Rock Talk Archives for September 2012
Celebrating Science
Earlier this month, I had the opportunity to participate in A Celebration of Science, where more than 1,000 leaders from across the scientific research and policy communities participated in a three-day event led by FasterCures and the Milken Institute. This celebration was held to reaffirm the importance of biomedical research and showcase the outcomes of research on people’s lives. The event took many forms, ranging from panels and presentations to a special concert event at the Kennedy Center.
The event came to NIH for a full day, where speakers and attendees convened at our Natcher Conference Center, not only to show the positive results of biomedical research on people’s lives, but also to engage in frank discussions of how to move forward.
These discussions included a topic my office is closely familiar with: supporting science in times of constrained budgets. It was promising to see Congressional representatives from both sides of the aisle—Representative Eric Cantor, the House majority leader, and Representative Steny Hoyer, the House minority whip—share the stage and acknowledge NIH’s vital role in supporting biomedical research.
But I was especially moved by the presentations from the individuals and families that have benefited from biomedical research progress. Dawn Averitt Bridge, an HIV-positive woman that later developed AIDS, spoke about how healthcare advances not only allowed her to survive, but also thrive in life, and become the mother of two non-HIV-infected children. Retta and Joe Beery, joined by their three children, described how whole genome sequencing changed their family’s life when it pinpointed the movement disorder affecting two of their children since birth—a treatable form of dystonia. It was extraordinarily meaningful to have patients share their personal stories and demonstrate the true value of NIH research—its ability to improve lives.
The day was inspiring and makes me proud to work at NIH and especially proud of the research you do as part of our incredible mission: to improve the health of the nation and the world. I encourage you to watch the highlight video below, or the full NIH Day morning session and evening session online. I hope it inspires you too.
What’s Next?—Reviewing Your Summary Statement and Thinking About Resubmitting
So you’re wearing your lucky shoes and are ready to take a first look at the results of your grant review. Whether you are anticipating doing a victory dance or getting ready to head out to the nearest kickboxing class, it’s a good time to think about what comes next.
Some of you have noticed that the summary statements now include a link to a new online resource to address just this question. Especially if you are new to NIH funding, I encourage you to check out this “Next Steps” page, which was put together to help NIH grant applicants with the “What’s next?” questions following receipt of the summary statement.
If you aren’t in the position to be preparing Just-in-Time information for an award, but instead are considering resubmission, you may want to consider some of the data that have appeared in my previous blog posts, in addition to the resources available on grants.nih.gov. For example, in the post “Correlation Between Overall Impact Scores and Criterion Scores”, I show how approach, innovation, and significance factor heavily into the overall impact scores. As you look at your summary statement, talk to your NIH program official, and discuss your ideas with colleagues, it might be useful to keep this in mind.
Additionally our podcast series, All About Grants, includes conversations with NIH staff to help you understand how your grant is reviewed, such as these two episodes on summary statement basics and resubmission advice.
Whether you’re new to the grant application process or an experienced applicant, we hope you find these resources useful.
Partnering with FDA to Support Research to Inform Tobacco Regulation
I am excited to tell you about a collaboration we have launched with the FDA to support research to inform the development and evaluation of regulations on the manufacturing, distribution, and marketing of tobacco products. FDA was granted authority to regulate tobacco products by the Family Smoking Prevention and Tobacco Control Act (Tobacco Control Act), which was signed into law in 2009.
FDA has identified 56 research priorities among 7 research areas related to tobacco use and public health. In collaboration with the FDA Center for Tobacco Products (CTP), which oversees implementation of the Tobacco Control Act, we have recently announced several funding opportunities to support scientific research that will inform CTP in carrying out its regulatory authorities. These include a research center program (P50) and ongoing support of a variety of research project grants (R01, R03, and R21). The awards made under these funding announcements will be administered by NIH using designated funds from the FDA CTP for tobacco regulatory science.
This collaboration is a great benefit for both agencies. Several NIH institutes and centers have long supported tobacco-related research. In fiscal year 2011, approximately $362 million was awarded by NIH to support research projects related to tobacco use.
We’re looking forward to helping the FDA CTP in achieving their goals—preventing tobacco use, especially among youth, encouraging current users trying to quit, and decreasing the harms from tobacco product use. We hope researchers are also looking forward to contributing their expertise to this interdisciplinary effort.
For more information about this collaboration, as well as links to research resources and contact information for further questions, check out the “Tobacco Control Regulatory Science: Understanding the Family Smoking Prevention and Tobacco Control Act” website at http://www.cancercontrol.cancer.gov/nih-fda/.
NIH RePORT up for a People’s Choice Award from HHSinnovates—Voting Is Now Open!
 I’m excited to let you know that NIH RePORT (Research Portfolio Online Reporting Tools) is a finalist for the HHSinnovates program, and for the first time ever the public will have the opportunity to vote on RePORT and the other finalists. We have often heard from you about the value that RePORT provides to the biomedical community, so now is the time to show your appreciation through your vote.
I’m excited to let you know that NIH RePORT (Research Portfolio Online Reporting Tools) is a finalist for the HHSinnovates program, and for the first time ever the public will have the opportunity to vote on RePORT and the other finalists. We have often heard from you about the value that RePORT provides to the biomedical community, so now is the time to show your appreciation through your vote.
