Re-engineering Translational Sciences
For patients diagnosed with deadly or debilitating diseases, and for their families, the announcement of a new treatment offers hope for a better future. However, developing new diagnostics and therapeutics is a lengthy, complex, costly and risk-laden endeavor spanning a continuum of research activities, including:
- Initial identification of the intervention idea.
- Product prototype development.
- Testing for safety, accuracy and efficacy in model systems.
- Several types of clinical trials.
- Approval by the U.S. Food and Drug Administration.
- Integration of the new treatment into clinical care.
- Continued monitoring of efficacy and any side effects associated with long-term use.
The upstream component of the developmental pipeline (basic research — the study of the fundamental mechanisms of disease and behavior) is fueled by dramatic technological advances and new insights into disease mechanisms, largely with the support of NIH and other funding agencies. The downstream end of the pipeline (clinical trials in preparation for FDA submission) is primarily the domain of the biopharmaceutical industry due to its expertise in assessing promising interventions.
Preclinical translational research is the bridge between basic research and human medicine. Preclinical translation relies on a wide variety of activities, including experiments conducted with cell and animal models; samples of human or animal tissues; and computer-assisted modeling of drug, device or diagnostic interactions with living systems.
Due to the multidimensional complexity, limited understanding of the underlying science, and resulting high failure and cost in this middle zone, it is sometimes called the Valley of Death, and most product ideas never emerge from it. Many of the processes in this zone have been performed in much the same way for a decade or more, with little innovation or systematic study of the reasons for success or failure.
NIH recognizes that the process for translating scientific discoveries into new tools and treatments is thus ripe for innovation.
The Time Is Right
The mission of NCATS is to catalyze the generation of innovative methods and technologies that will enhance the development, testing and implementation of diagnostics and therapeutics across a wide range of human diseases and conditions. By improving the process by which diagnostics and therapeutics are developed, NCATS aims to make translational science more efficient, less expensive and less risky. In this way, NCATS complements — and does not compete with — the work of the private sector and the other NIH Institutes and Centers.
NCATS has solicited ideas from stakeholders about aspects of translational science that are ripe for the scientifically rigorous approach the Center offers. Among these ideas:
- Therapeutic target validation
- Virtual drug design
- Preclinical toxicology
- Tissue chip and other novel models of safety and efficacy
- Drug rescue and repurposing
- Adaptive clinical trial design
The most promising programs will be funded through NIH's grant- and contract-awarding process.
Concentrating Translational Capabilities
To succeed in its objectives as a catalytic hub for translational science, NCATS will assemble a wide range of preclinical and clinical capabilities from within NIH and reshape these components into an integrated scientific enterprise.
Approximately 60 U.S. academic institutions have received NIH Clinical and Translational Science Awards (CTSAs), collectively representing one of NCATS's most valuable assets. This consortium of CTSA centers offers expertise in pre-clinical science, clinical trials, comparative effectiveness research, training and community engagement.
To further efforts to re-engineer translational science, NCATS is now home to the NIH Chemical Genomics Center (NCGC) and the Toxicology in the 21st Century (Tox21) initiative.
The average length of time from target discovery to approval of a new drug currently averages about 13 years, the failure rate exceeds 95 percent, and the cost per successful drug exceeds $1 billion, after adjusting for all of the failures.
— Francis S. Collins, M.D., Ph.D., NIH Director
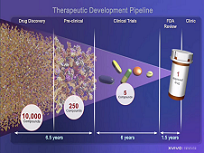
Therapeutic Development Process
Social Media Links