For many decades, fluorescence imaging has been used widely in cell biology to study cellular structures and processes, particularly in living cells. More recently, a number of significant technological advances have led to exponential growth in the application of fluorescence imaging in biology. For example, the development of new instruments, probes and imaging strategies have allowed investigators to use fluorescence techniques to make observations and measurements across many different biology systems and across multiple length scales with a detection limit currently approaching 20 nanometers. The toolbox for fluorescence imaging now includes an array of different techniques that can be adapted to diverse biological problems and settings, such as Total Internal Fluorescence (TIRF); fluorescence correlation spectroscopy (FCS); speckle spectroscopy; and supra-resolution imaging, including STORM or PALM. Here are a few research examples using fluorescence techniques.
Microtubule Networks During Cell Division
 This image captures a cell undergoing division, during which the genetic material is segregated to the two ends of the cell before completing the process. The microtubule network that forms the mitotic spindle (green) is stained with antibodies coupled to a fluorescent dye, and the chromosomes (red) are stained with a fluorescent dye that binds directly to the DNA. PtK1 cells were used in this experiment, and the final image is comprised of a series of sections taken along the vertical axis that were averaged to minimize the background. Credit: Julie Canman, Columbia University; Ted Salmon, University of North Carolina, Chapel Hill.
This image captures a cell undergoing division, during which the genetic material is segregated to the two ends of the cell before completing the process. The microtubule network that forms the mitotic spindle (green) is stained with antibodies coupled to a fluorescent dye, and the chromosomes (red) are stained with a fluorescent dye that binds directly to the DNA. PtK1 cells were used in this experiment, and the final image is comprised of a series of sections taken along the vertical axis that were averaged to minimize the background. Credit: Julie Canman, Columbia University; Ted Salmon, University of North Carolina, Chapel Hill.
Fluorescence Speckle Microscopy
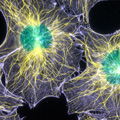 Fluorescence speckle microscopy (FSM) generated this image, which shows the intricate network of microtubule (yellow) and actin filament (purple) fibers that builds a cell's structure. The nuclei appear in greenish-blue color. In FSM, only a small fraction of the protein is labeled, significantly reducing out-of-focus fluorescence and greatly improving visibility of fluorescently labeled structures and their dynamics in thick regions of living cells. This image was digitally false colored to emphasize each component. Credit: Clare Waterman-Storer, NIH.
Fluorescence speckle microscopy (FSM) generated this image, which shows the intricate network of microtubule (yellow) and actin filament (purple) fibers that builds a cell's structure. The nuclei appear in greenish-blue color. In FSM, only a small fraction of the protein is labeled, significantly reducing out-of-focus fluorescence and greatly improving visibility of fluorescently labeled structures and their dynamics in thick regions of living cells. This image was digitally false colored to emphasize each component. Credit: Clare Waterman-Storer, NIH.
Controlling the Motility of Living Cells
 A new approach to manipulating cell activity uses a genetically encoded photoactivatable derivative of Rac1, a key protein that regulates actin cytoskeletal dynamics. The derivative can be reversibly and repeatedly activated to precisely localize cell protrusions and ruffling. In this video, a blue laser beam activates Rac1, which then stimulates the movement of a human cancer cell. The cell first responds by creating ruffles at the edge. It then stretches the cell forward. This new light-based approach can turn Rac1—and potentially many other proteins—on and off at exact times and places in living cells, and it could offer a new tool for studying embryonic development, nerve regeneration and cancer. Credit: Yi Wu, the Hahn lab, University of North Carolina. Article abstract
A new approach to manipulating cell activity uses a genetically encoded photoactivatable derivative of Rac1, a key protein that regulates actin cytoskeletal dynamics. The derivative can be reversibly and repeatedly activated to precisely localize cell protrusions and ruffling. In this video, a blue laser beam activates Rac1, which then stimulates the movement of a human cancer cell. The cell first responds by creating ruffles at the edge. It then stretches the cell forward. This new light-based approach can turn Rac1—and potentially many other proteins—on and off at exact times and places in living cells, and it could offer a new tool for studying embryonic development, nerve regeneration and cancer. Credit: Yi Wu, the Hahn lab, University of North Carolina. Article abstract