Physical Activity Guidelines Advisory Committee Report
Part G. Section 2: Cardiorespiratory Health
List of Figures
List of Tables
Introduction
Cardiovascular diseases (CVD) account for the majority of premature
morbidity and mortality in the developed world. The influence of physical
activity and the prevention and treatment of cardiovascular disease is
therefore of great importance. In considering the effects of physical activity
on cardiovascular health, one must address not only its influence on the
development of symptomatic disease (e.g., heart attack and stroke) but also the
influence on risk factors that are known to contribute to the development of
symptomatic disease and are often indicative of sub-clinical asymptomatic
vascular pathology. Most of the modifiable risk factors for cardiovascular
diseases are metabolic in nature and are, in turn, modifiable by changes in
physical activity. These metabolic risk factors include hypertension,
atherogenic dyslipidemia, the axis of insulin resistance to metabolic syndrome
to frank type 2 diabetes, and obesity. In turn, both physical inactivity and
poor cardiorespiratory fitness are major risk factors for cardiovascular
diseases.
Review of the Science
Overview of Questions Addressed
In this critical review of the knowledge base about the relations
between cardiovascular disease and physical activity, cardiovascular disease
should be construed to include coronary heart disease, cerebrovascular disease,
and peripheral arterial disease. This section of the report reviews the data
regarding this relation in two parts, sequentially addressing a series of
questions about the presence and the nature of the relationship between
physical activity and cardiorespiratory health. First, the section addresses
the primarily observational data about physical activity and cardiovascular
disease in separate sections dealing with coronary heart disease,
cerebrovascular disease and stroke, and peripheral arterial disease. Then,
using data from experimental studies, it explores the evidence of the relation
between physical activity and several cardiovascular disease risk markers:
hypertension, atherogenic dyslipidemia, vascular health and cardiorespiratory
fitness. Influences of physical activity on insulin resistance, glucose
control, metabolic syndrome and diabetes are addressed in
Part G. Section 3: Metabolic
Health and relations between physical activity and obesity
are addressed in Part G. Section 4: Energy
Balance. Within each disease or risk factor category, this
section reviews the supporting evidence and provides conclusions about the
following 3 questions.
- What is the nature of the relationship with physical activity?
- What is known about the dose-response relationship with different
characteristics of physical activity?
- What is known about whether the effects of physical activity exposure
can be obtained in smaller multiple bouts per day (accumulation) versus single
daily bouts?
Data Sources and Process Used To Answer
Questions
The Cardiorespiratory Subcommittee focused its review on studies
performed since the publication of the Surgeon General's Report on Physical
Activity and Health in 1996 (1), emphasizing disease
prevention as opposed to disease treatment. The subcommittee drew heavily from
the Physical Activity Guidelines for Americans Scientific Database
(see Part F: Scientific Literature Search
Methodology, for a detailed description of the Scientific
Database). In addition, the subcommittee relied on expert knowledge of the
authors to identify specific published studies that are critical for the
knowledge base that may predate 1996, post-date the collation of the Scientific
Database, or for outcomes that were not identified as part of the Scientific
Database process (e.g., vascular health markers). Also, reviews in some subject
areas (hypertension and atherogenic dyslipidemia) relied in part upon
meta-analyses. Finally, for some topics (e.g., cardiorespiratory fitness),
separate literature searches were performed in the PubMed database.
All of the prospective cohort and case-control studies included in this
review provide self‑report information on the habitual physical activity
of the subjects, a standardized assessment of CVD clinical events and a
comparison of event rates in subjects assigned to 2 or more categories of
physical activity. For interventional experimental studies, the analysis was
restricted to randomized controlled trials (RCTs) that had a sedentary (non
physical activity intervention control arm or period) and studied at least 25
subjects per arm, unless the findings were highly significant with a lower
number.
In general, the reviews and discussions address physical activity
performed in the context of dedicated sessions of exercise. The assumption is
that the specified exercise activity is performed in addition to and on top of
normal physical activity performed as a part of activities of daily living. The
data are primarily confined to dynamic aerobic (endurance) exercise, as the
long-term cardiovascular prevention benefits of resistance and flexibility
exercises are relatively little studied to date (2). An
exception to this approach occurs when measures of total activity or
occupational activity are use as exposure variables in prospective cohort or
case-control studies.
Special Considerations and Limitations
The relation between dynamic aerobic exercise and cardiovascular health
outcomes, including cardiorespiratory fitness is complex and can be thought of
as a series of point estimates within a 3-dimensional matrix of continuous
variables: exercise exposure, disease activity, and the magnitude of the
response. The major limitation to exercise exposure recommendations for
cardiovascular health outcomes is that any recommendation poorly conveys the
concept that the location of any point estimate along each of these 3 axes is
along a continuum of exposure and response, and should not be viewed as an
absolute threshold below which no benefits accrue and above which benefits
always accrue.
Continuum of Exercise Exposure
It is well accepted that aerobic exercise exposures can be characterized
by an interaction between bout intensity, frequency, duration, and longevity of
the program (3;4). The product of these
characteristics can be thought of as volume and can be represented by the total
energy expenditure (EE) of the exercise exposure. Exercise volume is referred
to as the major focus of the exercise recommendation in some recent statements
(5), thus allowing for the mixing of exercise bouts of
varying intensity, frequency and duration. As recommendations are intended to
be adopted for an individual's life-time, longevity is not considered here.
However, it is clear that most benefits resulting from changes in physical
activity and exercise patterns accrue over days, weeks, months and even years
of exposure, and that the study and understanding of such time lines are of
scientific and clinical interest and should be investigated further. Most of
the data from experimental studies presented here regarding dose-response
associations address the issue of varying intensities of exercise and do not
control for bout duration, frequency, or total volume of the exercise exposure.
In most observational studies, the major variable used as an exposure is
activity amount (e.g., minutes, metabolic equivalent [MET]-minutes per day,
miles per week) with the other exposure frequently being activity intensity.
However, because total weekly EE usually is not controlled, it is possible that
the effects of higher intensities observed in these studies might reflect the
higher volumes performed, and that the volume of the activity exposure is the
important operative. As will be apparent from the relation of exercise volume
to the other variables, one cannot fix volume and also simultaneously study
either intensity, frequency, or duration effects while controlling the other
two. Relatively few interventional experimental studies examine exercise
intensity while controlling for EE and even fewer study frequency or duration
effects while controlling for EE. This makes the construction of a precise
exercise dose for any given response problematic.
Continuum of Disease Progression
Cardiovascular disease is a continuum from asymptomatic fatty vascular
streaks, to severe symptomatic coronary heart disease, to fatal myocardial
necrosis and death. The same is true for cerebrovascular disease and stroke.
The goal of this section is to focus primarily on primary cardiovascular
disease prevention. As part of that process, we have explored some treatment
effects on cardiovascular risk factors (e.g., atherogenic dyslipidemia and
hypertension), the favorable modulation of which, by pharmacologic or lifestyle
therapy, have been shown to be related to reductions in cardiovascular risk as
well. The modulation of these risk markers may be the mechanism through which
physical activity acts to reduce cardiovascular clinical events, as well. One
should be aware that the activity exposure beneficial for primary
cardiovascular health (the factors studied in this chapter) and prevention may
or not apply to patients with clinically active and apparent cardiovascular
disease, such as those in rehabilitation programs.
Role of Physical Inactivity in Disease
Progression
A note about the importance of acknowledging the health risks of
inactivity in studies of the effects of physical activity on cardiovascular
risk factors is indicated here. In studies that include a sedentary inactive
non-intervention control group for comparison to the exercise intervention
groups, the inactive group consistently tends to demonstrate a worsening in
health parameters over time. This is the health cost of physical inactivity, to
be contrasted with the health benefits of regular physical activity. That is,
the lack of physical activity in normal life leads to worsening in some
parameters absent other life style changes, such as in diet. In some instances,
the lack of worsening in some parameters over time demonstrated in intervention
groups would appear to be an indication that the exercise or physical activity
intervention has no effect, whereas, in fact, when compared to inactive control
groups, a significant difference in response over time is observed.
Continuum of the Response
The response of biological parameters to dynamic aerobic exercise, and
likely to resistance training as well, is a continuum from undetectable changes
to highly significant, robust and clinical important ones that are highly
dependent on the exercise exposure variables previously discussed.
Consequently, it is likely that no given minimal intensity, frequency, duration
or volume of exercise will result in a favorable response for any given
outcome. Similarly, it is unlikely that any of these exercise variables has a
level for optimal outcome. Furthermore, increases in exercise
exposure do have tangible adverse outcomes that are primarily musculoskeletal
and cardiovascular (see Part G. Section
10: Adverse Events). Thus, potential increases in favorable
outcomes of increasing exercise exposure must be balanced by the potential for
increases in unfavorable outcomes.
Question 1: What Is the Relationship
Between Physical Activity and Cardiovascular Morbidity and Mortality?
Conclusion
The results of recently published studies continue to support a strong
inverse relation between the amount of habitual physical activity performed and
CHD and CVD morbidity or mortality. For both men and women at middle age or
older, remaining sedentary is a major independent risk factor, with persons
reporting moderate amounts of activity having a 20% lower risk and those
reporting activity of higher amounts or intensity having approximately a 30%
lower risk than least active persons. These may be underestimates of the risk
reductions (with the underestimate being on the order of 10%) because
multivariate models in many studies include adjustments for hypertension,
dyslipidemia, and glucose tolerance, conditions that may represent biological
intermediates in the causal pathway. Although still limited, data also indicate
habitual physical activity benefits the cardiovascular health of people of
various races and ethnicities.
Introduction
Physical Activity and Health: A Report of the Surgeon General
concluded by saying, "The epidemiologic literature supports an inverse
association and a dose-response gradient between physical activity level or
cardiorespiratory fitness and both CVD in general and CHD in particular. A
smaller body of research supports similar findings for hypertension. The
biological mechanisms for these effects are plausible and supported by a wealth
of clinical and observational studies. It is unclear whether physical activity
provides a protective role against stroke" (1, p.112).
Since 1996, a large volume of research has been directed at better defining the
relation between physical activity and various CVD clinical outcomes, the
mechanisms by which the cardiovascular benefits of physical activity are likely
mediated, and the characteristics of the dose of activity (type, intensity,
frequency, session duration, and total volume) associated with lower CVD
clinical event rates.
The following material provides an overview of the scientific literature
since 1996 directed at establishing the effects of physical activity on various
clinical cardiovascular outcomes and the issue of dose-response. The main focus
is on the primary prevention of clinical events; therefore, most of the
evidence comes from prospective cohort studies of at-risk populations. All of
the studies included in this review provide self-report information on the
habitual physical activity of the subjects, a standardized assessment of
cardiovascular clinical events, and a comparison of event rates in subjects
assigned to 2 or more categories of physical activity. These comparisons
consisted of a measure of the relative risk (RR) for the groups and 95%
confidence intervals for the measure of risk, including risk ratios, hazard
ratios or odds ratios. In all the cited studies, the multivariate adjusted
relative risks were recorded and used in any analysis. These adjustments varied
from study to study but usually included at a minimum age, body mass index
(BMI), cigarette smoking, blood pressure, and blood lipid concentrations. It is
understood that using multivariate adjustments, which in some cases include
measures of BMI, blood pressure, and blood lipids, could inappropriately
decrease the magnitude of the relation between the physical activity exposure
and the clinical outcome because some of the benefit of the activity might be
mediated through these variables ("intermediate" or "mediator" variables).
However, we considered this a more conservative approach than adjusting just
for age and other selected demographic variables. In studies where RRs for more
active versus the least active persons are presented using both limited
adjustments and multivariate adjustments that accounted for potential
"intermediate" variables, the RRs for limited adjustments show greater effects
in the range of 10% (6-8). To determine whether a
dose-response pattern existed between physical activity characteristics and the
clinical outcome, data for at least 3 activity categories needed to be
provided. The Physical Activity Guidelines for Americans Scientific
Database was used to identify eligible studies published between January 1996
and June 2007. Also, selected studies that did not meet criteria for inclusion
in the Database but provided ancillary data related to specific issues have
been considered in this review, including meta-analyses and systematic reviews.
Rationale
Between January 1995 and June 2007, more than 60 studies were published
that met the subcommittee's search criteria investigating the effects of
habitual physical activity on cardiovascular morbidity and/or mortality in men
and women throughout a wide age span and of various race and ethnicities. Much
of the self-reported physical activity was performed during leisure time, but
also included are data from occupational, household, and commuting activities.
A majority of these data come from prospective cohort studies with the results
from a limited number of case-control studies included. Studies tended to
report outcomes for various clinical manifestations of coronary heart
disease (e.g., fatal or nonfatal myocardial infarction, ischemic heart
disease, cardiac death), a more general category of cardiovascular disease
that could include a variety of manifestations of atherothrombotic vascular
disease (e.g., coronary heart disease, stroke, other vascular disorders), and
stroke or cerebrovascular disease. Data were organized from these
studies by CHD, CVD, and stroke and then by sex with an emphasis on the
magnitude of any relation and whether evidence of a dose response existed. The
relation between a measure of physical activity and a CVD clinical outcome was
considered significant if the 95% confidence interval did not include 1.0. A
significant dose-response relation usually was based on P for trend being
<0.05.
Coronary Heart Disease
The results of studies investigating the relation between habitual
physical activity and CHD morbidity and/or mortality published since 1996 quite
consistently show lower event rates in more physically active men and women
than for their least active counterparts. Most notable has been the large
increase in the number of studies that have included data on women, with 19
studies reporting data on women and 9 with data on men and women combined (see
Table G2-1 for a summary of the studies and
Table G2.A1 [PDF - 257 KB] for selected
data from individual studies).
The studies of women reporting CHD clinical events included more than
200,000 subjects aged 20 to 85 years. For the prospective cohort studies, the
median RR of having a CHD clinical event for women reporting participation in
moderate intensity or amount of physical activity compared to women reporting
no or only light intensity activity was 0.78, while the RR for women performing
vigorous or high amounts of activity as compared to women eporting no or light
activity was 0.62. These RRs are quite similar to those resulting from a
meta-analysis of many of the same studies that were published between 1996 and
2003 (9). The conclusion from this meta-analysis for CHD
was that physical activity was associated with a lower risk of CHD (as well as
CVD and stroke) in a dose-response fashion with pooled RRs for both moderate
amounts and high amounts being significant when compared to no or light
activity. In the 6 case-control studies reported for women, the median RR was
0.62 for moderate versus no or light activity and 0.44 for vigorous intensity
or high amounts of activity versus no or light activity.
Of the studies reporting on CHD in men, 16 were prospective cohort
studies and 4 were case-control studies. Approximately 124,000 men aged 15 to
96 years at baseline were included as subjects. Most studies reported on
leisure-time physical activity (LTPA) with a few studies including occupational
activity, commuting, and sports participation. Among the prospective cohort
studies, the median RR was 0.81 for moderate intensity or amount of activity
versus no or light activity and 0.68 for vigorous intensity or high amounts
versus light or no activity. For the 6 case-control studies, the median RR was
0.65 for moderate versus no or light activity and 0.53 for vigorous intensity
or high amounts versus no or light activity. These values are of a similar
magnitude to those reported in a systematic review of studies published between
1953 and 2000 (10) and in a meta-analysis published in
2001 that included data from studies published before and after the Surgeon
General's Report on Physical Activity and Health (11). The lower CHD event rate for more active men was
reported for both nonfatal and fatal CHD with no systematic difference in CHD
incidence versus CHD mortality.
Five prospective cohort studies and 4 case-control studies were
published in which the results for CHD events for men and women were combined.
In the prospective cohort studies, the median RR was 0.74 for moderate
intensity or amount versus no or light activity and 0.63 for high intensity or
amount versus no or light activity. In the case-control studies, the RR was
0.61 for moderate activity versus no or light activity and 0.48 for high
amounts or intensity versus no or light activity.
Table G2.1. Summary of Prospective Cohort
Studies and Case-Control Studies Published in the English Language Since 1996
Reporting on the Relation Between Habitual Physical Activity and the Prevention
of Coronary Heart Disease, Cardiovascular Disease, or Stroke
Data summaries for each study in this review are included in the
Appendix.
