FY 2009 Congressional Budget Justification
On this page:
- Organization Chart
- Budget Authority by Institute and Center
- Budget Authority by Mechanism
- Budget Authority by Program
- Justification of Budget Request
- Director’s Overview
- Program Narratives
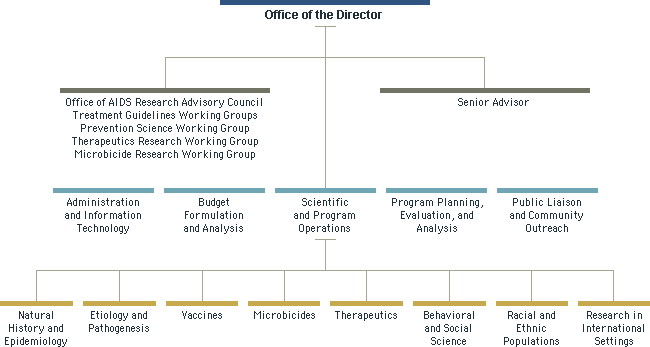
Organization Chart
Budget Authority by Institute and Center
| Institute/Center | FY 2007 Actual | FY 2008 Enacted | FY 2009 Estimate | Change |
|---|---|---|---|---|
| NCI | $253,666,000 | $254,499,000 | $254,499,000 | — |
| NHLBI | 67,360,000 | 65,360,000 | 65,360,000 | — |
| NIDCR | 19,688,000 | 19,741,000 | 19,741,000 | — |
| NIDDK | 30,898,000 | 31,031,000 | 31,031,000 | — |
| NINDS | 46,351,000 | 46,451,000 | 46,451,000 | — |
| NIAID | 1,491,341,000 | 1,494,222,000 | 1,494,222,000 | — |
| NIGMS | 53,485,000 | 53,628,000 | 53,628,000 | — |
| NICHD | 133,555,000 | 137,358,000 | 137,358,000 | — |
| NEI | 10,585,000 | 10,585,000 | 10,585,000 | — |
| NIEHS | 7,513,000 | 5,310,000 | 5,310,000 | — |
| NIA | 5,392,000 | 5,392,000 | 5,392,000 | — |
| NIAMS | 4,866,000 | 4,866,000 | 4,866,000 | — |
| NIDCD | 1,412,000 | 900,000 | 900,000 | — |
| NIMH | 178,590,000 | 179,153,000 | 179,153,000 | — |
| NIDA | 300,162,000 | 301,532,000 | 301,532,000 | — |
| NIAAA | 26,942,000 | 27,017,000 | 27,017,000 | — |
| NINR | 12,114,000 | 12,145,000 | 12,145,000 | — |
| NHGRI | 6,835,000 | 6,855,000 | 6,855,000 | — |
| NIBIB | 1,038,000 | 1,096,000 | 1,096,000 | — |
| NCRR | 160,992,000 | 161,525,000 | 161,525,000 | — |
| NCCAM | 2,285,000 | 2,385,000 | 2,385,000 | — |
| NCMHD | — | — | — | — |
| FIC | 22,983,000 | 23,138,000 | 23,138,000 | — |
| NLM | 7,376,000 | 7,399,000 | 7,399,000 | — |
| OD | 60,359,000 | 61,757,000 | 61,757,000 | — |
| B&F | — | — | — | — |
| TOTAL, NIH | 2,883,277,000 | 2,878,424,000 | 2,877,912,000 | -512,000 |
Budget Authority by Mechanism
(Dollars in thousands)
| Mechanism | FY 2007 Actual | FY 2008 Enacted | FY 2009 Estimate | |||
|---|---|---|---|---|---|---|
| No. | Amount | No. | Amount | No. | Amount | |
| Research Projects | ||||||
| Noncompeting | 1,950 | $1,157,109 | 1,912 | $1,207,757 | 1,861 | $1,156,757 |
| Administrative supplements | (141) | 53,744 | (104) | 43,565 | (130) | 54,696 |
| Competing | 647 | 350,803 | 762 | 321,086 | 869 | 355,324 |
| Subtotal, competing | 647 | 350,803 | 762 | 321,086 | 869 | 355,324 |
| Subtotal, RPGs | 2,597 | 1,561,656 | 2,674 | 1,572,408 | 2,730 | 1,566,777 |
| SBIR/STTR | 69 | 31,220 | 66 | 31,201 | 64 | 31,201 |
| Subtotal, RPGs | 2,666 | 1,592,876 | 2,740 | 1,603,609 | 2,794 | 1,597,978 |
| Research Centers | ||||||
| Specialized/comprehensive | 54 | 122,458 | 55 | 124,140 | 55 | 124,484 |
| Clinical research | 5 | 44,215 | 9 | 52,366 | 9 | 52,563 |
| Biotechnology | 1 | 3,450 | 3 | 3,958 | 3 | 3,958 |
| Comparative medicine | 16 | 57,982 | 15 | 60,144 | 15 | 60,144 |
