Science this week. The paper, together with a previous NIH-commissioned study ![]() describes a biomedical research pipeline with few students and postdocs from underrepresented groups, coupled with disparities in review outcomes when they do apply for an NIH research grant. NIH finds these results troubling. As someone who has been involved in designing and implementing government peer review programs for over 25 years and one who believes strongly in peer review as the best way to identify good science, I find these results troubling as well. At this time we don’t understand fully the basis of the disparate review outcomes. What I do know, however, is that the NIH reviewers are outstanding individuals, who tirelessly devote their expertise, time and effort to making the biomedical research enterprise and NIH program the model for the world. We greatly value our reviewers and their contributions to our success at supporting high quality science that has huge impact on people’s everyday lives.
describes a biomedical research pipeline with few students and postdocs from underrepresented groups, coupled with disparities in review outcomes when they do apply for an NIH research grant. NIH finds these results troubling. As someone who has been involved in designing and implementing government peer review programs for over 25 years and one who believes strongly in peer review as the best way to identify good science, I find these results troubling as well. At this time we don’t understand fully the basis of the disparate review outcomes. What I do know, however, is that the NIH reviewers are outstanding individuals, who tirelessly devote their expertise, time and effort to making the biomedical research enterprise and NIH program the model for the world. We greatly value our reviewers and their contributions to our success at supporting high quality science that has huge impact on people’s everyday lives.
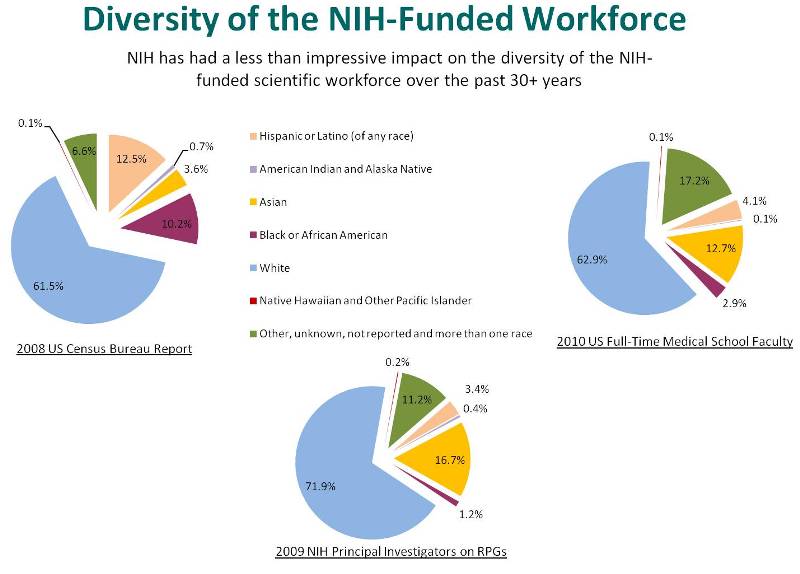
It is essential to include diverse groups in our efforts to improve the health of the nation. Diversity improves the ability to conduct research in all fields, but attracting the brightest minds who represent a broad range of interests is especially important for biomedical research. NIH has a long history of efforts towards achieving this goal, aiming to include, particularly in our training programs, underrepresented racial and ethnic groups, women, individuals with disabilities, and individuals from disadvantaged backgrounds. However, our efforts to establish a robust pipeline for a diverse workforce all the way through to principal investigators have been less than successful. Fewer than 2 percent of our PIs are Black, lower than the percentage of Black faculty at medical schools and much lower than the general population. The same holds true for Hispanics.
It is imperative that NIH rethinks its approach. As Drs. Tabak and Collins indicated in their commentary ![]() , we have launched new diversity-focused research programs, assembled new advisory groups, and are planning experiments to specifically identify issues that might be contributing to differential review outcomes.
, we have launched new diversity-focused research programs, assembled new advisory groups, and are planning experiments to specifically identify issues that might be contributing to differential review outcomes.
The NIH can’t do this alone, however. We need to work together with the biomedical research and academic communities, sharing our successes and learning from our failures. My hope is that there will be renewed efforts across these communities to diversify our workforce. Can we improve the way we support new faculty members as they apply for NIH funding? Can we become aware of unconscious attitudes around race and gender that may influence our judgment? How can we make careers in research more attractive to those from underrepresented groups? At what point in their lives are individuals making decisions about science as a career and are there interventions that we can do at these critical points to steer more underrepresented groups towards exciting research careers?