Men
Condition
Prevented |
Prospective Cohort Studies
Number of
Studies
Reporting RR |
Prospective Cohort Studies
Median RR
M/L |
Prospective Cohort Studies
Median RR
H/L |
Prospective Cohort Studies
Number of
Studies
Reporting
D-R |
Prospective Cohort Studies
Number of
Studies
D-R Sig. |
Case-Control Studies
Number of
Studies
Reporting RR |
Case-Control Studies
Median RR M/L |
Case-Control Studies
Median RR H/L |
Case-Control Studies
Number of
Studies
Reporting D-R |
Case-Control Studies
Number
of Studies
D-R Sig. |
| Coronary Heart Disease |
17 |
0.81 |
0.68 |
11 |
7 |
6 |
0.65 |
0.53 |
2 |
2 |
| Cardiovascular Disease |
10 |
0.78 |
0.70 |
3 |
2 |
1 |
0.65 |
0.67 |
0 |
0 |
| Total Stroke |
11 |
0.65 |
0.72 |
6 |
5 |
0 |
– |
– |
– |
– |
Women
Condition
Prevented |
Prospective Cohort Studies
Number of
Studies
Reporting RR |
Prospective Cohort Studies
Median RR
M/L |
Prospective Cohort Studies
Median RR
H/L |
Prospective Cohort Studies
Number of
Studies
Reporting
D-R |
Prospective Cohort Studies
Number of
Studies
D-R Sig. |
Case-Control Studies
Number of
Studies
Reporting RR |
Case-Control Studies
Median RR M/L |
Case-Control Studies
Median RR H/L |
Case-Control Studies
Number of
Studies
Reporting D-R |
Case-Control Studies
Number
of Studies
D-R Sig. |
| Coronary Heart Disease |
13 |
0.78 |
0.62 |
8 |
5 |
6 |
0.62 |
0.44 |
3 |
1 |
| Cardiovascular Disease |
12 |
0.80 |
0.72 |
6 |
5 |
1 |
0.89 |
0.71 |
0 |
0 |
| Total Stroke |
8 |
0.82 |
0.72 |
5 |
4 |
0 |
– |
– |
– |
– |
Men and Women (Data Combined)
Condition
Prevented |
Prospective Cohort Studies
Number of
Studies
Reporting RR |
Prospective Cohort Studies
Median RR
M/L |
Prospective Cohort Studies
Median RR
H/L |
Prospective Cohort Studies
Number of
Studies
Reporting
D-R |
Prospective Cohort Studies
Number of
Studies
D-R Sig. |
Case-Control Studies
Number of
Studies
Reporting RR |
Case-Control Studies
Median RR M/L |
Case-Control Studies
Median RR H/L |
Case-Control Studies
Number of
Studies
Reporting D-R |
Case-Control Studies
Number
of Studies
D-R Sig. |
| Coronary Heart Disease |
5 |
0.74 |
0.63 |
1 |
1 |
4 |
0.61 |
0.48 |
3 |
1 |
| Cardiovascular Disease |
5 |
0.87 |
0.72 |
2 |
1 |
0 |
– |
– |
– |
– |
| Total Stroke |
4 |
0.67 |
0.75 |
2 |
1 |
2 |
0.68 |
0.48 |
0 |
0 |
D-R, dose-response; H/L, high intensity or high amount
vs. light intensity/amount; M/L, moderate intensity/amount vs. light
intensity/amount; RR, relative risk (includes risk ratio, odds ratio or hazard
ratio); Sig., significant.
Cardiovascular Disease
In prospective cohort studies published since 1996 that included data on
the relation between habitual physical activity and CVD in women (n=12), the
median RR was 0.80 for those reporting moderate intensity or amount versus no
or light activity and 0.72 for vigorous versus no or light activity. In the one
case-control study reporting on CVD in women, the RR was 0.89 for moderate
intensity versus no or light activity and 0.71 for high versus no or light
activity. (See Table
G2.A2 [PDF - 221 KB] for selected data from these prospective cohort and case-control
studies.) Here again, the amount and quality of data evaluating the relation
between physical activity and CVD clinical events in women has substantially
increased since 1996, with at least 350,000 women included in the reported
studies. Overall, the CVD data reported on men are very similar to those for
women: In 10 prospective cohort studies the median RR for CVD events was 0.78
for moderate versus no or light activity and 0.70 for high intensity or amount
versus no or light activity. In the one case-control study, the RR was 0.65 for
moderate versus light activity and 0.67 for high versus no or light activity.
Although data are not provided in the reports, it is very likely that a
majority of the CVD events included in these studies were the result of
coronary heart disease.
Effects of Sex, Age, or Race and Ethnicity
Although the magnitude of median RRs for CHD for both moderate versus
light activity and high versus light activity are somewhat lower in women than
in men (Table G2-1), physically active men and
women both typically have a lower risk for CHD than do their least active
counterparts. Comparisons between the sexes are difficult across studies
because of some evidence that the activity levels in the least active women are
less than for the least active men, age distributions within age categories
(e.g., 40 to 65 years, 65 to 79 years) are different from study to study, and
CHD event rates within age categories differ between men and women. In the
studies that included data for both men and women (12-20),
even fewer presented results for men and women separately and in some studies
that do, the number of CHD events in women is relatively small, thus
substantially limiting the reliability of any analysis (19). In a case-control study published by Fransson and
colleagues (20) evaluating the association between various
types of physical activity and acute myocardial infarction, women appeared to
be somewhat more protected than men. The RR for fatal and nonfatal MI in women
comparing most active versus least active for total activity was 0.16 (95% CI
0.07-0.37), and the RR for the same comparison in men was 0.46 (95% CI
0.31-0.69). For women, the RR for LTPA more than 3 times per week versus seldom
was 0.31 (95% CI 0.15-0.66); for men the RR was 0.53 (95% CI 0.38-0.73). It
should be noted that rarely is a distinction made in these studies between
associations in pre- and post-menopausal women, and whether they are different
in these two populations when studied separately. Consequently, no evidence
exists that effects of physical activity on CHD are different whether the study
population is men, pre-menopausal, or post-menopausal women.
The inverse association between physical activity and CHD events has
been reported for adults across a wide range of ages, with the magnitude of the
association for older men and women (aged 65 years and older) at least as
strong as for younger adults. Because CVD morbidity and mortality rates are low
in men younger than age 45 years and women younger than age 55 years, very few
data are available on the relation between physical activity levels and CVD
clinical events in younger adults or youth. None of the meta-analyses on
physical activity and CVD events published since 1995 has evaluated the effect
of age on the magnitude of the relation, and only a limited number of studies
have compared different age categories within their population. Manson and
colleagues (21) had a sufficiently large sample of women
(n=73,743) and cardiovascular events (n=1,551) in the Women's Health Initiative
Observational Study to analyze the relation between LTPA and CVD incidence for
3 age groups, 50 to 59 years, 60 to 69 years, and 70 to 79 years. When activity
was classified by MET-hours per week in quintiles, all 3 age groups showed a
significant difference (P for trend <0.001) when the highest versus the
lowest quintiles were compared (RR = 0.45, 0.50 and 0.64, respectively) with
the lowest quintile being the reference (1.0) the adjusted RRs for quintiles 2
through 5 for women aged 50 to 59 years were 0.68, 0.63, 0.54, 0.45,
respectively. For women aged 60 to 69, the RRs were 0.79, 0.63, 0.56, 0.50,
respectively, and for women aged 70 to 79, they were 0.93, 0.86, 0.75, 0.64,
respectively. Other studies have not showed any meaningful difference in the
relation between physical activity level and CVD events in different age
categories. For example, women in the College Alumni Health Study contrasting
those younger than age 45 years versus those 45 years and older at baseline (22), combined data on men and women contrasting aged 65 years
and younger versus those older than 65 years (23), or
those aged 65 to 74 years versus aged 75 years and older (24). In a small prospective cohort study in men evaluating
various risk factors for CHD, high-intensity activity was related to CHD events
in those older than age 65 years (0.36, 95% CI 0.13-1.05) but not in those aged
65 years and younger (25). In the Buffalo Blood Pressure
Study, older women (aged 60 years and older) were not protected from CVD
mortality by high levels of total activity, though physical activity provided
some protection for women younger than aged 60 years. However the number of CVD
events was small in both groups (26).
Few studies conducted in the United States have had an adequate sample
size and clinical outcomes to evaluate the association between physical
activity and CVD clinical events in race or ethnic groups other than
non-Hispanic whites. The Women's Health Initiative Observational Study (21)
included 61,574 white women and 5,661 black women with a mean follow-up of 3.2
years. The relation between total physical activity level (quintiles of
MET-hours per week) and CVD clinical events was significant for both groups of
women with RR for the highest versus lowest quintile of activity for white
women being 0.56 (P for trend <0.001) and for black women 0.48 (P
for trend = 0.02). In contrast to these results, a report on the
Atherosclerosis Risk in Communities (ARIC) study population indicated that
although activity level and CVD clinical events had a significant inverse
relation in white men and women, no such relation was found for either back men
or women (19). The authors suggested that this lack of
association in blacks may be due to the limited number of blacks reporting
vigorous physical activity (5% in black men versus 15% in white men). However,
outside the United States, where the relation between physical activity level
and CVD clinical events has been evaluated in other race and ethnic
populations, there is no indication that the favorable association frequently
reported for non-Hispanic white men and women does not occur in other race and
ethnic populations. For example, physically active Japanese men and women
living in Japan (27) and older Japanese men living in
Hawaii (28) had lower CVD mortality rates than the least
active. Similar results have been reported for Chinese women living in Shanghai
(29) and Chinese men and women living in Hong Kong (30). In a case-control study including men and women
conducted in New Delhi and Bangalore India, at least 145 MET-minutes per day of
LTPA versus no activity had a RR for myocardial infarction of 0.44 (95% CI
0.27-0.41). Time spent in non-work sedentary activity also was directly
associated with risk of myocardial infarction (the RR for at least 215 minutes
per day of sedentary activity versus fewer than 70 minutes per day was 1.58
[95% CI 1.05-2.36]).
Change in Physical Activity and Cardiovascular Disease Clinical
Events
Most reports from prospective observational studies have presented the
relation between physical activity measured on one occasion and the rate of CVD
clinical events over various periods of follow-up. However, a few studies have
obtained self-reported activity 2 or more times, typically 3 to 15 years apart,
and related change in activity during this interval with CVD clinical events
during a follow-up period. The goal of this approach is to determine whether an
increase in activity is associated with lower event rates than observed for
subjects who remain inactive. Also, do subjects who move from an active to an
inactive category have higher CVD event rates than subjects who remain
physically active? Men in the Harvard Alumni Study who increased their physical
activity index to 2,000 kilocalories per week or more (measured in 1962 or 1964
and again in 1977) compared to men who remained inactive had a 17% lower CHD
death rate (P= 0.51), while men who took up moderately vigorous sports
had a 41% lower risk (P= 0.04) (31). Similar
results have been reported for British men. Those who reported an increase in
activity over 12 to 14 years had a RR for CVD mortality of 0.66 (95% CI
0.35-1.23) compared to men who remained sedentary, while men who remained
active had a RR of 0.54 (95% CI 0.31-0.94) compared to continuously sedentary
men (32).
Women in the Nurses' Health Study who reported increases in their LTPA
between 1980 and 1986 with follow-up to 1994 had lower CVD event rates than
women who remained sedentary (6). When the increase in
activity for women who were sedentary in 1980 was expressed in quartiles of
METs, the RRs for quartile 1 through quartile 4 were 0.85, 0.79, 0.67 and 0.71,
respectively (P for trend=0.03). Women aged 65 years of age and older
who had physical activity assessed twice (5.7 years apart) and changed from
being inactive to active had a RR for CVD mortality of 0.64 (95% CI 0.42-0.97)
compared to women who remained inactive, and women who remained active had a RR
of 0.68 (95% CI 0.58-0.82). Although data on the association between change in
activity and CVD clinical events in prospective observational studies does not
provide the same level of evidence as data from RCTs, these results do add to
the strength of the evidence linking higher levels of physical activity with
lower CVD risk. In the studies cited, the change in activity preceded the
clinical events and the direction of the association is consistent with an
increase in activity causing a reduction in risk.
Question 2: What Are the Dose-Response
Relations Between Physical Activity and Cardiovascular Morbidity and
Mortality?
Conclusion
The inverse association between CVD clinical events and habitual
physical activity exists across a wide range of types, amounts, and intensities
of activity. People at highest risk are those who are least active and spend
much of their day in activities that consume low amounts of energy. When
compared to very sedentary persons, men and women who perform small amounts of
moderate-intensity activity, such as 60 minutes per week of walking at a brisk
pace, exhibit fewer CVD clinical events. People who perform more activity
and/or at a faster pace are at an even lower risk, with much of the benefit
derived when men and women are performing 150 or more minutes per week of
moderate-intensity (3 to less than 6 METs) physical activity. Greater amounts
of activity appear to provide greater benefit but the shapes of any
dose-response relations have not been well defined. Vigorous-intensity activity
(equal to or more than 6 METs) when performed for a similar duration as
moderate-intensity activity results in greater energy expenditure and is
associated with lower CVD event-rates. Much of the recent data are based on
LTPA, but performing physical activity during an occupation, around the home,
or while commuting all appear to provide benefit as well.
Rationale
In the studies reporting on CHD or CVD, the median RR difference for
high levels of activity versus inactive or light activity categories was
somewhat greater than the difference in the median RR for moderate levels of
activity versus inactive or light activity, thus indicating a somewhat greater
benefit from higher amounts or intensities of activity versus moderate
intensity and amounts of activity. In the cohort studies that had 3 or more
physical activity levels, authors frequently evaluated dose-response by
calculating the linear trend and testing this trend for significance. If the P
for trend was ≤0.05, then the dose response was considered significant.
For CHD in women, 7 studies reported P values for dose response, and 3
of them were significant. Six studies reported dose response for CVD in women,
with 5 reaching significance. For men, 7 of 11 studies reporting dose response
for CHD were significant as were 2 of the 3 studies reporting on CVD. For
studies that combined data on men and women, the one study that reported
dose-response for CHD found it to be significant, and 1 of the 2 studies
reporting on CVD was significant.
From a public health perspective, it is important to recognize that when
the reference group in the population being studied is very sedentary, modest
amounts of moderate intensity activity are associated with significantly
reduced rates of CHD and CVD. For example, in 3 large prospective cohort
studies of women in the United States (6;7;21), those who walked in the range of 1
to 2 hours per week versus non-walkers produced RRs for CVD or CHD events of
0.75 (95% CI 0.63-0.89; (21), 0.70 (95% CI 0.51-0.95; (6)), and 0.49 (95% CI 0.28-0.86; (7)) (Figure G2-1). The P for trend with multivariate
adjustment for categories of walking amount (MET-minutes per week or duration
(minutes per week) was significant (P <0.001) in all 3
studies. Also, walking at a faster pace was associated with a lower risk of CHD
or CVD in these 3 studies, with those who walked at a pace 3.0 miles per hour
and greater having a significantly lower RR than non-walkers (0.76, 0.70 and
0.52). The P for trend across walking pace was significant for all 3
studies. Other studies have reported on walking and CVD with either
significantly lower RRs for men and women who walk regularly versus non-walkers
(24) or favorable but non-significant trends for increased
walking (22;28;33;34). There was no difference in a
large study of Chinese women living in Shanghai where the least active
reference group included walking from 0 to 3.4 MET-hours per week (29). In this study, the amount of walking in the reference
group of Chinese women was sufficiently high that additional walking may not
provided additional protection against CVD. Overall, these data on walking and
CVD indicate that when brisk walking is performed 3 hours per week by otherwise
sedentary persons, especially women, the CVD clinical event rate is
significantly lower than for persons who do little walking or other physical
activities.
Figure G2.1 Relative Risk of CVD in
Women — Walking Amount/Week

Figure G2.1. Data Points
| Studies |
1 |
2 |
3 |
4 |
5 |
| Manson, 1999 |
1 |
0.78 |
0.88 |
0.7 |
0.65 |
| Manson, 2002 |
1 |
0.91 |
0.75 |
0.75 |
0.68 |
| Lee, 2001 |
1 |
0.86 |
0.49 |
0.48 |
- |
Question 3: What Is the Relationship
Between Physical Activity and Cerebrovascular Disease and Stroke?
Conclusion
More physically active men and women generally have a lower risk of
stroke incidence or mortality than the least active, with more active persons
demonstrating a 25% to 30% lower risk for all strokes. A favorable relation
exists between physical activity level and stroke (both for ischemic and for
hemorrhagic stroke), but the data on these stroke subtypes are still quite
limited. The benefits appear to be derived from a variety of activity types,
including activity during leisure time, occupational activity, and walking.
Overall, the relationship between activity and stroke is not influenced by sex
or age, and very little data exist for race and ethnicity other than for
non-Hispanic whites.