| Research Centers in Minority Institutions | 3 | 10,213 | 3 | 11,830 | 3 | 11,830 |
| Subtotal, Centers | 79 | 238,318 | 85 | 252,438 | 85 | 252,979 |
| Other Research | ||||||
| Research careers | 276 | 38,784 | 275 | 38,870 | 275 | 38,870 |
| Cancer education | 0 | 15 | 0 | 15 | 0 | 15 |
| Cooperative clinical research | 13 | 28,016 | 13 | 25,650 | 13 | 25,650 |
| Biomedical research support | 1 | 1,961 | 0 | 1,462 | 0 | 1,462 |
| Minority biomedical research support | 1 | 311 | 1 | 311 | 1 | 311 |
| Other | 134 | 62,371 | 122 | 63,483 | 122 | 62,573 |
| Subtotal, Other Research | 425 | 131,458 | 411 | 129,791 | 411 | 128,881 |
| Total Research Grants | 3,170 | 1,962,652 | 3,236 | 1,985,838 | 3,290 | 1,979,838 |
| Ruth L. Kirschstein Training Awards: | FTTPs | FTTPs | FTTPs | |||
| Individual awards | 83 | 3,290 | 82 | 3,294 | 82 | 3,318 |
| Institutional awards | 738 | 32,519 | 725 | 32,884 | 725 | 33,259 |
| Total, Training | 821 | 35,809 | 807 | 36,178 | 807 | 36,577 |
| Research & development contracts | 226 | 457,634 | 222 | 443,089 | 222 | 445,671 |
| (SBIR/STTR) | (4) | (190) | (4) | (186) | (4) | (186) |
| Intramural research | 292,545 | 288,034 | 289,671 | |||
| Research management and support | 96,789 | 98,449 | 99,831 | |||
| Construction | 0 | 0 | 0 | |||
| Office of the Director | 60,359 | 61,757 | 61,757 | |||
| Total Budget Authority | 2,905,788 | 2,913,345 | 2,913,345 | |||
Budget Authority by Program
(Dollars in thousands)
| Area of Emphasis1 | FY 2005 Actual | FY 2006 Actual | FY 2007 Actual | FY 2008 Enacted | FY 2009 Estimate | Change |
|---|---|---|---|---|---|---|
| HIV Microbicides | — | $85,693 | $96,413 | $103,397 | $107,290 | $3,893 |
| Vaccines | $508,974 | 581,450 | 582,403 | 592,634 | 597,955 | 5,321 |
| Behavioral and Social Science | 418,106 | 406,217 | 420,395 | 417,954 | 425,684 | 7,730 |
| Therapeutics | 732,159 | 635,434 | 656,264 | 643,340 | 629,574 | -13,766 |
| Etiology and Pathogenesis | 741,662 | 716,239 | 692,816 | 701,907 | 702,025 | 118 |
| Natural History and Epidemiology | 297,070 | 269,835 | 239,396 | 235,002 | 233,293 | -1,709 |
| Training, Infrastructure, and Capacity Building | 168,645 | 160,686 | 192,065 | 192,778 | 190,377 | -2,401 |
| Information Dissemination | 42,765 | 27,723 | 26,036 | 26,333 | 27,147 | 814 |
| Subtotal | 2,909,381 | 2,883,277 | 2,905,788 | 2,913,345 | 2,913,345 | 0 |
| Roadmap | 11,130 | 18,582 | — | — | — | — |
| Total | $2,920,511 | $2,901,859 | $2,905,788 | $2,913,345 | $2,913,345 | $0 |
1 Beginning in FY 2008, HIV Microbicides will be a separate activity. Dollars for HIV Microbicides were previously included within other science areas, such as Therapeutics, Etiology and Pathogenesis, Behavioral and Social Science, and Vaccines. The FY 2006 and FY 2007 amounts are comparable budget figures.
