What are pharmacogenomics and pharmacogenetics?

Your genes determine a lot about how you look. They also play a key role in how your body responds to medicines.
The terms pharmacogenomics and pharmacogenetics are often used interchangeably to describe a field of research focused on how genes affect individual responses to medicines. Whether a medicine works well for you—or whether it causes serious side effects—depends, to a certain extent, on your genes.
Just as genes contribute to whether you will be tall or short, black-haired or blond, your genes also determine how you will respond to medicines. Genes are like recipes—they carry instructions for making protein molecules. As medicines travel through your body, they interact with thousands of proteins. Small differences in the composition or quantities of these molecules can affect how medicines do their jobs.
These differences can be due to diet, level of activity, or the medicines a person takes, but they can also be due to differences in genes. By understanding the genetic basis of drug responses, scientists hope to enable doctors to prescribe the drugs and doses best suited for each individual.
Aren't prescribed medicines already safe and effective?
While standard doses of most medicines work well for most people, some medicines don't work at all in certain people or cause annoying and sometimes dangerous side effects. For example, codeine is useless as a painkiller in nearly 10 percent of people, and an anticancer drug, 6-mercaptopurine, is extremely toxic in a small fraction of the population.
How do scientists gather pharmacogenomic information?
 Many pharmacogenomic findings are based on knowledge of biochemical pathways within cells. For example, scientists already knew a lot about the enzymes that break down the anticancer drug irinotecan when its toxic effects in certain patients came to light. This knowledge allowed researchers to rapidly pinpoint a genetic variant of one of these enzymes as the cause of the dangerous reaction. Scientists have developed a genetic test for this variant so that doctors can adjust the dosage for those at risk for serious side effects.
Many pharmacogenomic findings are based on knowledge of biochemical pathways within cells. For example, scientists already knew a lot about the enzymes that break down the anticancer drug irinotecan when its toxic effects in certain patients came to light. This knowledge allowed researchers to rapidly pinpoint a genetic variant of one of these enzymes as the cause of the dangerous reaction. Scientists have developed a genetic test for this variant so that doctors can adjust the dosage for those at risk for serious side effects.
Advances can also come from studies that accompany clinical drug trials. After obtaining permission from participants, some pharmaceutical companies collect DNA samples from people in clinical trials. Scientists then analyze the samples together with results of the clinical trial to identify genetic variations that correlate with a drug's effectiveness or toxicity.
Pharmacogenomic researchers have already identified many genes whose variations affect drug responses. They also know where to look for the numerous others they are bound to discover in the future. The availability of the human genome sequence, which was completed in 2003, led to the HapMap project, an international effort to catalog common genetic differences among human beings. These resources are providing a treasure trove of genetic information that is expected to speed advances in pharmacogenetics.
In what ways can doctors use pharmacogenetics to help them treat their patients?
For many diseases, there are a variety of treatment options. Pharmacogenomics can help doctors pick the right one for each patient.
Pharmacogenetics can be used by doctors to identify the optimal dose and/or medicine for each patient.
The right dose
Dosage is usually based on factors such as age, weight, and liver and kidney function. But for someone who breaks down a drug quickly, a typical dose may be ineffective. In contrast, someone who breaks down a drug more slowly may need a lower dose to avoid accumulating toxic levels of the drug in the bloodstream. A pharmacogenetic test can help reveal the right dose for individual patients.
The right drug—for cancer
Pharmacogenetics is used in targeted therapy for cancer to identify the best drug regimen for a particular tumor. Even tumors of the same type (such as lung, breast, or liver) vary at the genetic level. Cancer is fundamentally a genetic disease, but most of the genetic differences between cancer cells and normal cells are not inherited—they accumulate as the cancer develops. Analyzing specific genes in a patient's tumor helps doctors identify the drug combination to which the tumor will most likely respond. For example, the breast cancer drug Herceptin® is only effective when the tumor cells have accumulated extra copies of the HER2 gene and have high levels of the protein this gene encodes on their surfaces.