Rationale
The Surgeon General's Report on Physical Activity and Health
concluded that "the existing data do not unequivocally support an association
between physical activity and stroke risk" (1, p.103). This
conclusion was based on a review of 14 observational studies (4 included
women), of which 8 showed an inverse relationship between physical activity and
stroke. The other studies showed no significant association, with 2 studies
suggesting a U-shaped relationship with higher stroke risk in the least and
most active categories. Since 1996, studies meeting the criteria for this
review include data from studies on women (n=8), men (n=11), and men and women
combined (n=6). (See Table
G2.A3 [PDF - 189 KB] for selected data from these prospective cohort and case-control
studies.) In addition, 2 meta-analyses of physical activity and stroke have
been published (35;36). In most
studies, data are reported on all strokes, and in some studies data also are
provided separately for ischemic and hemorrhagic stroke. In women, the median
RR was 0.82 for all strokes combined for moderate-intensity activity versus no
or light activity and 0.72 for high-intensity or amount versus no or light
activity. For all strokes in men, the median RR was 0.65 for moderate-intensity
versus no or light activity and 0.72 for high-intensity or amount versus no or
light activity. In the studies reporting combined data on men and women, the
median RR for the prospective cohort studies (n=4) was 0.67 for
moderate-intensity versus no or light activity and 0.75 for high-intensity or
amount versus no or light activity. For the 2 case-control studies, the median
RR was 0.68 for moderate versus low activity and 0.48 for high versus low
activity.
The meta-analysis by Wendel-Vos and colleagues (36)
included data from 31 studies published in English before 2001, including 24
prospective cohort studies and 7 case-control studies. Based on these analyses,
the authors concluded that moderately active men and women had lower rates of
ischemic, hemorrhagic, and all strokes than did the least active subjects. When
persons who reported moderate-intensity occupational activity were compared
with persons who reported light-intensity occupational activity, the RR was
0.64 (95% CI 0.48-0.87). They also observed an RR of 0.85 (95% CI 0.78-0.93)
for moderate versus light LTPA. High-level occupational activity appears to
protect against ischemic stroke compared with both moderate (0.77, 95% CI
0.60-0.98) and inactive occupational levels (0.57, 95% CI 0.60-0.98). Persons
reporting high-level compared to low-level LTPA were at significantly lower
risk for all strokes (0.78, 95% CI 0.71-0.85), ischemic stroke (0.79, 95% CI
0.69-0.91), and hemorrhagic stroke (0.74, 95% CI 0.57-0.96). Both moderately
active men and women had a lower RR for hemorrhagic stroke than their inactive
counterparts (men = 0.54, 95% CI 0.36-0.81; women = 0.76, 95% CI 0.67-0.86;
P=0.07 for difference between men and women). Studies conducted in
Europe showed a stronger inverse association between active and inactive
persons (0.47, 95% CI 0.33-0.66) compared to studies conducted in the United
States (0.82, 95% CI 0.75-0.90). The overall results of the meta-analysis on
physical activity and stroke published a year earlier (35)
were similar to the results of this meta-analysis. When Lee and colleagues
included data from both cohort and case-control studies, the RR for stroke
incidence or mortality for the most active versus the least active was 0.73
(95% CI 0.67-0.79) and for moderately active versus the least active the RR was
0.80 (95% CI 0.74-0.86).
The inverse association between physical activity level and stroke risk
appears very similar for men and women in the few studies that report
sex-specific data. Vatten and colleagues (37) followed
34,868 Norwegian women and 32,872 men for 16 years and documented
cause-specific mortality. The P for trend for total activity and stroke
mortality was 0.009 for men and <0.001 for women, and the RR for high
activity versus never active was significant for both sexes. In Japan, 31,023
men and 42,242 women were followed for an average of 9.7 years, and walking and
sports participation were inversely related to CVD mortality (27). The relationship of walking time to all stroke or
ischemic stroke mortality was very similar for men and women as was the time
spent in sports participation. Because the occurrence of stroke is very low for
those younger than age 55 years, very few reports are available on the relation
of physical activity to stroke morbidity or mortality in younger and
middle-aged populations. Data from the National Health and Nutrition
Examination Survey Epidemiologic Follow-up Study (38)
indicate no systematic difference in the relationship of LTPA amount to either
total or non-hemorrhagic stroke in men or women aged 45 to 64 years versus 65
to 74 years at baseline (the age x low activity interaction term was not
significant). Overall, the strongest and most consistent association between
activity level and stroke in this study was seen in white women.
Although stroke rates tend to be higher in African American men and
women than in other race/ethnicities in the United States, no studies have
adequately addressed the relation of physical activity level and stroke risk in
any race/ethnicity other than non-Hispanic whites.
Question 4: What Is the Relationship
Between Physical Activity and Peripheral Arterial Disease?
Conclusion
No large RCTs have been conducted to investigate exercise training in
peripheral arterial disease (PAD). Little is known regarding exercise dose
response (intensity, duration or frequency) or different modalities (walking,
cycling, resistance training) of exercise to prevent PAD because most of the
studies have followed the same exercise prescription, which has used supervised
treadmill walking at a similar dose. Furthermore, even less is known about how
subpopulations differ in responses to exercise training, such as whether sexes
respond differently or whether an interaction exists between type 2 diabetes
and exercise responsiveness in persons with PAD.
Rationale
Exercise for Primary Prevention of Peripheral Arterial Disease
Only a handful of cross-sectional primary prevention studies have been
performed to relate ankle brachial index (ABI), an indicator of severity of
peripheral lower extremity arterial occlusion, with physical activity (Table G2.A4 [PDF - 99 KB] summarizes these
studies) Activity questionnaires have been used to examine retrospectively the
relationship between physical activity and abnormal ABIs. In the Edinburgh
Artery Study (39), for example, the amount of physical
activity performed between the ages of 35 to 45 years was inversely related to
prevalence of PAD at ages 55 to 74 years, but only in men. Further, this
relation held only for men who had smoked at some time in the past. Gardner and
colleagues (40) observed that the amount of physical
activity was related to ABI measures in those without PAD, suggesting that
regular habitual exercise may be related to the presence of sub-clinical
asymptomatic PAD.
Exercise for Secondary Prevention of Peripheral Arterial Disease
Exercise training is a powerful secondary preventive measure for those
with established PAD (Tables G2.A5 [PDF - 123 KB] and
G2.A6 [PDF - 126 KB] summarize these
studies). Several meta-analyses and review articles summarize this body of
literature (Table G2.A7 [PDF - 120 KB]
summarizes these studies) (41-49). Although these studies
unequivocally demonstrate exercise training to be beneficial for improving
maximal walking ability, many lack necessary criteria such as large sample
sizes, randomized and controlled designs, assessments of sex and dose-response
effects, and differential responses in symptomatic (intermittent claudication)
versus asymptomatic individuals needed to make strong specific clinical
exercise recommendations. Despite these shortcomings, the data demonstrate that
adherence to a structured supervised exercise program is currently regarded as
the most effective treatment for symptomatic PAD. In all of the clinical
studies noted above, the 2 most commonly measured variables used to determine
the effectiveness of a PAD therapy are peak walking time (PWT) and claudication
onset time (COT). It is clear that exercise improves both PWT and COT in
patients with PAD (50-64).
Based on the evaluation of meta-analyses and clinical studies, the
average improvement following exercise training in PWT is near 100%, with COT
improving consistently to an even greater degree (to the magnitude of 130% or
more). Other responsive variables, primarily measured in small studies, are
peak oxygen consumption, walking economy, daily physical activity, 6-minute
walk time, leg blood flow, and quality of life. Interestingly, although some
studies have demonstrated improved leg blood flow and ABI, these indices have
not convincingly been related to functional markers. It appears that improved
oxidative metabolism in the skeletal muscle may explain some of the
improvements in exercise tolerance (50;52). Whether increased growth of small blood vessels
(angiogenesis) and oxidative machinery (enzymes, mitochondria) are responsible
for the improved muscle metabolism following exercise training is being
explored. Findings also suggest that improvements can be augmented beyond those
resulting from a traditional 12-week exercise program. As much as an additional
50% improvement in PWT may be achieved with continued therapy to up to 24 weeks
(51). Twelve to 24 weeks of exercise training produced
improvements in free-living accelerometer-derived daily physical activity,
walking economy measured by constant workload oxygen consumption (slow
component of VO2). Although a traditional exercise prescription for
PAD recommends that patients endure a moderate rather than severe level of
claudication pain during training bouts, limited evidence indicates that a
lower exercise intensity than the pain threshold elicits similar results as
exercise above the pain threshold as long as the same dose in minutes is
maintained (63).
The Relationship Between Daily Physical Activity and Peripheral
Arterial Disease
Studies have confirmed that the severity of PAD is related to daily
free-living physical activity. (Table G2.A8 [PDF - 112 KB] summarizes these
studies.) Studies show that, among individuals with PAD, daily physical
activity is reduced approximately 40% compared to matched healthy controls and
that the degree of claudication (as measured by ABI and PWT) is related to
daily physical activity within a PAD population (65;66). These findings have been confirmed using accelerometers
and performance score questionnaires that have related the decrease in daily
physical activity to impairments in the lower extremity. A progressive decline
in leisure-time activities of both moderate and high intensities has been
identified in individuals with PAD (67). The loss of daily
physical activity corresponds with decreasing ABI values and COT. Furthermore,
a relation appears to exist between free-living physical activity and
microcirculation in the calf muscle (66). The natural
progression of PAD has been assessed and determined to be inversely related to
self-reported physical activity as assessed by COT, 6-minute walk test, and
calf blood flow (68). All of these studies demonstrate
that, despite a lack of randomized controlled exercise studies to evaluate the
effect of exercise training on preventing PAD, a lack of exercise contributes
to disease progression, symptom status, and additional inactivity in those who
have PAD.
Although most studies comparing supervised versus home-based programs
conclude that supervised exercise is better, this remains inconclusive. No
study has investigated the effects of an exercise program on asymptomatic
patients with known PAD to determine whether exercise can prevent the onset of
claudication or disease worsening. In addition, little is known about the role
of resistance training, as no definitive trial has directly compared
traditional walking exercise to resistance training in the PAD population.
Question 5: What Is the Relationship
Between Physical Activity and Hypertension?
Conclusion
Both aerobic and progressive resistance exercise yield important
reductions in systolic and diastolic blood pressure (BP) in adult humans,
although the evidence for aerobic exercise is more convincing. Traditional
aerobic training programs of 40 minutes of moderate- to high‑intensity
exercise training 3 to 5 times per week and that involve more than 800
MET-minutes of aerobic exercise per week appear to have reproducible effects on
BP reduction.
Rationale
In this section we update the evidence of the effects of chronic
exercise on resting BP in adults generated since the release of the Surgeon
General's Report on Physical Activity and Health (1).
This update is limited to a review of previous meta-analyses that met the
following criteria: (1) RCTs only, (2) meta-analyses published in the English
language between January 1, 1995 and September 30, 2007, (3) adults aged 18
years and older, (4) aerobic or progressive resistance training as the
only intervention, (5) non-intervention control group, (6) resting or
ambulatory systolic and diastolic BP as a primary outcome in each
meta-analysis.
Relationship Between Aerobic Exercise and Blood Pressure
Ten meta-analyses dealing with the effects of aerobic exercise on
resting BP in adults have been published since 1996 (69-78). Six of these
meta-analyses were comprehensive (69;72;74-77) and the remaining 4 focused on
either women (71), older adults (73),
overweight and obese subjects (70), or walking as the only
intervention (78). The most recent and inclusive
meta-analysis that included data partitioned according to hypertensive,
prehypertensive, and normotensive adults included a total of 72 studies, 105
exercise groups, and 3,936 men and women with a between-study age range of 21
to 83 years (median age = 47 years) (76). Across all
categories, mean reductions in resting BP ranged from 2 to 5 mmHg (2% to 4%)
for resting systolic BP and 2 to 3 mmHg (2% to 3%) for resting diastolic BP.
Reductions were greater in hypertensive subjects (systolic BP, −6.9 mmHg;
diastolic BP, −4.9 mmHg) than in prehypertensive (systolic BP, −3.1
mmHg; diastolic BP, −1.7 mmHg) and normotensive (systolic BP, −2.4
mmHg; diastolic BP, −1.6 mmHg) subjects. Changes were equivalent to
relative reductions of approximately 5% for both resting systolic and diastolic
BP in hypertensive subjects, 1% (systolic BP) and 2% (diastolic BP) in
prehypertensive subjects, and 2% for both resting systolic and diastolic BP in
normotensive subjects. Significant reductions of 3.3 mmHg (2%) and 3.5 mmHg
(4%) also were observed for daytime ambulatory systolic and diastolic BP with
no significant change in nighttime BP. Changes in ambulatory BP are especially
noteworthy because the assessment of the measure may better predict target
end-organ damage (79). Changes in both resting and
ambulatory BP were independent of changes in body weight (76). Similar changes in resting BP also were found for the
other inclusive meta-analyses (69;72;74-77) as well as meta-analyses that
focused on women (71), older adults (73), overweight and obese subjects (70),
and walking (78).
Dose-Response Relations Between Aerobic Exercise and Blood
Pressure
The vast majority of studies included in the meta-analyses conducted
since the release of the Surgeon General's Report on Physical Activity and
Health (1) have tended to follow traditional
guidelines for the prescription of aerobic exercise in adults as recommended by
the American College of Sports Medicine (5;80;81). For example, for the most
recently published meta-analysis dealing with the effects of aerobic exercise
on resting BP (77), the pooled median length of training
was 16 weeks, with a frequency of 3 days per week. However, the analysis
included studies in which subjects exercised up to 7 days per week, with a
duration of 40 minutes per session and intensity of 65% of maximal heart rate
reserve. No consistent relations were observed between changes in resting
systolic and diastolic BP and the length, frequency, duration, and intensity of
training (77). The most common forms of exercise used in
these RCTs were walking, jogging, and stationary cycling, although other types
of exercise, such as aerobic dance, also were included. Other meta-analyses
also have failed to find a relation between training program characteristics
and changes in resting BP (69-72;74-76;78). In contrast, one
meta-analysis did report larger decreases in resting systolic and diastolic BP
with a greater duration (minutes) of training per session as well as greater
decreases in resting systolic BP with lower training intensities (73).
Relation Between Progressive Resistance Exercise and Blood
Pressure
Since the release of the Surgeon General's Report on Physical
Activity and Health (1), 3 meta-analyses (45;77;82) have been conducted on the
effects of progressive resistance exercise on resting systolic and diastolic
BP. However, as 2 of these included the same data (77;82), this discussion is limited to the one that contained
more complete data on progressive resistance training (82). This meta-analysis included 9 RCTs and 12 exercise
groups comprising 341 men and women aged 20 to 72 years (median age = 69
years). The vast majority of subjects were not hypertensive (baseline resting
systolic/diastolic BP values, 131.6/80.9 mmHg) (82). With
the one static (isometric) training study deleted from the analysis, a
statistically significant reduction of 3.1 mmHg was found for resting diastolic
BP with a trend for a reduction in systolic BP of 3.1 mmHg. Similar and
statistically significant reductions of 2% and 4% also were found for resting
systolic and diastolic BP in an earlier meta-analysis that excluded static
training studies (45).
Progressive Resistance Exercise and Blood Pressure
For the most recent meta-analysis (82) progressive
resistance training took place over a mean duration of 16.4 weeks, 2 to 3 days
per week at 61% of one-repetition maximum. The mean number of exercises was 10
while the number of sets was 2. Omitting the static study, the number of
repetitions ranged from 8 to 25. Ten of the 12 groups (83%) used exercises that
involved both the upper and lower body. Three of the 9 studies in the
meta-analysis used a circuit training protocol, one used a static protocol, and
the remainder used more conventional types of training protocols. No
differences in resting systolic and diastolic BP were found between traditional
and circuit training protocols.
Significance of Exercise-Induced Reductions in Blood Pressure
Although the reductions in BP as a result of aerobic and progressive
resistance training may appear to be small, especially for normotensive and
prehypertensive groups, they are clinically significant. It has been estimated
that as little as a 2 mmHg reduction in population average resting systolic BP
can reduce mortality from coronary heart disease, stroke, and all causes by 4%,
6% and 3%, respectively, while a reduction of 5 mmHg can reduce mortality risk
by 9%, 14%, and 7% (83). The potential numbers of annual
lives saved in the United States as a result of these reductions has been
estimated at 11,800 for a 2 mmHg reduction in resting systolic BP and 27,600
for a 5 mmHg reduction (83).
Question 6. What Is the Relationship
Between Physical Activity and Atherogenic Dyslipidemia?
