For Consumers
The Evolution, and Revolution, of Flu Vaccines
 Get Consumer Updates by E-mail
Get Consumer Updates by E-mail
|
|
 Share this article (PDF 207 K)
Share this article (PDF 207 K)
The flu vaccine that you get at your doctor's office or pharmacy is the work of highly skilled microbiologists, epidemiologists, physicians and other public health experts too numerous to mention. It also likely required a hen and a rooster monitored by a veterinarian inside a henhouse that met biosecurity requirements.
Sound complicated? It is. The manufacturing of flu vaccines is a highly-orchestrated and complex process.
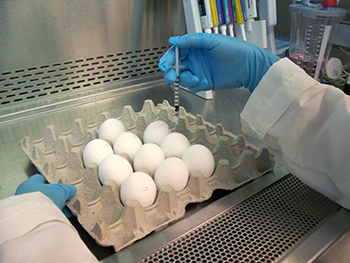
Vaccine manufacturers each year use millions of fertilized eggs as a culture to grow influenza viruses that, after numerous steps requiring about six months of expert work, become that season's flu vaccine. And while this tried-and-true method will continue to provide safe and effective vaccines for the foreseeable future, flu vaccines using new, more advanced technologies are arriving on the scene. Some don't require eggs at all.
The Food and Drug Administration (FDA) and its parent, the U.S. Department of Health and Human Services, have long encouraged the development of new technologies for producing flu vaccines. For example, cell culture technology is used to make vaccines to prevent other infectious diseases, and FDA has been working for a number of years, both on the research and regulatory fronts, to facilitate this for flu vaccines. A major push for cell-based flu vaccines occurred in 2006 as part of a plan to be ready in case of a world-wide epidemic.
In 2010, FDA issued final guidance to assist manufacturers working to develop safe and effective viral vaccines that grow in specially prepared cell lines. In the last two months, FDA has approved two new flu vaccines that, instead of using eggs to grow the influenza virus, use cell lines from either a mammal or insects.
"This is an important advance that will supplement current egg-based vaccines," said Jerry P. Weir, Ph.D., director of the Division of Viral Products in FDA's Center for Biologics Evaluation and Research. "The more manufacturing alternatives there are available, the better we can respond to public health emergencies in a timely manner."
Although egg-based production remains vital, cell technology has some advantages. Unlike eggs, cells can be frozen for later use to grow large volumes of cells. That advantage offers the potential for faster start-up of the vaccine manufacturing process for any unexpected need. This would also be critical if egg supplies were compromised. In addition, some flu virus strains don't grow that well in eggs at first and may grow better and faster in cells, helping speed vaccine production and availability. Moreover, alternatives to egg-based products provide an option for people with egg allergies.
The Making of the Vaccine
The job of producing a new vaccine for the next flu season starts well before the current flu season ends. For FDA, it's a year-round initiative.
The composition of vaccines for other diseases can stay the same year after year. But because influenza viruses are constantly evolving, and the viruses that circulate among people often change from one year to another, new flu vaccine needs to be made every year. To that end, scientists around the world collect samples to identify which flu strains are most likely to be circulating in the next flu season.
In February—well before a new flu season begins—FDA recommends the different strains of influenza viruses that should be included in vaccines that are going to be produced for the upcoming U.S. flu season starting in the fall.
Both traditional and new manufacturing methods for FDA-approved vaccines require high-tech processes and manufacturing facilities that have been inspected by FDA. Typically, seed viruses are produced and then distributed to manufacturers.
In egg based manufacturing, each virus strain is injected into eggs. The eggs are incubated for several days to allow the virus to multiply. The virus-loaded fluid from the egg is then harvested and undergoes purification. Manufacturers and the FDA test for potency and safety, using a type of test developed and calibrated by FDA.
Eventually, the strains are mixed together to formulate the vaccine into standard dosages. The product is then put into containers such as vials, syringes and, for the nasal vaccine, sprayers.
Each vaccine undergoes quality control tests, including testing for sterility. Manufacturers submit the results of their testing, along with sample vials from each lot to FDA for "lot release." FDA releases lots and the manufacturers begin shipping vaccine throughout the United States for use by the public.
Influenza vaccine works mainly by triggering the immune system to produce antibodies that help the body to fight off the infection.
New Vaccines Approved
The first flu vaccine to use cell-based rather than egg-based technologies, Flucelvax, was approved by FDA in November 2012 for use in people ages 18 and older. The manufacturing process for Flucelvax is similar to the egg-based production method, but the virus strains included in the vaccine are grown in cells of mammalian origin instead of eggs.
A second flu vaccine that is manufactured using another new technology was approved on January 16, 2013 for use in people 18 through 49 years of age. Called Flublok, it does not require the use of actual influenza viruses and eggs are not used at any point in the manufacturing process.
Flublok uses an influenza virus protein that is made by genetically modifying a virus that infects insect cells to produce the flu vaccine protein. The protein, as in other flu vaccines, then triggers the immune system of the person receiving the vaccine to make protective antibodies.
Similar technology, Weir noted, has been used successfully before in other licensed vaccines, such as the vaccines used to prevent cervical cancer. Finding a safe and effective way to use the technology to prevent influenza "represents a breakthrough," said Weir. "It provides another important option to fight a disease associated with the deaths of thousands in the United States every year."
This article appears on FDA's Consumer Updates page, which features the latest on all FDA-regulated products.
January 18, 2013