The right drug—for HIV
For patients with a bacterial or viral infection, analyzing the genes of the infectious agent can reveal the most suitable drug treatment. For example, the Food and Drug Administration (FDA) has approved a genotyping kit that detects genetic variations in HIV that make the virus resistant to some antiretroviral drugs. If drug resistance is discovered, doctors can prescribe other medications.
The right drug—for depression
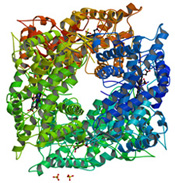
This computer-generated image shows the molecular skeleton of a liver enzyme called Cytochrome P450 2D6, which helps people process a wide range of medicines. Because the enzyme comes in several varieties, it is of great interest to pharmacogenomic researchers.
Depression can be treated with a variety of different medicines, and it is often time-consuming and difficult to find the drug(s) that works best for each person. In the future, genetic testing may take some of the guesswork out of choosing a drug regimen. These tests are likely to involve analyzing a person's liver enzymes, especially those in the cytochrome P450 family, which are largely responsible for processing antidepressants.
Other tests that may prove useful to psychiatrists will detect differences in the molecules targeted by antidepressants, such as the serotonin transporters targeted by a large class of antidepressants called selective serotonin reuptake inhibitors (SSRIs). Scientists have uncovered evidence for a link between a person's response to SSRIs and variations in serotonin transporters and other biological molecules that act on serotonin.
The right drug—for cardiovascular disease
Statins, the most widely prescribed drugs worldwide, help prevent cardiovascular disease by reducing the level of “bad” cholesterol in the bloodstream. While statins work well for many patients, responses are highly variable and doctors must adjust the dosage for each person.
Researchers have discovered that variants in a number of molecules—including those that break down or transport statins, as well as the statins' molecular target in the cholesterol production pathway—contribute to the variable response among individuals. Using results of genetic tests, doctors may one day be able to prescribe the right dose from the start and more quickly reduce their patients' risk of dangerous cardiovascular events such as heart attack and stroke.
Does the FDA require that doctors test patients for genetic differences before prescribing any drugs currently on the market?
Identifying small genetic variations between people is key to pharmacogenetics.
The labels of more than 20 medications now mention the availability of tests for genetic variations that impact the drug's action. However, in many cases, such as the anticancer drugs azathioprine and irinotecan, which can build up to toxic levels in a small fraction of people, testing is optional.
How do I get a pharmacogenetic test?
Ask your doctor, who can order a test from a medical laboratory. Some major institutions, such as the Mayo Clinic, the Indiana University School of Medicine, and St. Jude Children's Hospital, also offer pharmacogenetic testing. If you take a test, a technician will draw a sample of your blood or rub a cotton swab along the inside of your cheek to collect cells. The lab will extract genetic material from the sample and carry out the test. These tests typically cost a few hundred dollars and may be covered by your health insurance company.
Cancer biopsy samples are also often subjected to genetic tests. The results can help guide therapy and predict the likelihood of recurrence. Several such tests have been approved by the FDA.
Are the results of pharmacogenetic tests confidential?
While pharmacogenetic tests are designed to help people, some fear that the results could be used against them, such as to discriminate against them in a job setting or to deny them health insurance coverage. A person's genetic information is protected through the Health Insurance Portability and Accountability Act (HIPAA), which was passed by Congress in 1996. Many states also have laws in place that protect the privacy of health information, including genetic data.
How will pharmacogenetics affect the design, development, and availability of new medicines?
Pharmacogenetic research can lead to safer, more effective medicines and better health care.
Pharmacogenomic knowledge will enable pharmaceutical companies to design, develop and market drugs for people with specific genetic profiles. Testing a drug only in those likely to benefit from it could streamline its development and maximize its therapeutic benefit.
The FDA, which monitors the safety of all drugs in the United States, considers pharmacogenomics to be a valuable tool in the development of new medical products. To date, the FDA has approved a number of genotyping kits relevant to pharmacogenomics, including one that screens for variants in the cytochrome P450 enzymes, which process many kinds of drugs. In most cases the FDA encourages, but does not require, companies to submit pharmacogenomic data with new drug applications. This data is only required for medicines that were developed based on pharmacogenomics.