Conclusion
For the purposes of this review, atherogenic dyslipidemia is defined as
the presence of abnormally low serum concentrations of high-density lipoprotein
(HDL) cholesterol and elevated concentrations of high triglycerides (TG) and
small, dense low-density lipoprotein (LDL) cholesterol. The response of serum
lipoproteins to changes in habitual physical activity have been well studied.
In general, both HDL cholesterol and serum TG reproducibly and favorably
respond to changes in habitual physical activity, with increases in HDL
cholesterol and decreases in serum TG, mostly related to the volume of exercise
training and responding with threshold volumes in the range of 7 to 15 miles
per week of regular exercise (equating to an approximate 600 to 800
MET-minutes). Some evidence indicates that women are less responsive than men
to change in habitual exercise, perhaps due to the observation that those with
the largest baseline abnormalities (lower HDL and higher TG) gain the greatest
benefit and men on average have lower HDL and higher TG than do women. However,
when weekly volume or energy expenditure is controlled for men and women, the
sex-related differences seem to be mitigated. Some inconsistent evidence
suggests that LDL cholesterol may respond favorably to exercise training under
some conditions; when it does, it is at the same volume thresholds as observed
for HDL and TG. Finally, more recent studies have observed that fractionated
serum lipoproteins respond favorably to aerobic exercise training in a
dose-response fashion that is related to the weekly volume of exercise.
Rationale
A large volume of information is available on the exercise
responsiveness of serum lipoproteins and dose-response effects, much of it
accumulated before the 1996 Surgeon General's Report. For this review
of the literature regarding the relation between habitual exercise and serum
lipoproteins, we have relied mostly upon meta-analyses and reviews assembled
since 1996. The relevant information is well summarized in 2 relatively recent
reviews from Durstine and colleagues (84) and Leon and
Sanchez (85). Most of the information before 1996 is based
upon responses in total cholesterol and fractionated lipids (i.e., HDL, LDL,
and TG). Recently some new information has emerged on the response of
lipoprotein sub-fractions to exercise training (86;87).
The response of HDL cholesterol to exercise training traditionally has
been well studied. As illustrated in a recent meta-analysis of exercise-induced
effects on HDL cholesterol (88), the volume of exercise
exposure is the primary determinant of exercise-induced modulations of HDL at a
EE threshold of 10 to 12 MET-hours per week. Thus, although some evidence
exists that exercise intensity may be related to HDL increasing as a result of
exercise, this effect becomes insignificant when total exercise volume is
controlled.
Women seem to be more resistant to modulation of TG through exercise
interventions than are men, although this is not a consistent finding. In some
studies, TG appear to be more responsive to lower volumes of exercise training
than the volumes to which HDL is responsive, mimicking the responses in insulin
action to which TG levels are closely tied (87). However,
the sum of the literature seems to indicate that triglycerides are
consistently, reproducibly and robustly responsive to exercise training of
volumes that are comparable to those that induce changes in HDL (10 to 20
MET-hours per week) and that moderate-intensity exercise results in more
sustained changes in TG than does high-intensity exercise once the training
stimulus is removed (87).
LDL cholesterol is generally found not to be responsive to exercise
training interventions. However, in the few circumstances when LDL has been
observed to be modulated by exercise, it requires approximately 12 MET-hours
per week of exercise to favorably influence LDL. Recently, studies of the
modulation of fractionated lipoproteins with exercise training have shown that
HDL, TG, and LDL size and number are favorably modulated in a dose-response
fashion to exercise training related to training volume and that 800
MET-minutes of exercise per week was required for an effect different from that
of a sedentary control group, whose LDL parameters tended to worsen over time
in the absence of other lifestyle changes (87). More work
is needed to understand the magnitude, consistency, and mechanism of these
effects.
Question 7: What Is the Relationship
Between Physical Activity and Vascular Health?
Conclusion
Habitual aerobic exercise appears to induce favorable responses in
measures of vascular health. Exercise training initially increases brachial
artery flow-mediated dilation (BAFMD—a measure of endothelial vascular
health) with later normalization of BAFMD as vessels become structurally
larger. Habitual aerobic exercise appears to slow the progression of
age-related central arterial stiffening in healthy subjects. Increased levels
of habitual physical activity are associated with slowed progression of carotid
intimal medial thickening (CIMT) in cross-sectional and prospective cohort
studies. No significant dose-response data are available for any of these
measures.
Rationale
This section summarizes the effects of chronic aerobic exercise training
on measures of vascular health, including BAFMD, arterial stiffness, and CIMT.
Brachial Artery Flow-Mediated Dilation
Dysfunction of endothelial cells is an early event in the process of
atherosclerosis (89), and is associated with risk factors
for cardiovascular disease (90-92). These relations have
led to the use of endothelium-mediated vascular responsiveness as a surrogate
biomarker of vascular health. Brachial artery flow-mediated dilation, a
non-invasive measure of endothelial function, has been shown to correlate with
measures of coronary artery function (93;94) and independently predicts cardiovascular events in
patients with established disease (95-100). Due to its
non-invasive nature and relative ease of use, BAFMD has become increasingly
used as a research tool to monitor the efficacy of interventions on vascular
health.
This section provides a review of the current published data on the
effects of exercise training as the primary intervention on BAFMD. A total of
300 abstracts were initially retrieved and reduced to 22 separate intervention
groups (57;99;101-119). All data included were from RCTs with a minimum
exercise training intervention of at least 1 week and BAFMD data reported at
both pre- and post-exercise training. Studies include data from both apparently
healthy subjects as well as those with chronic heart failure, obesity,
dyslipidemia, coronary heart disease, metabolic syndrome, uncomplicated
myocardial infarction, heart transplant, and diabetes.
The results from this literature review provide convincing evidence that
exercise training produces significant changes in the vascular health biomarker
BAFMD. Figure G2-2 graphically illustrates the
effect sizes seen in all intervention groups. Fifteen of the 22 intervention
groups included in the analysis showed a statistically significant improvement
in BAFMD (confidence intervals did not contain zero) in response to exercise
training. Of the remaining 7 studies, only one produced a negative effect size
(107).
Figure G2.2. Effect Sizes Seen in
Interventions in Which BAFMD Is Used as a Vascular Health Biomarker
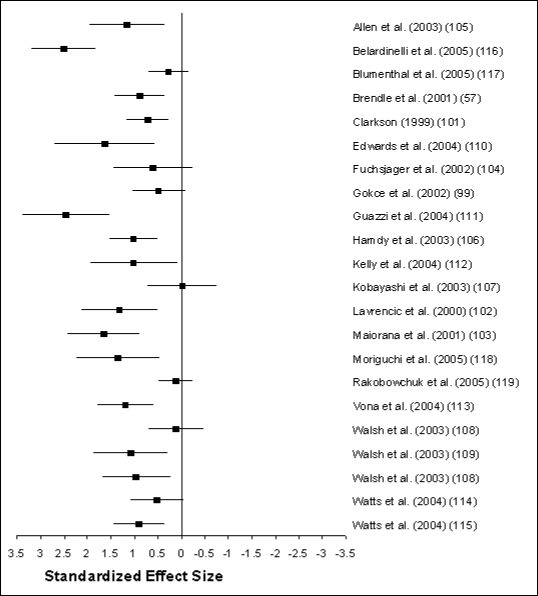
Figure developed from Clark O; Djulbegovic B. Forest
plots in Excel software (data sheet). 2001. Available at
www.evidencias.com
 . .
Figure G2.2. Data Points
Upper Limit of the
Confidance
Interval |
Lower Limit of the
Confidance
Interval |
Point
Estimate |
Studies |
| 1.96 |
0.36 |
1.16 |
Allen et al. (2003) (105) |
| 3.18 |
1.82 |
2.5 |
Belardinelli et al. (2005) (116) |
| 0.69 |
-0.14 |
0.27 |
Blumenthal et al. (2005) (117) |
| 1.42 |
0.35 |
0.88 |
Brendle et al. (2001) (57) |
| 1.15 |
0.27 |
0.71 |
Clarkson (1999) (101) |
| 2.7 |
0.56 |
1.63 |
Edwards et al. (2004) (110) |
| 1.44 |
-0.25 |
0.6 |
Fuchsjager et al. (2002) (104) |
| 1.04 |
-0.09 |
0.47 |
Gokce et al. (2002) (99) |
| 3.38 |
1.52 |
2.45 |
Guazzi et al. (2004) (111) |
| 1.52 |
0.52 |
1.02 |
Hamdy et al. (2003) (106) |
| 1.94 |
0.08 |
1.01 |
Kelly et al. (2004) (112) |
| 0.72 |
-0.76 |
-0.02 |
Kobayashi et al. (2003) (107) |
| 2.12 |
0.51 |
1.31 |
Lavrencic et al. (2000) (102) |
| 2.42 |
0.88 |
1.65 |
Maiorana et al. (2001) (103) |
| 2.23 |
0.46 |
1.35 |
Moriguchi et al. (2005) (118) |
| 0.48 |
-0.26 |
0.11 |
Rakobowchuk et al. (2005) (119) |
| 1.78 |
0.6 |
1.19 |
Vona et al. (2004) (113) |
| 0.71 |
-0.47 |
0.12 |
Walsh et al. (2003) (108) |
| 1.86 |
0.28 |
1.07 |
Walsh et al. (2003) (109) |
| 1.68 |
0.23 |
0.96 |
Walsh et al. (2003) (108) |
| 1.08 |
-0.04 |
0.52 |
Watts et al. (2004) (114) |
| 1.43 |
0.36 |
0.89 |
Watts et al. (2004) (115) |
Several factors modulate the magnitude of exercise-induced responses in
BAFMD. The most influential of these appears to be health status before the
exercise training intervention. That is, the magnitude of BAFMD improvement
following training is, in part, a function of the initial or pre-training
level. Subjects with cardiovascular disease exhibit greater improvements in
BAFMD following exercise training but start with a lower pre-training BAFMD.
Apparently healthy subjects also show improvement in BAFMD but not to the same
degree as those with cardiovascular disease. The data on apparently healthy
subjects come from only 3 studies and so should be interpreted with some
caution (101;105;119). Interestingly, age does not appear to influence the
magnitude of BAFMD response, suggesting it is modifiable in both young and
old.
A second important moderator of response is the type of exercise
performed. Changes in BAFMD were noted in most studies regardless of modality.
However, the greatest affect was seen in those studies using aerobic exercise
alone (14 studies) or in combination with resistance training (6 studies). The
evidence for resistance training alone (2 studies) are less convincing,
suggesting resistance training by itself may not be as effective in improving
BAFMD.
A third moderator of response is length of the training period. Shorter
periods of exercise training (8 weeks or less) result in larger changes in
BAFMD compared to longer periods of training (more than 8 weeks). This implies
that changes in BAFMD occur rapidly after initiating training but may diminish
with time. This is consistent with the theory that vascular responses to
aerobic exercise training consist of a series of stress-response-adaptation
responses, where exercise is the stressor, increased BAFMD is the initial
response, and structural vessel enlargement is the eventual adaptation (with
subsequent normalization of the BAFMD response) (120).
As noted, the modality-specific (aerobic versus resistance) exercise
training responses requires further study. Similarly, the dose-response effects
of aerobic exercise training are notably understudied.
Carotid Intimal-Medial Thickening
Most studies on this outcome are prospective or case-control
observational studies. Relatively few studies have examined the effects of
exercise training on CIMT or progression. From 7 available cross-sectional
studies, 4 report lower CIMT in subjects with higher physical activity levels
(121;122) or higher VO2peak
(123;124). The remaining 3
studies found no difference between active and sedentary groups (125-127). The discrepancies between these study results
could be related to differences in age and health of participants, methods of
activity measurement and reporting, concomitant lifestyle changes, length of
measurement, and differences in the techniques used to quantify CIMT.
The results from interventional studies make it even more difficult to
draw definitive conclusions. From 8 available studies (127-134), only 3 appear to have reported the effects of
exercise training isolated from other concurrent treatments (127;130;132) and
none of these showed significant effects (135).
Unfortunately, 2 of these studies were underpowered to detect CIMT progression,
and the third was a pharmaceutical trial where exercise served as a control and
no changes were observed after 4 years (132).
A lack of adequately powered exercise interventional studies is
understandable if one considers the small size of the pooled annual rates of
changes in CIMT progression that occur among control groups from randomized
placebo-controlled trials. For studies using multiple IMT measures from several
interrogation angles and carotid segments, the mean maximum progression rate
was 0.0176 millimeters per year with a median SD of 0.05 (136). Given that sample size calculations rely heavily on
rates of change, precision of the measurement, and projected effectiveness of
the intervention, the subject numbers required and length of exercise training
assessment period would have to be much longer than is traditional in such
studies. For example, for a 30% treatment effect, average change in mean-max
CIMT of 0.0352 ± 0.05 millimeters over 2 years, and using as two-tailed
alpha, one would need 468 subjects in each arm of the trial to have 90%
power.
Arterial Stiffness
Central arterial stiffening occurs with aging (137) but is often both a consequence and mechanism of
atherosclerotic vasculopathy. The investigation of arterial stiffness has
increased in recent years due to the development of noninvasive assessment
techniques (138-140). However, there appears to be a lack
of consensus regarding the most accurate and reliable method to measure
arterial stiffness, complicating the determination of the efficacy of exercise
training responses. The most frequently reported assessment methodologies are
pulse wave velocity, pulse wave analysis, and distensibility/compliance (change
in diameter/change in pressure).
Using these outcome measures, habitual aerobic exercise appears to slow
the progression of age-related central arterial stiffening in healthy subjects
as reported in several cross-sectional studies (137;141-143). Furthermore, 4 training intervention studies
report significant improvements in measures of central stiffness across sex and
age ranges (141-144). Interestingly, the benefits in
central elastic arteries were not replicated in peripheral muscular arteries
(142;145), suggesting that
training-specific responses, or different mechanisms are active in different
arterial beds.
The benefits of short-term aerobic exercise training on central
stiffness in patient populations are less clear. One study reported a decrease
in aortic pulse wave reflection in chronic hemodialysis patients following 3
months of aerobic training. This measure returned to pre-training levels 1
month after training ceased (detraining) (146). Another
showed favorable changes in coronary artery disease subjects within 12 weeks
(110), although other studies reported no effects of
training in hypertensive (147;148)
or diabetic (149) subjects.
Finally, the effects of resistance training on central arterial
stiffness are conflicting. Two cross-sectional studies report a decrease in
central but not peripheral arterial compliance in comparison to sedentary
controls (143;150). In contrast, of
4 available case controlled interventional studies, 2 report increases in
measures of central arterial stiffness (151;152) and 2 report no significant effect (153;154). These differences appear to
be related to intensity, with higher training intensities eliciting higher
central stiffness values. Clearly, large-scale prospective studies are
warranted to clarify these discrepancies and to further elucidate the possible
mechanisms involved in the observed changes.
Question 8: What Is the Relationship
Between Physical Activity and Cardiorespiratory Fitness?
Conclusion
Cardiorespiratory fitness is a sensitive and useful measure of changes
in response to physical activity. It demonstrates dose-response relations with
overall exercise volume and also with each of the various components of
exercise volume (intensity, frequency, duration, and longevity). It appears
that one can acquire improvements in cardiorespiratory fitness in bouts as
small as 10 minutes each, while holding volume constant. It is unclear whether
there is a relation between the duration of exercise bouts and fitness
responses, when total volume is held constant, especially for vigorous
intensity exercise. Changes in fitness during exercise interventions correspond
with changes in cardiovascular risk, but do not always correspond with changes
in cardiovascular risk factors.
Rationale
Cardiorespiratory fitness, as measured by a number of relatively simple
and inexpensive clinical maneuvers, provides strong and independent prognostic
information about overall morbidity and mortality. This relationship extends to
men, women, and adolescents. It is valid in apparently healthy individuals; in
patients with a broad range of maladies, including several types of cancer and
cardiovascular disease; and in at-risk individuals with type 2 diabetes,
metabolic syndrome, and hypertension (1;155;156). Fitness is also a marker for
functional capacity and ability to perform activities of daily living,
especially in older individuals. Finally, it is used as an outcome measure of
adherence and physical activity exposure in intervention studies. For example,
men who improve their fitness (as assessed by exercise duration) improve their
cardiovascular risk. In one report, long-term cardiovascular risk decreased by
8% for every minute increase in exercise capacity (157).
