New Ultrasound Technique Developed for Diagnosing and Tracking Liver Fibrosis and
Tumors
Researchers at Duke University have developed a new ultrasound imaging technique
that non-invasively detects tumors and fibrosis in the liver, thus avoiding the
pain and complications associated with biopsy. These researchers are also extending
the technique to aid in diagnosing other diseases, as well.
NIBIB grantees Kathy Nightingale, Ph.D., the James L. Vincent Associate Professor
of Biomedical Engineering at Duke, and Gregg Trahey, PhD, Professor of Biomedical
Engineering at Duke and their co-investigators, developed the technique, called
Acoustic Radiation Force Impulse imaging (ARFI). The technique has been licensed
and adopted for use in new Siemens ultrasound imaging systems in Europe, and clinical
studies there and in Asia are showing results consistent with findings from Dr.
Nightingale’s lab. While this tool is not yet available in the U.S., Siemens is
currently pursuing FDA approval to market it in the U.S.
Background
As with most diseases, the ability to detect liver fibrosis in its early stages
has a significant impact on whether or not a patient survives. The current gold
standard for diagnosing liver fibrosis is biopsy —a procedure that is painful,
expensive, can cause complications, and cannot be performed frequently enough to
effectively track the progression of the disease. When the liver has been damaged
-- whether by excessive alcohol intake, hepatitis, nonalcoholic fatty liver disease,
or from some inherited conditions — healthy liver tissue begins to transform
into stiff scars that disrupt the activity of the liver, a condition known as cirrhosis.
Chronic liver disease and cirrhosis affect more than 5.5 million people in the United
States, causing over 31,000 deaths due to liver failure in 20101.
In addition, non-alcoholic fatty liver disease (NAFLD), occurs when there is excessive
accumulation of fat in the liver, and currently afflicts about 80 million Americans.
This condition can progress to cirrhosis and, in its extreme stage to liver cancer.
What’s New
ARFI is an ultrasound technique, and so, does not produce ionizing radiation (unlike
X-rays, CT, and PET scans), and is relatively inexpensive compared to other imaging
modalities. This means it can be used more frequently to track the progression of
fibrosis. In addition, it can image the entire liver, as opposed to biopsy, which
can only examine a small sample of the liver. While the quality of an ultrasound
image can often be operator-dependent, this test only requires that the operator
locate the liver in an image display and then keep the detector in place; this makes
it relatively easy to get a clear image of the liver and possible fibrotic tissue
in a short amount of time.
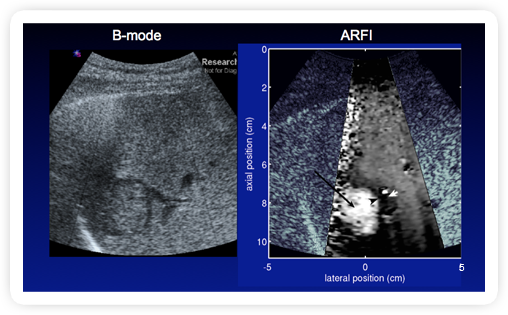
|
Fig 1. ARFI: In Vivo - Liver - Hepatocellular Carcinoma2
- Patient with liver cirrhosis, hepatitis C, HIV+
- HCC appears softer than surrounding cirrhotic liver tissue
Source: Duke Biomedical Engineering (Click on image to enlarge)
|
The ARFI technique uses focused, high intensity sound beams to produce “push-pulses”
that generate shear waves (secondary waves that extend in a direction perpendicular
to the direction of the push pulse) within tissue and then monitors the tissue response
with ultrasonic methods. The tissue response is related to the stiffness properties
and structure of the liver, and is displayed as high resolution, qualitative elastographic
images of the liver (Figure 1). The speed of the shear waves is proportional to
the stiffness of tissue; thus ARFI can also produce quantitative stiffness measurements
based on the speed of the shear waves. These measurements are used to quantify specific
levels of fibrosis that can be used to classify different stages of liver fibrosis
(Fig. 2).
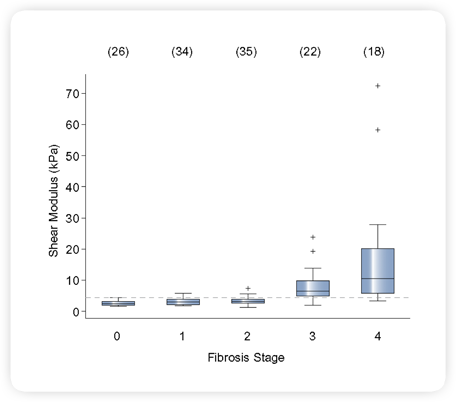
|
Fig 2. ARFI Study: NASH Patient Population Noninvasive Hepatic Fibrosis Staging3
Shear modulus (μ) distinguished low (F0-F2) from high (F3-F4) fibrosis
stages using a threshold of 4.2 kPa, with a sensitivity and specificity of 90%,
and AUC of 0.9.