Because this issue is so central to the operation of NIH and the success of our programs, NIH is committed to moving in new directions and sharing findings with the community as they emerge. I invite you to work with us and to share your suggestions as we as a community work through these important issues.
Heads Up: Workforce Request for Information on the Way
As I’ve mentioned before, a working group of the Advisory Committee to the Director is examining the future of the research biomedical workforce (January 31 and June 3). It’s now time to ask for your input on this very important topic. On Wednesday, we will publish a request for information in the NIH Guide for Grants and Contracts outlining the issues being considered by the group and asking for your views on those and any other issues you think the group should consider. The RFI will be open for comment until October 7. I’d like to get input from as much of the biomedical research community as possible, so please let your colleagues know about the opportunity to comment. Check back here for a link and more information in the coming weeks.
Update 8/17/11 – View the RFI: http://grants.nih.gov/grants/guide/notice-files//NOT-OD-11-106.html
Weigh in on New Ideas for NIH Common Fund Programs!
What do the NIH Director’s Pioneer Awards, Early Independence Awards, Transformative R01 Awards, Clinical and Translational Science Awards, and the Human Microbiome Project all have in common? They are just a few of the programs supported by NIH’s Common Fund. The Common Fund supports exceptionally innovative programs that are trans-NIH and are expected to take advantage of emerging opportunities quickly to catalyze progress in biomedical research. Since the subjects of these programs usually don’t fit into a single NIH institute or center (IC), they foster collaboration between ICs in innovative and high impact areas of science.
We are asking for input from internal and external stakeholders on proposed concepts to help us shape new Common Fund programs. We want you to weigh in on ideas for 2013 that have the potential to fundamentally change how we think about, support, or do research in a specific field, or even that create a new field all together. Take a look at the current Common Fund programs to see from where we have come and start thinking about where we should go.
I am always happy to hear your thoughts on the blog, of course, but the best way to participate in the brainstorming for the Common Fund is to provide your input by Wednesday, September 14 to http://commonfund.nih.gov/strategicplanning. I look forward to your ideas.
Get Out From Under Educational Debt
 We are welcoming our first Rock Talk guest blogger this week, Dr. Milton Hernandez. Milton is director of NIH’s Loan Repayment Programs.
We are welcoming our first Rock Talk guest blogger this week, Dr. Milton Hernandez. Milton is director of NIH’s Loan Repayment Programs.
Last week, The New York Times published in a blog “The Hidden Costs of Medical Student Debt ![]() .” In this, Dr. Pauline W. Chen highlights that young doctors who graduated from medical school in 2010 had an average debt of $158,000 and notes that:
.” In this, Dr. Pauline W. Chen highlights that young doctors who graduated from medical school in 2010 had an average debt of $158,000 and notes that:
“for some young people, looming debts mean eschewing a calling to serve a particularly needy, less lucrative patient population or practice, and instead pursuing a well-compensated subspecialty that caters to the comfortably insured.”
This large debt is also a barrier for young doctors and scientists who want to pursue research careers, and it has been a hot topic in the scientific community for many years. We need to keep talented young scientists in research careers if we are to address our nation’s current and future health needs. In 2001, Congress established the NIH extramural Loan Repayment Programs (LRPs). These programs repay up to $35,000 of student loan debt annually for investigators who commit to at least two years of biomedical or behavioral research funded by a domestic nonprofit, university or government entity.
Each year, NIH Institutes and Centers fund about 1,600 LRP contracts for investigators who perform clinical, pediatric, health disparities, infertility or contraception research. The programs are open to M.D.s as well as Ph.D.s and will help repay graduate and undergraduate debts. After the initial award, recipients may apply for one or two-year competitive renewals as long as they have sufficient educational debt remaining and meet the other eligibility criteria. The success rate for new applicants is 42 percent, and the success rate for renewals is 70 percent.
As of 2011, nearly 8,000 researchers have benefited from LRPs, and an evaluation conducted in 2009 showed that program participants stay in research careers longer, apply for and receive more research grants, and are more likely to become independent investigators than peers who do not receive LRP funding. Our success stories illustrate that many of our alumni are running their own research labs, and some, like New York State Health Commissioner Dr. Nirav R. Shah, represent the next generation of public health leaders.
If you are a young researcher who has educational debt, I encourage you to apply for these programs. The next application cycle opens September 1, and the deadline is at 8 p.m. ET on November 15. As you prepare your application, be sure to check out my webinar for tips and advice. To learn more and/or apply, go to http://www.lrp.nih.gov.