Due to the correlation between fitness and health status, the responsiveness to
changes in physical activity, and its usefulness as a marker of physical
activity levels, cardiorespiratory fitness is an important health outcome
measure in and of itself and is often quoted as an outcome in health-related
physical activity studies. That said, favorable changes in fitness do not
always correspond to change in health outcomes in response to exercise
recommendations (158). The data for this section was
acquired from an independent literature search of the PubMed database using
"cardiorespiratory fitness" as a search term and identifying meta-analyses and
review articles from both the 1996 date to the present and before 1996.
Cardiovascular fitness, as measured by any one of a number of parameters
associated with exercise testing (peak VO2, resting heart rate,
lactate level or heart rate at submaximal exercise level, VO2 at
ventilatory threshold, time to exhaustion, and others) is extremely sensitive
to changes in physical activity levels and habitual exercise. This is often
referred to as a training effect. The training effect shows a strong
dose-response relation to changes in exercise pattern of various types. Changes
in fitness are dependent upon the frequency, duration, and intensity of
exercise bouts and are also dependent upon the longevity of the exercise
training program or intervention (reviewed in 3). The
product of exercise frequency, bout duration, and intensity over time is often
referred to as exercise volume and is proportional to exercise-related energy
expenditure. A rich literature exists about the specific relation between the
characteristics of exercise exposure and changes in cardiorespiratory fitness
in the short and long term (3;4;159;160) in
individuals of all ages, including older men and women (161-163). An example of the changes in cardiorespiratory
fitness with training programs of various intensities and amounts (volumes) is
demonstrated in Figure G2-3 (164). As shown, effects on cardiorespiratory fitness of
exercise occur both with increasing intensity at the same volume, and
increasing volume at the same intensity. The groups are clearly distinguishable
by differences in group mean differences over time. However, it is also clear
that baseline fitness and the ability to respond to an exercise intervention
have numerous inputs other than physical activity pattern, one of which is
genetic (165). Using the same study population as in the
previous figure, when individual responses to training stimuli are displayed as
individual data points ordered by magnitude of response, it is apparent that
the identical stimulus can result in a broad range of responses, from negative
to positive (Figure G2-4). That is, even a strong
stimulus (high-volume, high-intensity exercise) can result in no significant
improvement or even deterioration in cardiorespiratory fitness in some
individuals, while resulting in a large magnitude of change, much larger than
the group mean, in others. This observation has implications for the
construction of physical activity recommendations, depending on whether the
goal is to significantly move the population mean (e.g., 50%) or to affect a
significant response in the vast majority of individuals, in which case a more
robust exposure may be required.
As previously noted, changes in cardiorespiratory fitness in response to
an exercise intervention depend upon a number of parameters, including the
characteristics of the exercise stimulus, baseline fitness, sex, age, body mass
index, and others. In addition, health benefits that accrue with an exercise
program are often, but not always, correlated with changes in fitness (160). Two recent studies illustrate the dose-response
relations between exercise exposure and fitness, as well as to several seminal
cardiovascular risk markers. The results from the DREW (166) and STRRIDE (158;164) studies are summarized in Table G2‑2. Cardiorespiratory fitness
(peak VO2) can be expressed in absolute terms (liters of oxygen
consumption per minute) or relative to body mass (ml/kg/min). Exercise exposure
in volume can be expressed as energy expenditure or as multiples of resting
oxygen consumption (METs), times duration (e.g., MET-hour), where 1 MET
approximately equals 3.5 ml/kg/min).
Figure G2.3. Changes in Peak
VO2 by Exercise Group

Figure G2.4. Changes in Peak
VO2 by Exercise Group and Ordered by Change
Figure G2.4. Data Points
Control Peak
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 26.88 |
21.22 |
0.79 |
-0.21 |
| 28.7 |
22.67 |
0.79 |
-0.21 |
| 24.55 |
20.13 |
0.82 |
-0.18 |
| 27.9 |
23.62 |
0.85 |
-0.15 |
| 23.8 |
20.2 |
0.85 |
-0.15 |
| 31.5 |
26.9 |
0.85 |
-0.15 |
| 29.55 |
25.4 |
0.86 |
-0.14 |
| 25.71 |
22.2 |
0.86 |
-0.14 |
| 35 |
30.4 |
0.87 |
-0.13 |
| 39.7 |
34.6 |
0.87 |
-0.13 |
| 29.4 |
26.6 |
0.90 |
-0.10 |
| 29.27 |
26.52 |
0.91 |
-0.09 |
| 25.3 |
23 |
0.91 |
-0.09 |
| 30.85 |
28.08 |
0.91 |
-0.09 |
| 20.4 |
18.6 |
0.91 |
-0.09 |
| 20.5 |
19.1 |
0.93 |
-0.07 |
| 42.4 |
39.6 |
0.93 |
-0.07 |
| 21.9 |
20.5 |
0.94 |
-0.06 |
| 32.39 |
30.44 |
0.94 |
-0.06 |
| 35.3 |
33.3 |
0.94 |
-0.06 |
| 28.1 |
26.6 |
0.95 |
-0.05 |
| 29.3 |
27.8 |
0.95 |
-0.05 |
| 37.77 |
35.9 |
0.95 |
-0.05 |
| 25.1 |
23.97 |
0.95 |
-0.05 |
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 27.6 |
26.5 |
0.96 |
-0.04 |
| 27.2 |
26.2 |
0.96 |
-0.04 |
| 21.4 |
20.7 |
0.97 |
-0.03 |
| 15.17 |
14.73 |
0.97 |
-0.03 |
| 36 |
35 |
0.97 |
-0.03 |
| 22.8 |
22.2 |
0.97 |
-0.03 |
| 30.69 |
30 |
0.98 |
-0.02 |
| 33.7 |
33.1 |
0.98 |
-0.02 |
| 28.5 |
28 |
0.98 |
-0.02 |
| 26.8 |
26.4 |
0.99 |
-0.01 |
| 33.5 |
33.4 |
1.00 |
0.00 |
| 34.9 |
34.8 |
1.00 |
0.00 |
| 21.6 |
21.6 |
1.00 |
0.00 |
| 26.5 |
26.5 |
1.00 |
0.00 |
| 27 |
27 |
1.00 |
0.00 |
| 26 |
26.1 |
1.00 |
0.00 |
| 37.3 |
37.8 |
1.01 |
0.01 |
| 21 |
21.5 |
1.02 |
0.02 |
| 29.4 |
30.1 |
1.02 |
0.02 |
| 18.7 |
19.4 |
1.04 |
0.04 |
| 17.9 |
18.7 |
1.04 |
0.04 |
| 34.98 |
36.57 |
1.05 |
0.05 |
| 21.3 |
22.3 |
1.05 |
0.05 |
| 21.3 |
22.3 |
1.05 |
0.05 |
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 24.4 |
25.8 |
1.06 |
0.06 |
| 23.1 |
24.7 |
1.07 |
0.07 |
| 30.4 |
32.7 |
1.08 |
0.08 |
| 17.57 |
19.23 |
1.09 |
0.09 |
| 25.3 |
28 |
1.11 |
0.11 |
| 21 |
23.9 |
1.14 |
0.14 |
| 24.1 |
27.6 |
1.15 |
0.15 |
| 25.8 |
29.9 |
1.16 |
0.16 |
| 19.81 |
23.27 |
1.17 |
0.17 |
| 18.1 |
21.7 |
1.20 |
0.20 |
| 22.4 |
26.9 |
1.20 |
0.20 |
| 27.9 |
|
|
|
| 21.4 |
|
|
|
| 33.8 |
|
|
|
| 22.5 |
|
|
|
| 22.7 |
|
|
|
| 23.5 |
|
|
|
| 28.6 |
|
|
|
| 19.1 |
|
|
|
| 33.6 |
|
|
|
| |
32.75 |
|
|
| 28.7 |
|
|
|
| 33.43 |
|
|
|
| 19.2 |
|
|
|
Mild Peak
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 23.9667 |
20.4667 |
0.85 |
-0.15 |
| 24.3333 |
21.7667 |
0.89 |
-0.11 |
| 30.6 |
29.2 |
0.95 |
-0.05 |
| 43 |
41.2 |
0.96 |
-0.04 |
| 20.5667 |
19.8667 |
0.97 |
-0.03 |
| 33.2966 |
32.1774 |
0.97 |
-0.03 |
| 29.6 |
29.1 |
0.98 |
-0.02 |
| 26.3 |
25.9 |
0.98 |
-0.02 |
| 22.1 |
21.8 |
0.99 |
-0.01 |
| 32.5 |
32.1 |
0.99 |
-0.01 |
| 21.3067 |
21.1 |
0.99 |
-0.01 |
| 26.4 |
26.3 |
1.00 |
0.00 |
| 23.8 |
23.9 |
1.00 |
0.00 |
| 18.1 |
18.2 |
1.01 |
0.01 |
| 24.8 |
25.1 |
1.01 |
0.01 |
| 27.6 |
28.1 |
1.02 |
0.02 |
| 24 |
24.5 |
1.02 |
0.02 |
| 42.5 |
43.6 |
1.03 |
0.03 |
| 22.2 |
22.8 |
1.03 |
0.03 |
| 31.3 |
32.2 |
1.03 |
0.03 |
| 24.815 |
25.5333 |
1.03 |
0.03 |
| 21.7 |
22.5 |
1.04 |
0.04 |
| 33.5 |
34.8 |
1.04 |
0.04 |
| 27.6 |
28.7 |
1.04 |
0.04 |
| 23.8833 |
24.8667 |
1.04 |
0.04 |
| 21.3 |
22.2 |
1.04 |
0.04 |
| 30.6 |
32.1 |
1.05 |
0.05 |
| 35.7 |
37.5 |
1.05 |
0.05 |
| 24.6 |
25.9 |
1.05 |
0.05 |
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 29.3 |
30.9 |
1.05 |
0.05 |
| 21.2 |
22.4 |
1.06 |
0.06 |
| 26.5 |
28.1 |
1.06 |
0.06 |
| 26.1 |
27.8 |
1.07 |
0.07 |
| 35 |
37.3 |
1.07 |
0.07 |
| 28.5 |
30.4 |
1.07 |
0.07 |
| 21.5907 |
23.0738 |
1.07 |
0.07 |
| 22.5 |
24.1 |
1.07 |
0.07 |
| 30.3 |
32.5 |
1.07 |
0.07 |
| 24.6 |
26.4 |
1.07 |
0.07 |
| 33.4 |
36.2 |
1.08 |
0.08 |
| 38.4 |
41.7 |
1.09 |
0.09 |
| 26.4373 |
28.7136 |
1.09 |
0.09 |
| 21.9033 |
23.9333 |
1.09 |
0.09 |
| 18 |
19.8 |
1.10 |
0.10 |
| 29.4755 |
32.803 |
1.11 |
0.11 |
| 31.4 |
35.1 |
1.12 |
0.12 |
| 19.5 |
21.8 |
1.12 |
0.12 |
| 24.1 |
27.2 |
1.13 |
0.13 |
| 17.84 |
20.3333 |
1.14 |
0.14 |
| 33.2833 |
38 |
1.14 |
0.14 |
| 26.5213 |
30.2995 |
1.14 |
0.14 |
| 20.1333 |
23.2 |
1.15 |
0.15 |
| 39.1 |
45.8 |
1.17 |
0.17 |
| 21.5 |
26.1 |
1.21 |
0.21 |
| 30 |
36.9 |
1.23 |
0.23 |
| 19.3667 |
24.8 |
1.28 |
0.28 |
| 24.6 |
32.4 |
1.32 |
0.32 |
| 22.023 |
29.503 |
1.34 |
0.34 |
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 22.8 |
30.9 |
1.36 |
0.36 |
| 17.7 |
|
|
|
| 19.7 |
|
|
|
| 24 |
|
|
|
| 29.8 |
|
|
|
| 21.3 |
|
|
|
| 26.9 |
|
|
|
| 22.3 |
|
|
|
| 20.2 |
|
|
|
| 24.3 |
|
|
|
| 27.9 |
|
|
|
| 26 |
|
|
|
| 28 |
|
|
|
| 20.8 |
|
|
|
| 32.1 |
|
|
|
| 27.3 |
|
|
|
| 26.6 |
|
|
|
| 26.3 |
|
|
|
| 20.5 |
|
|
|
| 22 |
|
|
|
| 27.2 |
|
|
|
| 22.4 |
|
|
|
| 23.52 |
|
|
|
| 17.94 |
|
|
|
| 27.24 |
|
|
|
| 27.89 |
|
|
|
| 20.22 |
|
|
|
| 33.6 |
|
|
|
| 27.33 |
|
|
|
| 22.35 |
|
|
|
Moderate Change Peak
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 28.3712 |
23.6123 |
0.83 |
-0.17 |
| 29.3333 |
28.6 |
0.98 |
-0.02 |
| 23.8 |
23.4 |
0.98 |
-0.02 |
| 26.8 |
26.7 |
1.00 |
0.00 |
| 35.7 |
35.6 |
1.00 |
0.00 |
| 21.5 |
21.7 |
1.01 |
0.01 |
| 26.7667 |
27.0333 |
1.01 |
0.01 |
| 37.2 |
37.8 |
1.02 |
0.02 |
| 36.3 |
36.9 |
1.02 |
0.02 |
| 39.3 |
40 |
1.02 |
0.02 |
| 39.7 |
40.8 |
1.03 |
0.03 |
| 32.4 |
33.3 |
1.03 |
0.03 |
| 35.1 |
36.4 |
1.04 |
0.04 |
| 20.7 |
21.5 |
1.04 |
0.04 |
| 25 |
26 |
1.04 |
0.04 |
| 33.188 |
34.5905 |
1.04 |
0.04 |
| 27 |
28.2 |
1.04 |
0.04 |
| 27.7 |
29 |
1.05 |
0.05 |
| 33.3 |
35 |
1.05 |
0.05 |
| 36.4 |
38.3 |
1.05 |
0.05 |
| 32.5 |
34.3 |
1.06 |
0.06 |
| 31.4333 |
33.2333 |
1.06 |
0.06 |
| 39.6 |
42 |
1.06 |
0.06 |
| 35.1 |
37.3 |
1.06 |
0.06 |
| 34.4 |
36.7 |
1.07 |
0.07 |
| 34.4 |
36.8 |
1.07 |
0.07 |
| 31.7 |
34 |
1.07 |
0.07 |
| 24.4 |
26.2 |
1.07 |
0.07 |
| 38.3 |
41.3 |
1.08 |
0.08 |
| 20.7 |
22.4 |
1.08 |
0.08 |
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 35.5 |
38.5 |
1.08 |
0.08 |
| 24 |
26.2 |
1.09 |
0.09 |
| 23.7 |
25.9 |
1.09 |
0.09 |
| 25.0233 |
27.5 |
1.10 |
0.10 |
| 26.1 |
28.8 |
1.10 |
0.10 |
| 25.4 |
28.1 |
1.11 |
0.11 |
| 33.1 |
36.9 |
1.11 |
0.11 |
| 31.2 |
35 |
1.12 |
0.12 |
| 28.6 |
32.1 |
1.12 |
0.12 |
| 30.2 |
34.2 |
1.13 |
0.13 |
| 34.0955 |
38.633 |
1.13 |
0.13 |
| 23.1 |
26.2 |
1.13 |
0.13 |
| 19.3 |
21.9 |
1.13 |
0.13 |
| 37.1 |
42.1 |
1.13 |
0.13 |
| 24.4 |
28.2 |
1.16 |
0.16 |
| 32.8 |
38 |
1.16 |
0.16 |
| 22.1533 |
25.7333 |
1.16 |
0.16 |
| 36.5 |
42.5 |
1.16 |
0.16 |
| 34 |
39.7 |
1.17 |
0.17 |
| 26.2 |
30.7 |
1.17 |
0.17 |
| 24 |
28.6 |
1.19 |
0.19 |
| 20.2 |
24.1 |
1.19 |
0.19 |
| 23.8 |
28.7 |
1.21 |
0.21 |
| 25.378 |
30.603 |
1.21 |
0.21 |
| 34.9 |
42.3 |
1.21 |
0.21 |
| 17.5 |
21.3 |
1.22 |
0.22 |
| 26.4 |
32.2 |
1.22 |
0.22 |
| 21.3 |
26.1 |
1.23 |
0.23 |
| 34.8 |
42.7 |
1.23 |
0.23 |
| 28 |
35.5 |
1.27 |
0.27 |
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 31.9 |
41 |
1.29 |
0.29 |
| 30.4 |
39.1 |
1.29 |
0.29 |
| 27.2 |
35.1 |
1.29 |
0.29 |
| 35.9016 |
46.5667 |
1.30 |
0.30 |
| 25.8 |
33.5 |
1.30 |
0.30 |
| 19.64 |
27.1667 |
1.38 |
0.38 |
| 17.8155 |
31.978 |
1.79 |
|
| 21 |
|
|
|
| 23.1 |
|
|
|
| 22.8 |
|
|
|
| 20.5 |
|
|
|
| 19.3 |
|
|
|
| 22.9 |
|
|
|
| 31.3 |
|
|
|
| 28.4 |
|
|
|
| 23.9 |
|
|
|
| 22.7 |
|
|
|
| 17.3 |
|
|
|
| 38.4 |
|
|
|
| 37.5 |
|
|
|
| 41.8 |
|
|
|
| 37 |
|
|
|
| 23 |
|
|
|
| 26.1 |
|
|
|
| 39.9 |
|
|
|
| 33.1 |
|
|
|