Trans-NIH AIDS Research Budget Justification
| FY 2007 Actual | FY 2008 Estimate | FY 2009 Estimate | Change | |
|---|---|---|---|---|
| Budget Authority | $2,905,788,000 | $2,913,345,000 | $2,913,345,000 | — |
Director’s Overview
Mission
The Office of AIDS Research (OAR) coordinates the scientific, budgetary, legislative, and policy elements of all National Institutes of Health (NIH) AIDS research. OAR, located within the NIH Office of the Director, is authorized to plan, coordinate, and evaluate all AIDS research conducted or supported by NIH. OAR develops an annual strategic plan and budget for all NIH AIDS activities.
The HIV/AIDS Pandemic
Globally1
- 33.2 million people estimated to be currently living with HIV/AIDS infection.
- More than 25 million men, women and children have already died.
- In 2007, an estimated 2.5 million new HIV infections occurred worldwide.
- In 2007, 2.1 million people died from AIDS.
AIDS in the United States2
- More than 1 million people are living with HIV/AIDS
- In 2005, an estimated 40,000 people were newly infected with HIV, and 16,300 died from AIDS
- Since 1981, 953,000 people have been diagnosed with AIDS, of whom 530,000 have died
- Racial and ethnic populations are disproportionately affected by HIV/AIDS.
1UNAIDS. AIDS Epidemic Update, 2007,
2CDC. HIV/AIDS Surveillance Report, 2005. Vol. 1717. Rev. ed.
The OAR has used these authorities to establish unique trans-NIH planning, budgeting, and portfolio assessment processes. These processes promote collaboration, minimize duplication, and ensure that NIH AIDS research dollars are invested in high priority research that ultimately will lead to the development of new tools for use in the global fight against AIDS.
NIH AIDS Research Impacts Global Health
NIH has established the largest and most significant AIDS research program in the world: a comprehensive and global trans-NIH research effort. NIH supports and conducts a comprehensive program of basic, clinical, and behavioral research on HIV infection and its associated coinfections, opportunistic infections, malignancies, and other complications. No other disease so thoroughly transcends every area of clinical medicine and basic scientific investigation, crossing the boundaries of the NIH Institutes and Centers (ICs).
NIH-funded research has lead to the discovery of antiretroviral therapies and regimens that have resulted in improved quality of life and life expectancy for those with access to these drugs. In addition, NIH research has led to the development of treatments for some HIV-associated co-infections and co-morbidities, including malignancies, neurological complications, tuberculosis, and other clinical manifestations. NIH research also has led to a number of advances in HIV prevention, including strategies for the prevention of mother-to-child transmission and the demonstration that medically supervised circumcision of adult men can reduce risk of heterosexual HIV acquisition. Despite these important advances, the epidemic continues to expand, and improved prevention strategies and therapeutic regimens are critically necessary.
AIDS Research Conducted in International Settings
(Dollars in millions)
| FY 2007 Actual | FY 2008 Enacted | FY 2009 Estimate |
|---|---|---|
| $362 | $364 | $366 |
Annual Trans-NIH AIDS Research Strategic Planning and Budget Formulation Efforts
OAR manages and coordinates the multifaceted and complex NIH AIDS research agenda. Each year, the OAR develops the Trans-NIH Plan for HIV-Related Research to ensure the AIDS budget is used to fund the highest priority AIDS-related research. The Plan shapes the NIH investment in biomedical and behavioral AIDS-related research and provides the framework to translate critical research findings into improved prevention and treatment strategies. It is developed in collaboration with scientists from NIH, other government agencies, and non-governmental organizations, as well as community representatives. During the planning process, the state of the science is reviewed, newly emerged and critical public health needs assessed, and scientific opportunities identified. The annual process culminates with the identification of the top strategic priorities and critical research needs. The fiscal year (FY) 2009 Trans-NIH Plan for HIV-Related Research can be accessed at http://www.oar.nih.gov.
