With new imaging technology, Recovery Act grantee discovers a cause of diabetics’ fragile skeletons
By Susan Johnson
October 6, 2011
Recovery Act Investment: “Cortical Bone Porosity Identifies Diabetes Subjects with Fragility Fractures”; Thomas M. Link; University of California, San Francisco; 2009: $419,410 (1RC1AR058405-01); 2010: $420,898 (5RC1AR058405-02). Funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the NIH Office of the Director.
Publications listing this Recovery Act Investment as providing grant support: Burghardt AJ, et al. High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. Journal of Clinical Endocrinology and Metabolism, 2010 Nov; 95(11):5045–5055.
Li X, et al. Quantification of vertebral bone marrow fat content using 3 Tesla MR spectroscopy: reproducibility, vertebral variation, and applications in osteoporosis. Journal of Magnetic Resonance Imaging, 2011 Apr; 33(4):974–979.
The Problem:
Twenty-five million Americans have type 2 diabetes, a chronic condition with potentially devastating consequences. Especially if it is not controlled by medication or lifestyle changes, diabetes can cause blindness or a loss of the sense of touch. Some people with the disease develop “fragility fractures,” or bone fractures that are caused by very small impacts, like a stumble.
However, the standard test for assessing bone strength shows that the density of calcium and other minerals in these people’s bones appear to be normal or even higher than normal. If this is the case, why do the bones break so easily?
Dr. Thomas Link suspected that the problem wasn’t the mineral content of the bone, but how the minerals were arranged. For the last 15 years, Link had been developing ways to measure bone strength beyond bone mineral density. He thought that the right method would uncover the reason for these skeletal weaknesses. This knowledge could form a basis for early detection and targeted bone-strengthening treatments to prevent fragility fractures before they occur.
To pursue this question, he had a powerful tool at his disposal: a high-resolution computed tomography (CT) scanner, which uses x-rays to visualize three-dimensional structures inside the body.
“We could potentially give these patients a medication to prevent fractures. But of course, first of all you have to identify them [the patients at risk]. If you don’t have any good tests, you have to wait until the patient actually has a fracture.” — Link
Imaging a few volunteer patients with this machine, Link and his colleagues saw hints of weaknesses in the bones’ hard outer shell, the cortex. They were eager to look into this finding in more detail.
But there was one major problem: Because this scanner was a custom-built machine, maintaining it was prohibitively expensive. The custom features and high resolution of the machine were essential to Link’s work, but costs associated with its upkeep and operation made his research unaffordable.
“When you build a house, how much wood you’re using is not as critical as how you arrange the wood to make the house stable. It’s the same with bone architecture.” — Link
Help From the American Recovery and Reinvestment Act (ARRA):
With his exciting preliminary data and a scientifically sound research plan, Link successfully applied for ARRA funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of NIH. He was supported through a new NIH initiative, the Challenge Grants in Health and Science Research, which was launched with ARRA funding to quickly spur progress toward meeting specific needs in health and science. Other than limited seed funding from the university, says Link, there was no other source of support for the kind of study he and his colleagues proposed.

Thomas M. Link, M.D., Ph.D., Clinical Director of Musculoskeletal Quantitative Imaging Research and Chief of the Musculoskeletal Imaging Section of the Department of Radiology and Biomedical Imaging at the University of California, San Francisco
With ARRA support through their Challenge Grant, Link and his colleagues pursued the intriguing hints they had uncovered about bone quality in diabetes. The research group also used this new support to pay the salary of their research coordinator, whom they would have been forced to lay off otherwise. The coordinator was vital for accelerating the pace of their research, says Link, ensuring that it stayed within the tight two-year ARRA funding period. The funds also partially supported several part- and full-time research positions, including Link’s.
In their recently published study, the researchers scanned the bones of 38 women just above the ankle and the wrist. Half of the participants had type 2 diabetes, and two of these participants had had a fragility fracture in the past. In addition to examining bone structure, Link and colleagues used the imaging data to calculate the bones’ strength by analyzing different parts of the bones separately.
Answers Emerge
The researchers’ striking results were consistent with the hints they had gotten from their earlier work. The bone cortex of most of the women with diabetes was slightly more porous than that of the women without diabetes, but the most dramatic results came from the diabetic women with histories of fractures. These women’s cortical bone was riddled with small holes. In what was perhaps an attempt to make up for the cortex’s weakness, these patients’ soft, inner bone had grown much harder than it should have been. The investigators’ calculations showed that these bones were much less flexible than the other participants’. Link and his collaborators believe that these combined factors are responsible for the fracture risk.
“NIH has allowed us to create a data set that is unique worldwide. The really amazing part about the ARRA funding is that our study has generated a lot of interest and raised a lot of questions that go far beyond just looking at cortical porosity. For us, this funding was a seed that was planted and will generate much more work in the future… It’s going to be very exciting.” — Link
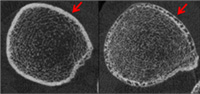
These high-resolution CT scans clearly show the hole-filled bone cortex of a diabetic patient with a history of fragility fractures (right). (The white ring is the cortex, as indicated by the red arrow.) The patient on the left is also diabetic, but her bone cortex is not structurally compromised.
On the basis of these more solid results, the researchers attracted additional funding that will sustain their work into the future. The work also attracted a great deal of attention, says Link, from the research community and from the pharmaceutical industry, which is interested in developing drugs to strengthen the weakened bone cortex.
Link expects that the data he has gathered so far will keep him and his colleagues busy for several years. Currently, they are pursuing several interrelated research projects on diabetic fracture risk that are derived from the original ARRA-funded project. They have almost completed a larger-scale version of that project that they hope will strengthen their findings. Link and colleagues will soon release the results of a study using a different type of imaging, known as magnetic resonance spectroscopy, to investigate whether poorly controlled diabetes is associated with higher amounts of fat in a patient’s vertebral bone marrow. In osteoporosis, bone fat content is connected to fracture risk, and Link and colleagues are investigating whether there is such a connection in diabetes.
Link and his colleagues are considering how to adapt their methods to commercially available scanners. The next-generation commercial CT scanners have a much higher resolution than their predecessors, says Link. If his group figures out how to do it, diabetes clinics may be able to use these new scanners to screen their patients for fracture risk.
People with diabetes face many challenges, from foot injuries to skin infections to high medical bills. With the knowledge and momentum that have come from their ARRA-funded research, Link and his collaborators hope to make the challenge of fragile bones much easier to surmount.