| 24.3 |
|
|
|
| 22.3 |
|
|
|
| 26 |
|
|
|
| 21.9 |
|
|
|
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 24.7 |
|
|
|
| 35.4 |
|
|
|
| 24.3 |
|
|
|
| 32.9 |
|
|
|
| 24.1 |
|
|
|
| 25 |
|
|
|
| 37.8 |
|
|
|
| 37.4 |
|
|
|
| 36.2 |
|
|
|
| 27.2 |
|
|
|
| 26.1 |
|
|
|
| 26.6 |
|
|
|
| 22.3 |
|
|
|
| 20.7 |
|
|
|
| 21 |
|
|
|
| 27.4 |
|
|
|
| 23.9 |
|
|
|
| 21.8 |
|
|
|
| 37.16 |
|
|
|
| 26.8 |
|
|
|
| 23.73 |
|
|
|
| 22.9 |
|
|
|
| 34.9 |
|
|
|
| 15.8 |
|
|
|
| 31.8 |
|
|
|
| 26.04 |
|
|
|
| 12.2 |
|
|
|
| 17.81 |
|
|
|
| 21.56 |
|
|
|
| 18.01 |
|
|
|
High Peak VO2
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 31.2714 |
29.8931 |
0.96 |
-0.04 |
| 24.2333 |
24.24 |
1.00 |
0.00 |
| 24.7 |
25.2 |
1.02 |
0.02 |
| 38.5 |
39.8 |
1.03 |
0.03 |
| 30.69 |
31.7367 |
1.03 |
0.03 |
| 29.6 |
30.8 |
1.04 |
0.04 |
| 36.9 |
39.2 |
1.06 |
0.06 |
| 35.3 |
37.6 |
1.07 |
0.07 |
| 31.6 |
33.7 |
1.07 |
0.07 |
| 34.1965 |
36.8 |
1.08 |
0.08 |
| 32.75 |
35.3333 |
1.08 |
0.08 |
| 26.1 |
28.2 |
1.08 |
0.08 |
| 31.7 |
34.7 |
1.09 |
0.09 |
| 20.2 |
22.4 |
1.11 |
0.11 |
| 25.0167 |
27.8 |
1.11 |
0.11 |
| 21.8615 |
24.3222 |
1.11 |
0.11 |
| 34.3 |
38.2 |
1.11 |
0.11 |
| 29.7643 |
33.1667 |
1.11 |
0.11 |
| 27.3 |
30.5 |
1.12 |
0.12 |
| 24.5333 |
27.5333 |
1.12 |
0.12 |
| 31.5 |
35.6 |
1.13 |
0.13 |
| 23.7 |
26.8 |
1.13 |
0.13 |
| 30.5 |
34.55 |
1.13 |
0.13 |
| 32.4667 |
36.9 |
1.14 |
0.14 |
| 27.3 |
31.2 |
1.14 |
0.14 |
| 28.3 |
32.4 |
1.14 |
0.14 |
| 36.1633 |
41.5333 |
1.15 |
0.15 |
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 25.5 |
29.3 |
1.15 |
0.15 |
| 22.4267 |
25.8 |
1.15 |
0.15 |
| 23.9 |
27.5 |
1.15 |
0.15 |
| 21.8 |
25.1 |
1.15 |
0.15 |
| 19.933 |
23.013 |
1.15 |
0.15 |
| 29.7 |
34.5 |
1.16 |
0.16 |
| 31.5 |
36.6 |
1.16 |
0.16 |
| 26.5 |
30.8 |
1.16 |
0.16 |
| 36.4 |
42.4 |
1.16 |
0.16 |
| 30.1 |
35.2 |
1.17 |
0.17 |
| 21.8 |
25.5 |
1.17 |
0.17 |
| 21.5 |
25.3 |
1.18 |
0.18 |
| 29.9 |
35.3 |
1.18 |
0.18 |
| 19.4 |
23 |
1.19 |
0.19 |
| 34.6 |
41.1 |
1.19 |
0.19 |
| 19.5 |
23.3 |
1.19 |
0.19 |
| 24.8 |
29.7 |
1.20 |
0.20 |
| 32.2 |
38.8 |
1.20 |
0.20 |
| 30.8533 |
37.2 |
1.21 |
0.21 |
| 20.2 |
24.4 |
1.21 |
0.21 |
| 25.3 |
30.7 |
1.21 |
0.21 |
| 24.13 |
29.35 |
1.22 |
0.22 |
| 32.3 |
39.4 |
1.22 |
0.22 |
| 32.3667 |
39.6333 |
1.22 |
0.22 |
| 35.9 |
44.2 |
1.23 |
0.23 |
| 33.3 |
41.1 |
1.23 |
0.23 |
| 30.6 |
37.9 |
1.24 |
0.24 |
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 21.3 |
26.4 |
1.24 |
0.24 |
| 31.4 |
39.2 |
1.25 |
0.25 |
| 20.2355 |
25.323 |
1.25 |
0.25 |
| 30.3 |
38.1 |
1.26 |
0.26 |
| 34.2 |
43.4 |
1.27 |
0.27 |
| 23.5 |
30 |
1.28 |
0.28 |
| 25.158 |
32.6655 |
1.30 |
0.30 |
| 33.3 |
43.3 |
1.30 |
0.30 |
| 26.3 |
35.1 |
1.33 |
0.33 |
| 29.5 |
39.8 |
1.35 |
0.35 |
| 29.9333 |
41.2 |
1.38 |
0.38 |
| 30.4357 |
42.2 |
1.39 |
0.39 |
| 33.2 |
46.2 |
1.39 |
0.39 |
| 22 |
30.7 |
1.40 |
0.40 |
| 28.1 |
40.6 |
1.44 |
0.44 |
| 17.1 |
28.9 |
1.69 |
0.69 |
| 14.5 |
|
|
|
| 17.8 |
|
|
|
| 27.2 |
|
|
|
| 21.3 |
|
|
|
| 18.3 |
|
|
|
| 26.9 |
|
|
|
| 25.6 |
|
|
|
| 23.9 |
|
|
|
| 26.3 |
|
|
|
| 32.7 |
|
|
|
| 27.8 |
|
|
|
| RVO2_1 |
RVO2_2 |
Diff 2-1 |
1-DX |
| 35 |
|
|
|
| 19.1 |
|
|
|
| 26.5 |
|
|
|
| 16.8 |
|
|
|
| 30.6 |
|
|
|
| 37.3 |
|
|
|
| 18.8 |
|
|
|
| 30.9 |
|
|
|
| 30.5 |
|
|
|
| 27.9 |
|
|
|
| 27.3 |
|
|
|
| 21.6 |
|
|
|
| 20.9 |
|
|
|
| 23.9 |
|
|
|
| 23.7 |
|
|
|
| 38.63 |
|
|
|
| 25.33 |
|
|
|
| 21.34 |
|
|
|
| 27.12 |
|
|
|
| 13.95 |
|
|
|
| 23.39 |
|
|
|
| 26 |
|
|
|
| 27.16 |
|
|
|
| 26.89 |
|
|
|
| 18.79 |
|
|
|
| 22.6667 |
|
|
|
Table G2.2. Table of Baseline
Characteristics, Exercise Prescriptions, Training Programs, and Outcome
Measures in Two Randomized Controlled Aerobic Exercise Training Studies
Women: DREW (N~120) *
| Group Prescriptions Training Volume
(kcal/kg/wk) |
Group Prescriptions Training Intensity
(Percent Peak VO2) |
Baseline
Peak
VO2(mL/kg/min) |
Training
Program Training
Prescription
(MET-hr/wk) |
Training
ProgramTraining
Prescription
(MET-min/wk) |
Training
Program Training
METs |
Training
Program Training
Minutes
per
Week |
Change
in Peak
VO2 |
Outcomes Change in
Relative
Peak VO2
(mL/kg/min) |
Outcomes Change in
Peak
VO2
(METs) |
Outcomes Change in
Body
Mass
Index |
Outcomes Change in
Waist
Circumference |
Outcomes Change in
Blood
Pressure |
Outcomes Change
in Blood
Blood
Lipids |
Outcomes Change in
FBG/ISI |
| 4.0 |
50% |
15.5 |
3.8 |
229 |
2.2 |
72 |
4.5% |
0.70 |
0.20 |
NS |
Decrease |
NS |
NS |
NS |
| 8.0 |
50% |
14.9 |
7.6 |
457 |
2.2 |
136 |
7.0% |
1.04 |
0.30 |
NS |
Decrease |
NS |
NS |
NS |
| 12.0 |
50% |
16.0 |
11.4 |
685 |
2.3 |
192 |
8.5% |
1.36 |
0.39 |
NS |
Decrease |
Decr. SBP |
NS |
NS |
Women: STRRIDE (N~30) †
| Group Prescriptions Training Volume
(kcal/kg/wk) |
Group Prescriptions Training Intensity
(Percent Peak VO2) |
Baseline
Peak
VO2(mL/kg/min) |
Training
Program Training
Prescription
(MET-hr/wk) |
Training
Program Training
Prescription
(MET-min/wk) |
Training
Program Training
METs |
Training
Program Training
Minutes
per
Week |
Change
in Peak
VO2 |
Outcomes Change in
Relative
Peak VO2
(mL/kg/min) |
Outcomes Change in
Peak
VO2
(METs) |
Outcomes Change in
Body
Mass
Index |
Outcomes Change in
Waist
Circumference |
Outcomes Change in
Blood
Pressure |
Outcomes Change
in Blood
Blood
Lipids |
Outcomes Change in
FBG/ISI |
| 14.0 |
50% |
23.4 |
13.3 |
800 |
3.3 |
193 |
6.5% |
1.52 |
0.43 |
NS |
NS |
NS |
Decr. TG |
Lg. Incr. ISI |
| 14.0 |
75% |
23.9 |
13.3 |
800 |
5.1 |
134 |
14.3% |
3.42 |
0.98 |
NS |
NS |
NS |
NS |
Incr. ISI |
| 23.0 |
75% |
24.1 |
21.9 |
1,314 |
5.2 |
195 |
16.4% |
3.95 |
1.13 |
Decrease |
Decrease |
NS |
NS |
Incr. ISI |
Men: STRRIDE (N~30) †
| Group Prescriptions Training Volume
(kcal/kg/wk) |
Group Prescriptions Training Intensity
(Percent Peak VO2) |
Baseline
Peak
VO2(mL/kg/min) |
Training
Program Training
Prescription
(MET-hr/wk) |
Training
Program Training
Prescription
(MET-min/wk) |
Training
Program Training
METs |
Training
Program Training
Minutes
per
Week |
Change
in Peak
VO2 |
OutcomesChange in
Relative
Peak VO2
(mL/kg/min) |
Outcomes Change in
Peak
VO2
(METs) |
Outcomes Change in
Body
Mass
Index |
Outcomes Change in
Waist
Circumference |
Outcomes Change in
Blood
Pressure |
Outcomes Change
in Blood
Blood
Lipids |
Outcomes Change in
FBG/ISI |
| 14.0 |
50% |
30.0 |
13.3 |
800 |
4.3 |
161 |
7.4% |
2.22 |
0.63 |
Decrease |
Decrease |
NS |
Decr. TG |
Lg. Incr. ISI |
| 14.0 |
75% |
33.6 |
13.3 |
800 |
7.2 |
99 |
11.2% |
3.76 |
1.08 |
NS |
Decrease |
NS |
NS |
Incr. ISI |
| 23.0 |
75% |
31.0 |
21.9 |
1,314 |
6.6 |
152 |
20.0% |
6.20 |
1.77 |
Decrease |
Decrease |
Decr. SBP |
Incr. HDL/ Decr. TG |
Lg. Incr. ISI |
Men and Women: STRRIDE (N~60) †
| Group Prescriptions Training Volume
(kcal/kg/wk) |
Group Prescriptions Training Intensity
(Percent Peak VO2) |
Baseline
Peak
VO2(mL/kg/min) |
Training
Program Training
Prescription
(MET-hr/wk) |
Training
Program Training
Prescription
(MET-min/wk) |
Training
Program Training
METs |
Training
Program Training
Minutes
per
Week |
Change
in Peak
VO2 |
Outcomes Change in
Relative
Peak VO2
(mL/kg/min) |
Outcomes Change in
Peak
VO2
(METs) |
Outcomes Change in
Body
Mass
Index |
Outcomes Change in
Waist
Circumference |
Outcomes Change in
Blood
Pressure |
Outcomes Change
in Blood
Blood
Lipids |
Outcomes Change in
FBG/ISI |
| 14.0 |
50% |
26.8 |
13.3 |
800 |
3.8 |
176 |
7.0% |
1.88 |
0.54 |
NS |
Decrease |
NS |
Decr. TG |
Lg. Incr. ISI |
| 14.0 |
75% |
29.1 |
13.3 |
800 |
6.2 |
116 |
12.6% |
3.67 |
1.05 |
NS |
Decrease |
NS |
NS |
Incr. ISI |
| 23.0 |
75% |
28.2 |
21.9 |
1,314 |
6 |
170 |
18.5% |
5.22 |
1.49 |
Decrease |
Decrease |
NS |
Incr. HDL/ Decr. TG |
Lg. Incr. ISI |
* Church, JAMA, 2007 (166)
† Duscha, Chest, 2005 (164); Johnson, Am
J Cardiol, 2007 (158)
Decr., decreased; FBG, fasting
blood glucose; HDL, high-density lipoprotein cholesterol; Incr., increase; ISI,
insulin sensitivity index, a parameter of insulin sensitivity derived from a
frequently sampled glucose tolerance test; lg., large; MET, metabolic
equivalent; NS, not significant; SBP, systolic blood pressure; TG,
triglycerides.
Similarly, changes in fitness in response to an exercise intervention
can be expressed in percent change or absolute change. Examples of each of
these in the two study populations are illustrated in this table. Because
relative VO2 is normalized to body mass, it is relatively sensitive
to changes in body mass during interventions. The observation that relative
fitness measures (relative peak VO2) at baseline are 50% lower in
DREW women than in STRRIDE women, may be due in part to the higher BMIs of DREW
women (30-40 kg•m-2) than in STRRIDE women (25-30
kg•m-2) and demonstrates the sensitivity of maximal fitness
measures, and exercise prescriptions when expressed as a percentage of baseline
fitness to BMI. However, the difference in body mass between the women in these
two study groups does not completely account for the differences in
cardiorespiratory fitness, as the mean absolute peak VO2 for women
in DREW was approximately 1.2 L/min and 1.8 L/min in STRRIDE women. Similarly,
women generally have lower cardiorespiratory fitness than do men and,
therefore, the same relative intensity of exercise (e.g., 50% peak
VO2) represents a lower energy expenditure in women than it does in
men. Relative percent increases in fitness in response to a fixed intervention
is highly dependent on baseline fitness level, although absolute fitness
measures are not. Finally, it is apparent that fitness changes do not correlate
with all outcome measures in a monotonic and linear fashion (e.g., insulin
sensitivity). Examination of these two studies in combination seem to indicate
that at least 800 MET-minutes per week of physical activity are required to
produce improvements in health outcomes, irrespective of the relative percent
increases in cardiorespiratory fitness.
Effects of Daily Fractionization (Accumulation) of Exercise Bouts on
Cardiorespiratory Fitness and Cardiovascular Health
Many groups are highly interested in whether multiple short bouts of
exercise (e.g., 3 bouts of 10 minutes) is equivalent to one long bout (e.g., 1
bout of 30 minutes) per day for improving fitness levels. It should be evident
that the choice of interval over which one integrates and accumulates a
physical activity exposure (e.g., day, week, month, or year) is somewhat
artificial, but interest remains in the issue of whether the benefits of
activity are the same when total daily activity is divided over the course of
the day. Several investigators have compared short versus long exercise
regimens in an attempt to address this question (167-179). Data for this section were obtained from a
literature search. From the appendix table (Table G2.A9 [PDF - 136 KB] summarizes these
studies), it is apparent that these studies do not provide a clear answer to
effects on cardiorespiratory fitness. Among these 11 studies, a single long
bout of exercise was superior to multiple daily bouts in 3 studies of improving
cardiorespiratory fitness. Multiple, shorter bouts were more effective in 2
studies, no difference was observed in 5 studies, and 1 study reported no
improvement in either single long or multiple short exercise bouts. A pattern
does appear to form within the few well-designed studies, however. It appears
that both single long bouts and multiple shorter bouts of aerobic exercise
training do elicit significant improvements in fitness, and that the evidence
is relatively strong that comparable fitness responses can be achieved with
different fractionization of the volume, given that the daily volume of the
exposure is the same.