How will pharmacogenomics affect the quality of health care?
 In the future, pharmacogenomics will increasingly enable doctors to prescribe the right dose of the right medicine the first time for everyone. This would mean that patients will receive medicines that are safer and more effective, leading to better health care overall.
In the future, pharmacogenomics will increasingly enable doctors to prescribe the right dose of the right medicine the first time for everyone. This would mean that patients will receive medicines that are safer and more effective, leading to better health care overall.
Also, if scientists could identify the genetic basis for certain toxic side effects, drugs could be prescribed only to those who are not genetically at risk for these effects. This could maintain the availability of potentially lifesaving medications that might otherwise be taken off the market.
What are some of the challenges that face pharmacogenetics?
While pharmacogenetics is expected to be a useful tool to find the best dose of the right medicine for each patient, doctors are unlikely to be able to rely on it alone. Other factors will remain important, and may sometimes overshadow pharmacogenetics. These other factors include characteristics of the disease itself as well as the patient's diet, weight, lifestyle, and other medicines he or she is taking.
As with many new medical advances, it will take time before pharmacogenetics enters the mainstream and becomes a standard tool for making treatment decisions. Overcoming this barrier may be particularly tricky for pharmacogenetics because most medicines work well for most people and adverse reactions are rare.
Another challenge facing pharmacogenetics is the number and complexity of interactions a drug has with biological molecules in the body. Variations in many different molecules may influence how someone responds to a medicine. Teasing out the genetic patterns associated with particular drug responses could involve some intricate and time-consuming scientific detective work.
While routine pharmacogenetic testing could ultimately save our health care system billions of dollars by improving drug effectiveness and safety, the savings could be offset by the additional cost of genetic tests.
What is the role of the National Institutes of Health (NIH)?
In April 2000, NIH launched the Pharmacogenetics Research Network (PGRN), a nationwide collaboration of hundreds of scientists focused on understanding how genes affect the way a person responds to medicines. Since its inception, PGRN scientists have studied genes and medications relevant to a wide range of diseases, including asthma, depression, cancer, and heart disease. A key component of the PGRN is the Pharmacogenetics Knowledge Base, an online resource that contains pharmacogenetic data from the PGRN and others, and is freely available to the research community.

The NIH Pharmacogenomics Research Network has made many advances in understanding the way genes affect individual responses to medicines, including those for heart disease, asthma, depression, cancer, and many other diseases. For more information about this nationwide research team, go to
http://www.nigms.nih.gov/Research/FeaturedPrograms/PGRN/The PGRN was launched with grants totaling $140 million over 5 years. An additional $150 million is allocated for the period of 2005-2009. The PGRN is funded largely through the National Institute of General Medical Sciences and the National Heart, Lung, and Blood Institute, with additional support from the National Cancer Institute, the National Library of Medicine, the National Institute of Environmental Health Sciences, The National Institute on Drug Abuse, the National Institute of Mental Health, the National Human Genome Research Institute, and the Office of Research on Women's Health.
NIH takes seriously the ethical and legal implications of pharmacogenetics research and is working closely with several task forces and associations to maximize the benefits of this research and to prevent any potential harm to individuals or society.
More Information
Educational booklet on personalized medicines, Medicines for You
Educational booklet on genetic research in identified populations, Genes and Populations
Pharmacogenomics Research Network
National Center for Biotechnology Information, A Science Primer: The Promise of Pharmacogenomics
Department of Health and Human Services, HIPAA information page 
National Human Genome Research Institute, Human Genome Information page 
International HapMap 
U.S. Food and Drug Administration, How FDA Advances Personalized Medicine 
Contact
If you would like more information on pharmacogenomic research supported by the National Institute of General Medical Sciences, please contact Alisa Machalek at 301-496-7301 or alisa.machalek@nih.gov.
About NIGMS
NIGMS supports basic biomedical research that is the foundation for advances in the diagnosis, treatment, and prevention of disease. NIGMS is part of the National Institutes of Health, U.S. Department of Health and Human Services. To learn more about NIGMS, visit http://www.nigms.nih.gov.