HHSinnovates is a program run by the Department of Health and Human Services (HHS) to highlight innovations in programs and processes developed by HHS staff. HHS Secretary Kathleen Sebelius launched the program in 2010, but this year marks the first “People’s Choice Award” where the public can vote and rate the HHS projects they deem most innovative.
Out of the initial 60 projects submitted in this round, RePORT is one of six finalists. I’m especially proud of the advances RePORT has made – and continues to make – in increasing transparency and accessibility to information on the NIH research portfolio.
As I’ve discussed in previous blog posts, RePORT’s innovative interface gives you access to comprehensive information about NIH funding, and the website continues to grow. One of the newest features links RePORTER with EurekAlert!, the international science news service operated by the American Association for the Advancement of Science (AAAS). When looking up an NIH award in RePORTER, you can view the news releases published in EurekAlert! that describe research supported by that particular award.
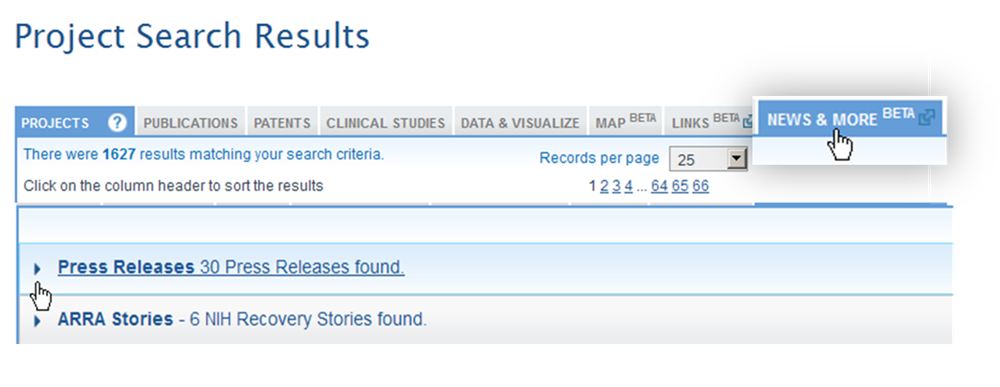
To demonstrate this tool, let’s build upon an example from my most recent blog post on RePORT, the search for active projects related to obesity. You’ll see in the screenshot below that the project search results page contains a “News & More” tab. At the time of writing this post, the tab shows that there are 30 press releases related to projects in the search results, along with 6 stories associated with projects funded through the Recovery Act. (The “News & More” feature is new. We expect the number of releases to increase quickly!)
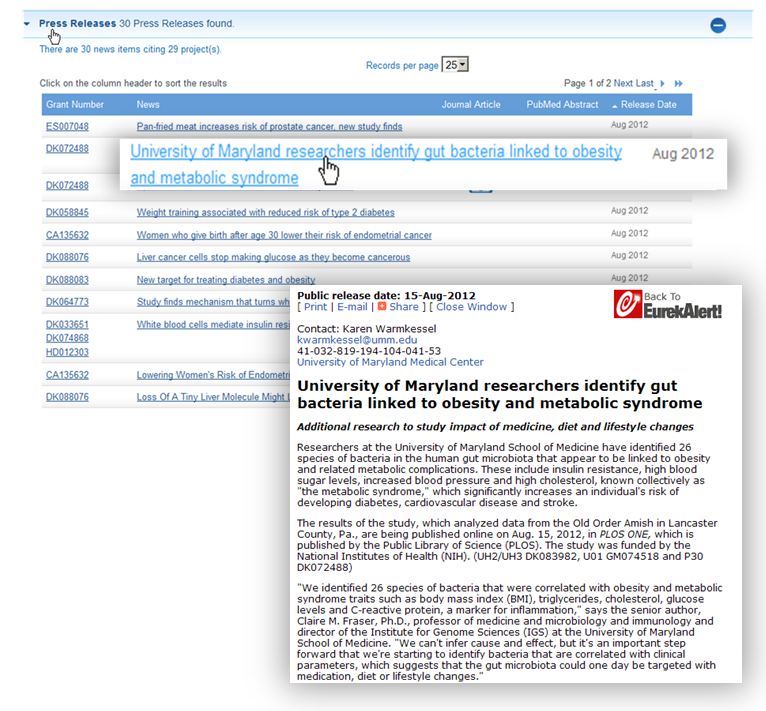
Clicking on the press release link will expand the list of 30, and you can sort by date to find the most recent news, for example, this August 15th release from the University of Maryland Medical Center.
So how does RePORTER identify these press releases? NIH notices of grant award require acknowledgement of federal funding in press releases and research manuscripts. When press offices of the NIH grantee institutions use EurekAlert! to publish their releases, they now have the ability to tag their press releases with relevant NIH grant numbers, similar to how PIs include their NIH grant numbers in the acknowledgements of published research manuscripts. If an institution does not use EurekaAlert! they can work with the communications office of the funding NIH institute or center to get the release linked to the grant record in RePORTER.
(Note: At the current time RePORTER does not link press releases to specific subprojects of multi-project grants. As a result, RePORTER search results containing subprojects will retrieve all news associated with the parent grant award and all of its other subprojects, including those in diverse areas of research.)
Congratulations to all the finalists, and I hope you will consider voting for NIH RePORT!