Several factors likely play a role in the variability of the findings.
Careful analysis of demographics and methods of each study indicate that the
populations under study differ widely, from college students to middle aged and
overweight individuals. It is possible that the more sedentary an individual is
at baseline (e.g., the lower the peak VO2), the less a difference is
observed in fitness responses when the exposure is fractionated over the course
of the day. This may be due to the fact that less fit individuals are
exercising at lower absolute intensities (e.g., walking) and that fractionation
has less influence on fitness responses when the intensity of the exercise is
lower. If true, then as fitness levels increase, fitness responses should be
more dependent on how the exposure is fractionated. This concept has not been
tested but begs for further work.
Second, these studies differed quite a bit in exercise exposures (e.g.,
intensity, frequency). The intervention length ranges from 8 weeks to 18 weeks,
while the intensity varies from 50% to 60% of predicted heart rate maximum to
70% to 80% of heart rate reserve. This variation is reflected in the large
range of fitness changes reports, from no change to as much as 19% improvement.
For example, in a study of young college students who trained at 50% to 60% of
predicted heart rate maximum, no improvement in cardiorespiratory fitness was
reported. It is very possible that the exercise exposure was not adequate for
this population. That is, it is possible that one cannot distinguish the
differences in responses between long and short bout activities when the total
volume of the stimulus is insufficient to generate optimal responses—for
example, where the total exercise time is fixed at 30 minutes of
moderate-intensity activity, and a longer period of moderate-intensity activity
or the same period of vigorous-intensity activity might better distinguish the
responses to bout duration when total exercise volume is held constant.
Moreover, although these studies report their results as fitness gains, not all
studies use the same fitness measures. Many of the studies do not perform a
maximal exercise test and only extrapolate a maximal value based upon a
sub-maximal test.
Third, the other outcomes in these studies, cardiovascular risk markers,
such as lipids, glucose control, and others show various responses to the
interventions. When responses differ, the continuous exposure regimens seem to
have more favorable outcomes than do fractionated regimens, although the data
are too limited to provide a reliable estimate of the effects of fractionated
exercise on such outcomes.
Overall Summary and Conclusions
The weight of evidence points toward a favorable relation between
increases in habitual dynamic aerobic exercise and cardiovascular health
outcomes, including coronary heart disease morbidity and mortality, stroke,
control of blood pressure, atherogenic dyslipidemia, vascular function
measures, and cardiorespiratory fitness. In addition, dynamic aerobic exercise
is considered a standard of therapy for increasing functional performance in
peripheral arterial disease. In many of these outcomes, including
cardiovascular morbidity and mortality, there appears to be a more favorable
response with increasing intensity of exercise bouts, although exercise volume
is poorly controlled in some studies and may be the important mediating
exercise parameter. Also, the more powerful relation between exercise intensity
and outcomes does not hold for all outcomes in experimental studies, especially
when weekly volume or energy expenditure is held constant (160). In many, if not most, cardiovascular outcomes,
favorable responses are notable and reproducible when the volume of physical
activity exceeds 800 MET-minutes per week. A combination of endurance exercise
bouts with different intensities, durations, and frequencies per day and week
can achieve this level of exercise, which is approximately equivalent to 12
miles per week of walking or jogging at any intensity. As energy expenditure at
a given perceived intensity is highly dependent upon baseline fitness level,
sex, and type of activity, a volume target can be individualized with
adjustment of bout intensity, duration, and frequency, both initially and as
greater fitness levels are achieved. Given that more volume is likely to result
in greater benefits but also higher injury and cardiovascular risk, the
ultimate volume goal should be approached gradually upon the initiation of a
program, especially in initially sedentary individuals.
Research Needs
In the course of reviewing the literature that contributed to the
information presented in this chapter, several significant deficiencies in the
published literature became apparent. More information addressing the following
issues would have significantly improved the information base used to formulate
physical activity recommendations. The Cardiorespiratory Health subcommittee
encourages governmental agencies to highlight research in these areas before
the next iteration of the Physical Activity Guidelines for
Americans.
- What is the time course of acquisition of the cardiovascular health
benefits resulting from increases in habitual physical activity?
- What are the cardiovascular health benefits of varying exercise bout
duration, frequency, and intensity, while controlling for total volume?
- What effect does daily exercise exposures accumulated in short bouts
have on the acquired cardiovascular health benefits of habitual physical
activity?
- What are the effects of resistance training on cardiovascular health
and what is the nature of dose-response effects (varying intensity, bout
volume, and frequency of programs)?
- Are there sex differences in cardiovascular health benefits of
habitual exercise when controlling for volume?
- What are the specific harmful effects of physical inactivity on
cardiovascular health?
- Are there responses that differ by ethnic and racial minority
differences?
- What are the specific effects of aerobic training, resistance
training, and a combination on selected biomarkers of vascular health, such as
brachial artery flow‑mediated dilation? What are the dose-response
effects?
- What are the main characteristics of an exercise program for
preventing and treating peripheral artery disease? What are the exercise
dose-response patterns, sex differences, exercise modality options, and
differential effects on diabetic patients with PAD, on asymptomatic patients,
and are there biomarkers to predict exercise responders?
Reference List
- United States Public Health Service. Office of the
Surgeon General, National Center for Chronic Disease Prevention and Health
Promotion, President's Council on Physical Fitness and Sports. Physical
activity and health: a report of the Surgeon General. Atlanta, GA; [Washington,
D.C.]; U.S. Dept. of Health and Human Services, Centers for Disease Control and
Prevention, National Center for Chronic Disease Prevention and Health
Promotion; President's Council on Physical Fitness and Sports; 1996.
- Williams MA, Haskell WL, Ades PA, Amsterdam EA,
Bittner V, Franklin BA, Gulanick M, Laing ST, Stewart KJ. Resistance exercise
in individuals with and without cardiovascular disease: 2007 update: a
scientific statement from the American Heart Association Council on Clinical
Cardiology and Council on Nutrition, Physical Activity, and Metabolism.
Circulation 2007 Jul 31;116(5):572-84.
- Wenger HA, Bell GJ. The interactions of intensity,
frequency and duration of exercise training in altering cardiorespiratory
fitness. Sports Med. 1986 Sep;3(5):346-56.
- Swain DP, Franklin BA. VO(2) reserve and the
minimal intensity for improving cardiorespiratory fitness. Med.Sci.Sports
Exerc. 2002 Jan;34(1):152-7.
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN,
Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and
public health: updated recommendation for adults from the American College of
Sports Medicine and the American Heart Association. Circulation 2007 Aug
28;116(9):1081-93.
- Manson JE, Hu FB, Rich-Edwards JW, Colditz GA,
Stampfer MJ, Willett WC, Speizer FE, Hennekens CH. A prospective study of
walking as compared with vigorous exercise in the prevention of coronary heart
disease in women. N.Engl.J.Med. 1999 Aug 26;341(9):650-8.
- Lee IM, Rexrode KM, Cook NR, Manson JE, Buring JE.
Physical activity and coronary heart disease in women: is "no pain, no gain"
passe? JAMA 2001 Mar 21;285(11):1447-54.
- Tanasescu M, Leitzmann MF, Rimm EB, Willett WC,
Stampfer MJ, Hu FB. Exercise type and intensity in relation to coronary heart
disease in men. JAMA 2002 Oct 23;288(16):1994-2000.
- Oguma Y, Shinoda-Tagawa T. Physical activity
decreases cardiovascular disease risk in women: review and meta-analysis.
Am.J.Prev.Med. 2004 Jun;26(5):407-18.
- Kohl HW, III. Physical activity and
cardiovascular disease: evidence for a dose response. Med.Sci.Sports Exerc.
2001 Jun;33(6 Suppl):S472-S483.
- Williams PT. Physical fitness and
activity as separate heart disease risk factors: a meta-analysis.
Med.Sci.Sports Exerc. 2001 May;33(5):754-61.
- Altieri A, Tavani A, Gallus S, La VC.
Occupational and leisure time physical activity and the risk of nonfatal acute
myocardial infarction in Italy. Ann.Epidemiol. 2004 Aug;14(7):461-6.
- Lovasi GS, Lemaitre RN, Siscovick DS,
Dublin S, Bis JC, Lumley T, Heckbert SR, Smith NL, Psaty BM. Amount of
leisure-time physical activity and risk of nonfatal myocardial infarction.
Ann.Epidemiol. 2007 Jun;17(6):410-6.
- Rastogi T, Vaz M, Spiegelman D, Reddy
KS, Bharathi AV, Stampfer MJ, Willett WC, Ascherio A. Physical activity and
risk of coronary heart disease in India. Int.J.Epidemiol. 2004
Aug;33(4):759-67.
- Rothenbacher D, Hoffmeister A,
Brenner H, Koenig W. Physical activity, coronary heart disease, and
inflammatory response. Arch.Intern.Med. 2003 May 26;163(10):1200-5.
- Wagner A, Simon C, Evans A, Ferrieres
J, Montaye M, Ducimetiere P, Arveiler D. Physical activity and coronary event
incidence in Northern Ireland and France: the Prospective Epidemiological Study
of Myocardial Infarction (PRIME). Circulation 2002 May 14;105(19):2247-52.
- Schnohr P, Lange P, Scharling H,
Jensen JS. Long-term physical activity in leisure time and mortality from
coronary heart disease, stroke, respiratory diseases, and cancer. The
Copenhagen City Heart Study. Eur.J.Cardiovasc.Prev.Rehabil. 2006
Apr;13(2):173-9.
- Sundquist K, Qvist J, Johansson SE,
Sundquist J. The long-term effect of physical activity on incidence of coronary
heart disease: a 12-year follow-up study. Prev.Med. 2005 Jul;41(1):219-25.
- Folsom AR, Arnett DK, Hutchinson RG,
Liao F, Clegg LX, Cooper LS. Physical activity and incidence of coronary heart
disease in middle-aged women and men. Med.Sci.Sports Exerc. 1997
Jul;29(7):901-9.
- Fransson E, De FU, Ahlbom A,
Reuterwall C, Hallqvist J, Alfredsson L. The risk of acute myocardial
infarction: interactions of types of physical activity. Epidemiology 2004
Sep;15(5):573-82.
- Manson JE, Greenland P, LaCroix AZ, Stefanick ML,
Mouton CP, Oberman A, Perri MG, Sheps DS, Pettinger MB, Siscovick DS. Walking
compared with vigorous exercise for the prevention of cardiovascular events in
women. N.Engl.J.Med. 2002 Sep 5;347(10):716-25.
- Sesso HD, Paffenbarger RS, Ha T, Lee IM. Physical
activity and cardiovascular disease risk in middle-aged and older women.
Am.J.Epidemiol. 1999 Aug 15;150(4):408-16.
- Khaw KT, Jakes R, Bingham S, Welch A, Luben R,
Day N, Wareham N. Work and leisure time physical activity assessed using a
simple, pragmatic, validated questionnaire and incident cardiovascular disease
and all-cause mortality in men and women: The European Prospective
Investigation into Cancer in Norfolk prospective population study.
Int.J.Epidemiol. 2006 Aug;35(4):1034-43.
- LaCroix AZ, Leveille SG, Hecht JA, Grothaus LC,
Wagner EH. Does walking decrease the risk of cardiovascular disease
hospitalizations and death in older adults? J.Am.Geriatr.Soc. 1996
Feb;44(2):113-20.
- Talbot LA, Morrell CH, Metter EJ, Fleg JL.
Comparison of cardiorespiratory fitness versus leisure time physical activity
as predictors of coronary events in men aged < or = 65 years and > 65
years. Am.J.Cardiol. 2002 May 15;89(10):1187-92.
- Dorn JP, Cerny FJ, Epstein LH, Naughton J, Vena
JE, Winkelstein W, Jr., Schisterman E, Trevisan M. Work and leisure time
physical activity and mortality in men and women from a general population
sample. Ann.Epidemiol. 1999 Aug;9(6):366-73.
- Noda H, Iso H, Toyoshima H, Date C, Yamamoto A,
Kikuchi S, Koizumi A, Kondo T, Watanabe Y, Wada Y, et al. Walking and sports
participation and mortality from coronary heart disease and stroke.
J.Am.Coll.Cardiol. 2005 Nov 1;46(9):1761-7.
- Hakim AA, Petrovitch H, Burchfiel CM, Ross GW,
Rodriguez BL, White LR, Yano K, Curb JD, Abbott RD. Effects of walking on
mortality among nonsmoking retired men. N.Engl.J.Med. 1998 Jan
8;338(2):94-9.
- Matthews CE, Jurj AL, Shu XO, Li HL, Yang G, Li
Q, Gao YT, Zheng W. Influence of exercise, walking, cycling, and overall
nonexercise physical activity on mortality in Chinese women. Am.J.Epidemiol.
2007 Jun 15;165(12):1343-50.
- Lam TH, Ho SY, Hedley AJ, Mak KH, Leung GM.
Leisure time physical activity and mortality in Hong Kong: case-control study
of all adult deaths in 1998. Ann.Epidemiol. 2004 Jul;14(6):391-8.
- Paffenbarger RS, Jr., Hyde RT, Wing AL, Lee IM,
Jung DL, Kampert JB. The association of changes in physical-activity level and
other lifestyle characteristics with mortality among men. N.Engl.J.Med. 1993
Feb 25;328(8):538-45.
- Wannamethee SG, Shaper AG, Walker M. Changes in
physical activity, mortality, and incidence of coronary heart disease in older
men. Lancet 1998 May 30;351(9116):1603-8.
- Weller I, Corey P. The impact of excluding
non-leisure energy expenditure on the relation between physical activity and
mortality in women. Epidemiology 1998 Nov;9(6):632-5.
- Davey SG, Shipley MJ, Batty GD, Morris JN, Marmot
M. Physical activity and cause-specific mortality in the Whitehall study.
Public Health 2000 Sep;114(5):308-15.
- Lee CD, Folsom AR, Blair SN. Physical activity
and stroke risk: a meta-analysis. Stroke 2003 Oct;34(10):2475-81.
- Wendel-Vos GC, Schuit AJ, Feskens EJ, Boshuizen
HC, Verschuren WM, Saris WH, Kromhout D. Physical activity and stroke. A
meta-analysis of observational data. Int.J.Epidemiol. 2004
Aug;33(4):787-98.
- Vatten LJ, Nilsen TI, Romundstad PR, Droyvold WB,
Holmen J. Adiposity and physical activity as predictors of cardiovascular
mortality. Eur.J.Cardiovasc.Prev.Rehabil. 2006 Dec;13(6):909-15.
- Gillum RF, Mussolino ME, Ingram DD. Physical
activity and stroke incidence in women and men. The NHANES I Epidemiologic
Follow-up Study. Am.J.Epidemiol. 1996 May 1;143(9):860-9.
- Housley E, Leng GC, Donnan PT, Fowkes FG.
Physical activity and risk of peripheral arterial disease in the general
population: Edinburgh Artery Study. J.Epidemiol.Community Health 1993
Dec;47(6):475-80.
- Gardner AW, Sieminski DJ, Montgomery PS. Physical
activity is related to ankle/brachial index in subjects without peripheral
arterial occlusive disease. Angiology 1997 Oct;48(10):883-91.
- Ernst E, Fialka V. A review of the clinical
effectiveness of exercise therapy for intermittent claudication.
Arch.Intern.Med. 1993 Oct 25;153(20):2357-60.
- Gardner AW, Poehlman ET. Exercise rehabilitation
programs for the treatment of claudication pain. A meta-analysis. JAMA 1995 Sep
27;274(12):975-80.
- Brandsma JW, Robeer BG, van den HS, Smit B,
Wittens CH, Oostendorp RA. The effect of exercises on walking distance of
patients with intermittent claudication: a study of randomized clinical trials.
Phys.Ther. 1998 Mar;78(3):278-86.