The trans-NIH AIDS research budget is developed by OAR in partnership with the ICs and is explicitly tied to the objectives of the strategic Plan. Each year, OAR reviews IC budget requests in relation to the scientific priorities and objectives articulated in the Plan, and to other IC submissions. This review reduces redundancy, promotes harmonization, and assures cross-Institute collaboration.
NIH AIDS Research Priorities for FY 2009
The FY 2009 Trans-NIH Plan for HIV-Related Research identifies two critical priorities that transcend all areas of AIDS research and shape the development of the budget:
- Prevention of acquisition and transmission of HIV: Prevention of HIV infection is NIH’s highest priority for HIV-related research. The NIH prevention research agenda includes basic, translational, and clinical research on microbicides and vaccines development; and behavioral and social sciences associated with HIV transmission and acquisition. There is an urgent need to expand the range of interventions for preventing HIV transmission beyond those currently available as new HIV infections continue at an unacceptably high rate globally, including in the United States. Studies of novel prevention strategies, such as circumcision to prevent heterosexual HIV acquisition in men, pre-exposure uses of antiretroviral therapy to prevent HIV infection, and strategies that can be used in resource-limited settings must be continued.
- Prevention and treatment of HIV-associated co-morbidities, co-mortalities, and co-infections: Recent epidemiologic studies and clinical reports show an increased incidence of HIV-associated co-morbidities, co-mortalities, and co-infections, including malignancies, neurological complications, tuberculosis, and other clinical manifestations, associated with long-term HIV disease and prolonged antiretroviral therapy. Research that will lead to a better understanding of these HIV-associated conditions is a high priority for the NIH, including research on how antiretroviral drugs may cause these manifestations and complications and the complex pathogenesis associated with HIV co-infections. In addition, translational and clinical studies are needed to transform fundamental research results into improved strategies for preventing and treating these HIV-associated co-morbidities, co-mortalities, and co-infections.
Program Narratives
Microbicides
HIV Microbicides: Microbicides, defined as antimicrobial products that can be applied topically for the prevention of HIV and other sexually transmitted infections, may offer one of the most promising primary preventive interventions. NIH supports a comprehensive microbicide research program that includes the screening, discovery, development, preclinical testing, and clinical evaluation of microbicide candidates, as well as fundamental research aimed at understanding how HIV transverses mucosal membranes and infects cells. In addition, NIH supports behavioral and social science research on the acceptability and use of microbicides among different populations.
NIH has initiated a series of administrative steps to increase the level of awareness and focus on microbicide research, including: the establishment of a Microbicide Research Working Group, comprised of non-government experts who will play a unique and critical role in guiding the formulation of the NIH microbicide agenda; establishment of a new microbicide research branch at NIAID; and the Microbicide Innovation Program, designed to accelerate the discovery and development of single and/or combination microbicides.
Budget Policy: The FY 2009 budget request for this activity is $107,290,000 which represents an increase of $3,893,000 over the FY 2008 Enacted level. HIV microbicide research is a top priority for NIH. In FY 2009, NIH will increase funding for the design, development, and evaluation of microbicide candidates. Key ongoing activities include support of the recently established microbicide clinical trials network and the necessary infrastructure to conduct microbicide trials, especially in developing countries; the development of criteria for selecting potential products to be evaluated in clinical trials and for advancing them through the different phases of clinical studies; and research on ethical and behavioral issues impacting these clinical trials. A number of working groups, conferences, workshops, and symposia also will be supported to foster innovative microbicide research that will lead to the development of products that prevent HIV transmission and acquisition. The increased funding for microbicide research will result from the realignment of funds that would have provided continued support for research in other program areas, particularly therapeutics.
