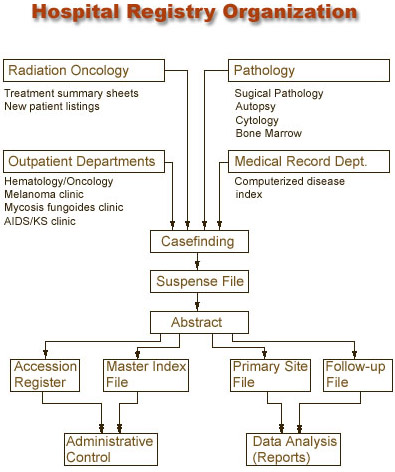
Casefinding
Casefinding is a system for locating every patient-inpatient or outpatient- who is diagnosed and/or treated with a reportable diagnosis. Casefinding is like casting a net far and wide to "capture" all of the reportable cancer cases.
In the casefinding process, a tracking system is kept so that the status of casefinding can be ascertained at any time. A printout of cases already entered is also kept in the registry; potential new cases can be checked against the list and eliminated if they have already been identified.
All registries must perform casefinding, including hospital-specific and central or population-based registries. Although these registries may use different source documents, the procedures involved in casefinding cycles are similar.
Most government agencies only require malignant (ICD-O behavior codes 2 and 3) cases to be included in the registry. However, hospital cancer committees or even some central registries may require the registry to include benign or borderline/uncertain cases. Examples include benign brain tumors or carcinoid tumors of the appendix.
The cancer committee must decide the data set and policy as to whether patient follow-up is done for the reportable cases and also to establish the reportable list. The reportable list should be posted in the registry's policies and procedures. The casefinding cycle would be the same for these cases as for the cases required by the government agencies.
The criteria for eligible cases in a registry depend upon the governing agencies of the registry. Along with state-specific reportable cases, registries participating in the Approvals Program of the Commission on Cancer (COC) of the American College of Surgeons (ACoS) must use the reportable list defined by the COC.
Casefinding is an important part of the cancer registry. A system to monitor prospective cases must be in place in different areas of an institution. The completeness of casefinding must be monitored for quality control purposes.