Source: Duke Biomedical Engineering (Click on image to enlarge)
|
For example, a type of liver cancer, called hepatocellular carcinoma (HCC), can
appear softer than surrounding cirrhotic liver tissue. The ARFI technique displays
an ultrasound image which highlights the softer tissue, so it appears as a mass
with a lighter shade of gray than surrounding tissue (Figure 1). On the other hand,
another type of liver cancer, called metastatic melanoma, is characterized as a
stiffer tissue, and appears as a darker mass than the surrounding non-fibrotic liver
tissue (Fig. 3).
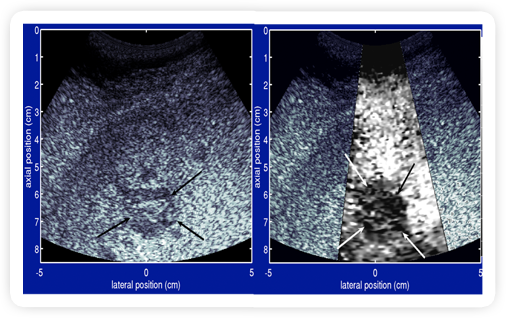
|
Fig 3. ARFI: In Vivo liver (metastatic melanoma)2
- Metastatic mass appears stiffer (darker) than surrounding liver tissue
- Patient with metastatic melanoma in liver, no known fibrosis
Source: Duke Biomedical Engineering (Click on image to enlarge)
|
An important part of developing this imaging technique was creating an algorithm
that allows detection of ARFI data with minimal background noise. Dr. Nightingale’s
lab designed an algorithm that creates precise images. Since shear wave speed increases
with the severity of the stage of fibrosis, this technique now makes it possible
to differentiate late stages of fibrosis from earlier stages. If fibrosis is indicated,
a biopsy will still most likely be required to confirm the finding, but this technique
can be used as an initial screening device to eliminate unnecessary biopsies.
Existing Technologies
The use of ultrasound and shear waves to detect fibrosis is not entirely new. The
FibroscanTM method is an existing technique that also uses ultrasound, plus an external
“punch” from a piston pressure source that directs both shear waves
and sound pressure waves at the liver. However, it does not produce anatomic or
elastographic images, but only numerical data which is used to detect fibrotic tissue.
Since this method does not display the precise location of specific regions or structures
within the liver that are fibrotic, it can confuse existing blood vessels or other
healthy structures with actual liver tissue. In addition, the use of the external
“punch” piston in this technique does not work in patients with fluid
around the liver (ascites).

|
|
Fig 4. Liver MRE
Image courtesy of Dr. Richard Ehman, Mayo Clinic (Click on
image to enlarge)
|
Another method for identifying liver fibrosis, which is already FDA-approved for
clinical use, is MR elastography. Like the ARFI method, this technique also uses
an external acoustic pressure pulse directed at the abdomen, which produces shear
waves within the tissue (i.e., the liver). This method also computes the relative
stiffness of tissue components, based on how they respond to the pressure wave,
and displays these relative stiffness values in a color-coded image, or MR elastogram
(Figure 4). Compared with the ultrasound ARFI technique, MR elastography has the
advantages of higher resolution (i.e., more detailed images) and larger area coverage.
However, the disadvantages include much higher costs, lack of availability in many
clinical settings (e.g. emergency rooms, rural areas, third world countries), and
lack of portability, since MRI machines are completely stationary.
What’s Next
In the future, those who are suspected of having fibrosis may first be screened
with this exam, and only after a positive indication of stiffness go through the
painful and sometimes dangerous biopsy procedure to confirm diagnosis. ARFI could
then monitor the progression of the disease on a more regular basis than is currently
available. In addition, Drs. Nightingale and Trahey have identified other diseases
such as breast, thyroid, and prostate cancer, in addition to cardiovascular diseases
that are associated with elevated stiffness and shear wave speed, thus increasing
the potential clinical uses of ARFI.
-- Jessica Meade
1
http://www.cdc.gov/nchs/data/nvsr/nvsr60/nvsr60_04.pdf
2 Fahey et al. “In vivo visualization of abdominal
malignancies with acoustic radiation force elastography”, Phys. Med. Biol.
53 (2008) 279–293.
3 Palmeri, M., et al, “Evaluating Liver in NAFLD
Patients Using Ultrasonic Acoustic Radiation Force-Based Shear Stiffness Quantification”,
Journal of Hepatology, 55(3):666-672, 2011.
 More Highlight
on Health articles
More Highlight
on Health articles
Last Updated On 12/10/2012