Vaccines
Vaccines: The best long-term hope for controlling the AIDS pandemic is the development of safe, effective, and affordable HIV vaccines. NIH supports a broad HIV vaccine research portfolio encompassing basic, preclinical, and clinical research. Moreover, NIH supports research to identify and better understand protective immune responses; and information gleaned from these studies is being used to inform the design and development of novel vaccine strategies.
In FY 2007, NIH supported a number of Phase I, II, and IIb clinical trials. Two large studies conducted by NIH in partnership with Merck & Co., Inc. were halted by the Data and Safety Monitoring Board after interim analyses of data because the vaccine candidate did not prevent HIV infection. Although disappointing, these results underscore the need for additional research to better understand the basic host immune responses to HIV infection. An important basic research study at the NIH Dale and Betty Bumpers Vaccine Research Center determined the long-sought picture of the precise interaction of the HIV surface protein gp120 as it looks when bound to an infection-fighting antibody, a finding that could have profound implications for HIV vaccine design.
Budget Policy: The FY 2009 budget request for this activity is $597,955,000, which is an increase of $5,321,000 above the FY 2008 Enacted level. Prevention of HIV acquisition remains the top priority for NIH research; and a HIV vaccine offers the best hope of stemming the pandemic. The disappointing results from the clinical studies of the Merck vaccine indicate a critical need to invest in basic research studies on the virus and host immune responses that can inform the development of new and innovative vaccine concepts; as well as the development of improved animal models to conduct pre-clinical evaluations of vaccine candidates. In FY 2009, NIH will fund additional basic research on HIV and host responses, as well as the design and development of new vaccine concepts and the pre-clinical/clinical development of vaccine candidates in the pipeline; however, funding for clinical research will be decreased. Specifically, the amount requested for vaccine research in FY 2009 includes $407,064,000 for basic research on HIV and host responses and for the design, development, and pre-clinical evaluation of vaccines, which is an increase of $17,222,000 above the FY 2008 Enacted level. The budget also includes $190,891,000 for clinical research, which is a decrease of $11,901,000 below the FY 2008 Enacted level. Funds that would have provided continued support for research in other areas will be redirected to support these changing priorities in HIV vaccine research.
Behavioral and Social Science
Behavioral and Social Science: NIH supports research to further our understanding of how to change the behaviors that lead to HIV acquisition, transmission, and disease progression—including preventing their initiation—and how to maintain protective behaviors once they are adopted. In addition, NIH supports research aimed at better understanding the social and cultural factors associated with HIV risk or protection, particularly in communities at high risk of HIV acquisition. This research will contribute to the implementation of a broader range of preventive and/or therapeutic strategies.
A behavioral research study recently demonstrated that an evidence-based prevention intervention program on practices to reduce exposure to sexually transmitted diseases (STDs), including HIV, improved the protective behaviors of inner-city African-American women for up to one year and actually decreased their risk of acquiring an STD. This addresses a significant area of health disparity. HIV prevention requires a combination of strategies, and these research results and those of other behavioral prevention intervention studies will be important components of future prevention efforts.
Budget Policy: The FY 2009 budget request for this activity is $425,684,000, which is an increase of $7,730,000 over the FY 2008 Enacted level. NIH will continue to fund research to develop and evaluate effective interventions to prevent HIV transmission and acquisition by reducing HIV-related risk behaviors and increasing protective behaviors. The amount requested for FY 2009 includes an increase of $5,157,000 for research on HIV prevention interventions. This increase will be funded through the re-alignment of funding that would have provided a continued level of support for research grants and contracts in lower-priority program areas.
Therapeutics
Therapeutics: Antiretroviral treatment has resulted in improved immune function in patients who are able to adhere to the treatment regimens and tolerate the toxicities associated with antiretroviral drugs; and it has delayed the progression of HIV disease, extending the time between initial infection and the development of AIDS. However, epidemiologic studies have revealed a number of co-infections and co-morbidities associated with long-term HIV disease, including tuberculosis, hepatitis C, malignancies, metabolic disorders, cardiovascular disease, and neurologic disorders. A better understanding of the underlying etiology of these HIV-associated conditions will lead to better prevention and treatment strategies. NIH supports a comprehensive therapeutics research program to design, develop, and test drugs and drug regimens to prevent and treat HIV infection and its associated co-infections and co-morbidities.
Results from one of the largest HIV/AIDS treatment trials ever conducted, known as the Strategies for Management of Anti-Retroviral Therapies (SMART) study, showed that a specific treatment strategy that includes interrupting antiretroviral therapy more than doubles the risk of progression to AIDS or death from any cause. This collaborative study provided important new data that will help physicians and their patients make critical treatment decisions. Another important international trial, the Children with HIV Early Antiretroviral Therapy (CHER) study, demonstrated that HIV-infected infants treated with antiviral therapy before three months of age will survive longer than infants whose treatment is delayed. These findings also highlight the importance of early HIV diagnosis and may lead to changes in standards of care in many parts of the world.
Budget Policy: The FY 2009 budget request for this activity is $629,574,000, which represents a decrease of $13,766,000 below the FY 2008 Enacted level. While improved therapeutic regimens for the treatment of AIDS and AIDS-associated co-infections and co-morbidities are urgently needed, especially regimens that can be deployed in resource-limited settings, funding for therapeutics research will be decreased in FY 2009 in order to increase funding for HIV prevention research, which was determined through the OAR FY 2009 trans-NIH strategic planning process to be NIH’s top priority for HIV-related research. Because the budget request for FY 2009 is the same as the FY 2008 Enacted level, a realignment of funding between research program areas is necessary in order to fund top priority HIV-related research. Therefore in FY 2009, funds that would have provided a continued level of support for research in this area will be re-allocated to support HIV prevention research, which includes the microbicide, vaccine, and behavioral and social science research programs.
Etiology and Pathogenesis
Etiology and Pathogenesis: NIH supports a comprehensive portfolio of research focused on gaining a better understanding of how HIV infection is established and maintained and what causes the associated profound immune deficiency and severe clinical complications. Research on basic HIV biology and AIDS pathogenesis has revolutionized the design of drugs, methodologies for diagnosis, and monitoring of the safety and effectiveness of antiviral therapies. Although ground-breaking strides have been made towards understanding the fundamental steps in the life-cycle of HIV, the host-virus interactions, and the clinical manifestations associated with HIV infection and AIDS; additional research is needed to further the understanding of the virus and how it causes disease, including studies to delineate how gender, age, ethnicity, and race influence vulnerability to infection and HIV-disease progression.
NIH-supported genomics studies recently identified genetic factors influencing the rate of viral suppression and the pace of HIV disease progression. This research could lead to improved HIV therapies and provide new targets for vaccine and microbicide development.
Budget Policy: The FY 2009 budget request for this activity is $702,025,000, which is an increase of $188,000 over the FY 2008 Enacted level. The results from recent microbicide and vaccine clinical studies have revealed gaps in knowledge and understanding of HIV etiology and pathogenesis, particularly with regards to host immune responses and how HIV interacts with and transverses mucosal surfaces. The amount requested for FY 2009 includes $68,379,000 for research on the biology of HIV transmission; this amount represents an increase of $7,108,000 over the amount of the FY 2008 Enacted level allocated for research in this area. Funding from lower priority research within this area, primarily research on pathogenesis of opportunistic infections, will be redirected within this program area in order to support higher priority etiology and pathogenesis research.
Natural History and Epidemiology
Natural History and Epidemiology: Natural history and epidemiologic research is needed to monitor epidemic trends, develop and evaluate prevention modalities, follow the changing clinical manifestations of HIV disease in different populations, and measure the effects of treatment regimens. NIH supports research in domestic and international settings to examine HIV transmission, HIV/AIDS disease progression (including the occurrence of coinfections and opportunistic infections, malignancies, metabolic complications, neurological and behavioral dysfunctions), the development of other HIV/AIDS-related conditions, and improved methodologies to support this research. Epidemiologic research is instrumental in identifying and describing AIDS-related co-morbidities, disentangling effects related to treatment from those related to HIV disease itself.
NIH researchers in two clinical trials demonstrated that heterosexual HIV acquisition was reduced by 50 percent in adult males who had been medically circumcised. These findings are of significant public health importance for HIV prevention. Ultimately, increased adult male circumcision could lead to fewer infections in women in those areas of the world where HIV is spread primarily through heterosexual intercourse.
Budget Policy: The FY 2009 budget request for this activity is $233,293,000, which represents a decrease of $1,709,000 from the FY 2008 Enacted level. NIH will continue to support high-priority epidemiology studies of groups and populations affected by HIV, as well as studies to investigate the mechanisms of disease progression, the impact of therapy in changing the spectrum of HIV disease, and the causes of death. Funds that would have provided continued support for research in this program area will be redirected to fund higher priority prevention research in other program areas.
Training, Infrastructure, and Capacity Building
Training, Infrastructure, and Capacity Building: NIH supports the training of domestic and international biomedical and behavioral AIDS researchers, as well as the equipment for the conduct of AIDS-related research and clinical studies. The expansion of NIH-funded HIV research globally has necessitated the development of research infrastructure in many locations, including resource-limited settings in Africa, the Caribbean, India, and Asia. Numerous NIH funded programs have increased the number of training positions for AIDS-related research, including programs specifically designed to recruit individuals from underrepresented populations into research careers and to build research infrastructure at minority-serving institutions. Additionally, the NIH Loan Repayment Program has attracted health professionals to NIH to engage in AIDS-related research.
NIH is working to improve international research and training to better address the challenges of AIDS in resource-constrained nations. One example is a trans-NIH initiative involving both intramural and extramural scientists to establish partnerships with scientists at Indian research institutions, particularly partnerships focusing on HIV prevention research.
Budget Policy: The FY 2009 budget request for this activity is $190,377,000, which represents a decrease of $2,401,000 below the FY 2008 Enacted level. Funds from expiring grant and contract commitments in this program area will be reallocated to support priorities in HIV prevention research. NIH will continue to support ongoing commitments for efforts to increase the supply of non-human primates, particularly rhesus macaques, for AIDS research and other areas of biomedical research both in the United States and abroad. NIH also will support training programs for U.S. and international researchers to build the critical capacity to conduct AIDS research both in minority communities in the United States and in developing countries. The amount requested for this activity for FY 2009 includes $77,297,000 for research training, an increase of $2,625,000 over the FY 2008 Enacted level; and $113,080,000 for infrastructure development, a decrease of $5,026,000 from the FY 2008 Enacted level.
Information Dissemination
Information Dissemination: Effective information dissemination approaches are integral to HIV prevention and treatment efforts and critical in light of the continuing advent of new and complex antiretroviral treatment regimens, issues related to adherence to prescribed treatments, and the need to translate behavioral and social prevention approaches into practice. The changing pandemic and the increasing number of HIV infections in specific population groups, such as racial and ethnic populations and women, also underscore the need to disseminate HIV research findings and other related information to communities at risk. The flow of information among researchers, health care providers, and the affected communities represents new opportunities to rapidly translate research results into practice and to shape future research directions.
NIH continues to support AIDSinfo (www.aidsinfo.nih.gov), a comprehensive resource for state-of-the science federal treatment and prevention guidelines. Guidelines are updated and posted on a continual basis. In 2007, the guidelines were downloaded more than 3.3 million times. AIDSInfo also provides information about participation in HIV therapeutics, vaccine, and microbicide clinical trials. In 2007, the website received over 5.4 million page views. In FY 2007, AIDSInfo launched a companion site, infoSIDA, to provide Spanish-language information about HIV/AIDS clinical trials and treatment information.
Budget Policy: The FY 2009 budget request for this activity is $27,147,000, which represents an increase of $814,000 above the FY 2008 Enacted level. As the number and complexity of clinical studies increases, additional resources must be invested in clinical trials-related information dissemination to ensure recruitment of an adequate number of participants. In addition, antiretroviral treatment guidelines as well as guidelines for the management of HIV complications for adults and children will all be updated and disseminated through the AIDSInfo website. In FY 2009, funds reallocated from other programs will be used to increase support for information dissemination associated with prevention research studies.