- Girolami B, Bernardi E, Prins MH, Ten Cate JW,
Hettiarachchi R, Prandoni P, Girolami A, Buller HR. Treatment of intermittent
claudication with physical training, smoking cessation, pentoxifylline, or
nafronyl: a meta-analysis. Arch.Intern.Med. 1999 Feb 22;159(4):337-45.
- Kelley GA, Kelley KS. Progressive resistance
exercise and resting blood pressure : A meta-analysis of randomized controlled
trials. Hypertension 2000 Mar;35(3):838-43.
- Leng GC, Fowler B, Ernst E. Exercise for
intermittent claudication. Cochrane.Database.Syst.Rev. 2000;(2):CD000990.
- Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT.
Exercise training for claudication. N.Engl.J.Med. 2002 Dec
12;347(24):1941-51.
- Bendermacher BL, Willigendael EM, Teijink JA,
Prins MH. Supervised exercise therapy versus non-supervised exercise therapy
for intermittent claudication. Cochrane.Database.Syst.Rev.
2006;(2):CD005263.
- Wind J, Koelemay MJ. Exercise therapy and the
additional effect of supervision on exercise therapy in patients with
intermittent claudication. Systematic review of randomised controlled trials.
Eur.J.Vasc.Endovasc.Surg. 2007 Jul;34(1):1-9.
- Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel
EE, Brass EP. Benefit of exercise conditioning for patients with peripheral
arterial disease. Circulation 1990 Feb;81(2):602-9.
- Hiatt WR, Wolfel EE, Meier RH, Regensteiner JG.
Superiority of treadmill walking exercise versus strength training for patients
with peripheral arterial disease. Implications for the mechanism of the
training response. Circulation 1994 Oct;90(4):1866-74.
- Hiatt WR, Regensteiner JG, Wolfel EE, Carry MR,
Brass EP. Effect of exercise training on skeletal muscle histology and
metabolism in peripheral arterial disease. J.Appl.Physiol 1996
Aug;81(2):780-8.
- Patterson RB, Pinto B, Marcus B, Colucci A, Braun
T, Roberts M. Value of a supervised exercise program for the therapy of
arterial claudication. J.Vasc.Surg. 1997 Feb;25(2):312-8.
- Womack CJ, Sieminski DJ, Katzel LI, Yataco A,
Gardner AW. Improved walking economy in patients with peripheral arterial
occlusive disease. Med.Sci.Sports Exerc. 1997 Oct;29(10):1286-90.
- Gardner AW, Katzel LI, Sorkin JD, Killewich LA,
Ryan A, Flinn WR, Goldberg AP. Improved functional outcomes following exercise
rehabilitation in patients with intermittent claudication. J.Gerontol.A
Biol.Sci.Med.Sci. 2000 Oct;55(10):M570-M577.
- Izquierdo-Porrera AM, Gardner AW, Powell CC,
Katzel LI. Effects of exercise rehabilitation on cardiovascular risk factors in
older patients with peripheral arterial occlusive disease. J.Vasc.Surg. 2000
Apr;31(4):670-7.
- Brendle DC, Joseph LJ, Corretti MC, Gardner AW,
Katzel LI. Effects of exercise rehabilitation on endothelial reactivity in
older patients with peripheral arterial disease. Am.J.Cardiol. 2001 Feb
1;87(3):324-9.
- Gardner AW, Katzel LI, Sorkin JD, Bradham DD,
Hochberg MC, Flinn WR, Goldberg AP. Exercise rehabilitation improves functional
outcomes and peripheral circulation in patients with intermittent claudication:
a randomized controlled trial. J.Am.Geriatr.Soc. 2001 Jun;49(6):755-62.
- Degischer S, Labs KH, Hochstrasser J, Aschwanden
M, Tschoepl M, Jaeger KA. Physical training for intermittent claudication: a
comparison of structured rehabilitation versus home-based training. Vasc.Med.
2002 May;7(2):109-15.
- Gardner AW, Katzel LI, Sorkin JD, Goldberg AP.
Effects of long-term exercise rehabilitation on claudication distances in
patients with peripheral arterial disease: a randomized controlled trial.
J.Cardiopulm.Rehabil. 2002 May;22(3):192-8.
- Tsai JC, Chan P, Wang CH, Jeng C, Hsieh MH, Kao
PF, Chen YJ, Liu JC. The effects of exercise training on walking function and
perception of health status in elderly patients with peripheral arterial
occlusive disease. J.Intern.Med. 2002 Nov;252(5):448-55.
- Killewich LA, Macko RF, Montgomery PS, Wiley LA,
Gardner AW. Exercise training enhances endogenous fibrinolysis in peripheral
arterial disease. J.Vasc.Surg. 2004 Oct;40(4):741-5.
- Gardner AW, Montgomery PS, Flinn WR, Katzel LI.
The effect of exercise intensity on the response to exercise rehabilitation in
patients with intermittent claudication. J.Vasc.Surg. 2005
Oct;42(4):702-9.
- Sanderson B, Askew C, Stewart I, Walker P, Gibbs
H, Green S. Short-term effects of cycle and treadmill training on exercise
tolerance in peripheral arterial disease. J.Vasc.Surg. 2006
Jul;44(1):119-27.
- Sieminski DJ, Gardner AW. The relationship
between free-living daily physical activity and the severity of peripheral
arterial occlusive disease. Vasc.Med. 1997 Nov;2(4):286-91.
- Gardner AW, Womack CJ, Sieminski DJ, Montgomery
PS, Killewich LA, Fonong T. Relationship between free-living daily physical
activity and ambulatory measures in older claudicants. Angiology 1998
May;49(5):327-37.
- Gardner AW, Clancy RJ. The relationship between
ankle-brachial index and leisure-time physical activity in patients with
intermittent claudication. Angiology 2006 Oct;57(5):539-45.
- Gardner AW, Montgomery PS, Killewich LA. Natural
history of physical function in older men with intermittent claudication.
J.Vasc.Surg. 2004 Jul;40(1):73-8.
- Halbert JA, Silagy CA, Finucane P, Withers RT,
Hamdorf PA, Andrews GR. The effectiveness of exercise training in lowering
blood pressure: a meta-analysis of randomised controlled trials of 4 weeks or
longer. J.Hum.Hypertens. 1997 Oct;11(10):641-9.
- Fagard RH. Physical activity in the prevention
and treatment of hypertension in the obese. Med.Sci.Sports Exerc. 1999
Nov;31(11 Suppl):S624-S630.
- Kelley GA. Aerobic exercise and resting blood
pressure among women: a meta-analysis. Prev.Med. 1999 Mar;28(3):264-75.
- Fagard RH. Exercise characteristics and the
blood pressure response to dynamic physical training. Med.Sci.Sports Exerc.
2001 Jun;33(6 Suppl):S484-S492.
- Kelley GA, Sharpe KK. Aerobic exercise and
resting blood pressure in older adults: a meta-analytic review of randomized
controlled trials. J.Gerontol.A Biol.Sci.Med.Sci. 2001
May;56(5):M298-M303.
- Kelley GA, Kelley KA, Tran ZV. Aerobic exercise
and resting blood pressure: a meta-analytic review of randomized, controlled
trials. Prev.Cardiol. 2001;4(2):73-80.
- Whelton SP, Chin A, Xin X, He J. Effect of
aerobic exercise on blood pressure: a meta-analysis of randomized, controlled
trials. Ann.Intern.Med. 2002 Apr 2;136(7):493-503.
- Cornelissen VA, Fagard RH. Effects of endurance
training on blood pressure, blood pressure-regulating mechanisms, and
cardiovascular risk factors. Hypertension 2005 Oct;46(4):667-75.
- Fagard RH, Cornelissen VA. Effect of exercise on
blood pressure control in hypertensive patients. Eur.J.Cardiovasc.Prev.Rehabil.
2007 Feb;14(1):12-7.
- Murphy MH, Nevill AM, Murtagh EM, Holder RL. The
effect of walking on fitness, fatness and resting blood pressure: a
meta-analysis of randomised, controlled trials. Prev.Med. 2007
May;44(5):377-85.
- Chobanian AV, Bakris GL, Black HR, Cushman WC,
Green LA, Izzo JL, Jr., Jones DW, Materson BJ, Oparil S, Wright JT, Jr., et al.
The Seventh Report of the Joint National Committee on Prevention, Detection,
Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003
May 21;289(19):2560-72.
- American College of Sports Medicine
position stand. The recommended quantity and quality of exercise for developing
and maintaining cardiorespiratory and muscular fitness in healthy adults.
Med.Sci.Sports Exerc. 1990 Apr;22(2):265-74.
- American College of Sports Medicine
Position Stand. The recommended quantity and quality of exercise for developing
and maintaining cardiorespiratory and muscular fitness, and flexibility in
healthy adults. Med.Sci.Sports Exerc. 1998 Jun;30(6):975-91.
- Cornelissen VA, Fagard RH. Effect of resistance
training on resting blood pressure: a meta-analysis of randomized controlled
trials. J.Hypertens. 2005 Feb;23(2):251-9.
- Stamler J, Rose G, Stamler R, Elliott P, Dyer A,
Marmot M. INTERSALT study findings. Public health and medical care
implications. Hypertension 1989 Nov;14(5):570-7.
- Durstine JL, Grandjean PW, Davis PG, Ferguson MA,
Alderson NL, DuBose KD. Blood lipid and lipoprotein adaptations to exercise: a
quantitative analysis. Sports Med. 2001;31(15):1033-62.
- Leon AS, Sanchez OA. Response of blood lipids to
exercise training alone or combined with dietary intervention. Med.Sci.Sports
Exerc. 2001 Jun;33(6 Suppl):S502-S515.
- Kraus WE, Houmard JA, Duscha BD, Knetzger KJ,
Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, et al. Effects
of the amount and intensity of exercise on plasma lipoproteins. N.Engl.J.Med.
2002 Nov 7;347(19):1483-92.
- Slentz CA, Houmard JA, Johnson JL, Bateman LA,
Tanner CJ, McCartney JS, Duscha BD, Kraus WE. Inactivity, exercise training and
detraining, and plasma lipoproteins. STRRIDE: a randomized, controlled study of
exercise intensity and amount. J.Appl.Physiol 2007 Aug;103(2):432-42.
- Kodama S, Tanaka S, Saito K, Shu M, Sone Y,
Onitake F, Suzuki E, Shimano H, Yamamoto S, Kondo K, et al. Effect of aerobic
exercise training on serum levels of high-density lipoprotein cholesterol: a
meta-analysis. Arch.Intern.Med. 2007 May 28;167(10):999-1008.
- Verma S, Buchanan MR, Anderson TJ. Endothelial
function testing as a biomarker of vascular disease. Circulation 2003 Oct
28;108(17):2054-9.
- Celermajer DS, Sorensen KE, Georgakopoulos D,
Bull C, Thomas O, Robinson J, Deanfield JE. Cigarette smoking is associated
with dose-related and potentially reversible impairment of
endothelium-dependent dilation in healthy young adults. Circulation 1993
Nov;88(5 Pt 1):2149-55.
- Celermajer DS, Sorensen KE, Spiegelhalter DJ,
Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with
endothelial dysfunction in healthy men years before the age-related decline in
women. J.Am.Coll.Cardiol. 1994 Aug;24(2):471-6.
- Taddei S, Virdis A, Mattei P, Ghiadoni L,
Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in
normotensive subjects and patients with essential hypertension. Circulation
1995 Apr 1;91(7):1981-7.
- Anderson TJ, Uehata A, Gerhard MD, Meredith IT,
Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, et al. Close
relation of endothelial function in the human coronary and peripheral
circulations. J.Am.Coll.Cardiol. 1995 Nov 1;26(5):1235-41.
- Takase B, Uehata A, Akima T, Nagai T, Nishioka
T, Hamabe A, Satomura K, Ohsuzu F, Kurita A. Endothelium-dependent
flow-mediated vasodilation in coronary and brachial arteries in suspected
coronary artery disease. Am.J.Cardiol. 1998 Dec 15;82(12):1535-8.
- Neunteufl T, Katzenschlager R, Hassan A, Klaar U,
Schwarzacher S, Glogar D, Bauer P, Weidinger F. Systemic endothelial
dysfunction is related to the extent and severity of coronary artery disease.
Atherosclerosis 1997 Feb 28;129(1):111-8.
- Kaku B, Mizuno S, Ohsato K, Murakami T, Moriuchi
I, Arai Y, Nio Y, Hirase H, Nagata M, Takahashi Y, et al. The correlation
between coronary stenosis index and flow-mediated dilation of the brachial
artery. Jpn.Circ.J. 1998 Jun;62(6):425-30.
- Neunteufl T, Heher S, Katzenschlager R, Wolfl G,
Kostner K, Maurer G, Weidinger F. Late prognostic value of flow-mediated
dilation in the brachial artery of patients with chest pain. Am.J.Cardiol. 2000
Jul 15;86(2):207-10.
- Perticone F, Ceravolo R, Pujia A, Ventura G,
Iacopino S, Scozzafava A, Ferraro A, Chello M, Mastroroberto P, Verdecchia P,
et al. Prognostic significance of endothelial dysfunction in hypertensive
patients. Circulation 2001 Jul 10;104(2):191-6.
- Gokce N, Holbrook M, Hunter LM, Palmisano J,
Vigalok E, Keaney JF, Jr., Vita JA. Acute effects of vasoactive drug treatment
on brachial artery reactivity. J.Am.Coll.Cardiol. 2002 Aug 21;40(4):761-5.
- Brevetti G, Silvestro A, Schiano V, Chiariello
M. Endothelial dysfunction and cardiovascular risk prediction in peripheral
arterial disease: additive value of flow-mediated dilation to ankle-brachial
pressure index. Circulation 2003 Oct 28;108(17):2093-8.
- Clarkson P, Montgomery HE, Mullen MJ, Donald
AE, Powe AJ, Bull T, Jubb M, World M, Deanfield JE. Exercise training enhances
endothelial function in young men. J.Am.Coll.Cardiol. 1999
Apr;33(5):1379-85.
- Lavrencic A, Salobir BG, Keber I. Physical
training improves flow-mediated dilation in patients with the polymetabolic
syndrome. Arterioscler.Thromb.Vasc.Biol. 2000 Feb;20(2):551-5.
- Maiorana A, O'Driscoll G, Dembo L, Goodman C,
Taylor R, Green D. Exercise training, vascular function, and functional
capacity in middle-aged subjects. Med.Sci.Sports Exerc. 2001
Dec;33(12):2022-8.
- Fuchsjager-Mayrl G, Pleiner J, Wiesinger GF,
Sieder AE, Quittan M, Nuhr MJ, Francesconi C, Seit HP, Francesconi M,
Schmetterer L, et al. Exercise training improves vascular endothelial function
in patients with type 1 diabetes. Diabetes Care 2002 Oct;25(10):1795-801.
- Allen JD, Geaghan JP, Greenway F, Welsch MA.
Time course of improved flow-mediated dilation after short-term exercise
training. Med.Sci.Sports Exerc. 2003 May;35(5):847-53.
- Hamdy O, Ledbury S, Mullooly C, Jarema C, Porter
S, Ovalle K, Moussa A, Caselli A, Caballero AE, Economides PA, et al. Lifestyle
modification improves endothelial function in obese subjects with the insulin
resistance syndrome. Diabetes Care 2003 Jul;26(7):2119-25.
- Kobayashi N, Tsuruya Y, Iwasawa T, Ikeda N,
Hashimoto S, Yasu T, Ueba H, Kubo N, Fujii M, Kawakami M, et al. Exercise
training in patients with chronic heart failure improves endothelial function
predominantly in the trained extremities. Circ.J. 2003 Jun;67(6):505-10.
- Walsh JH, Yong G, Cheetham C, Watts GF,
O'Driscoll GJ, Taylor RR, Green DJ. Effects of exercise training on conduit and
resistance vessel function in treated and untreated hypercholesterolaemic
subjects. Eur Heart J 2003 Sep;24(18):1681-9.
- Walsh JH, Bilsborough W, Maiorana A, Best M,
O'Driscoll GJ, Taylor RR, Green DJ. Exercise training improves conduit vessel
function in patients with coronary artery disease. J.Appl.Physiol 2003
Jul;95(1):20-5.
- Edwards DG, Schofield RS, Lennon SL, Pierce GL,
Nichols WW, Braith RW. Effect of exercise training on endothelial function in
men with coronary artery disease. Am.J.Cardiol. 2004 Mar 1;93(5):617-20.
- Guazzi M, Reina G, Tumminello G, Guazzi MD.
Improvement of alveolar-capillary membrane diffusing capacity with exercise
training in chronic heart failure. J.Appl.Physiol 2004 Nov;97(5):1866-73.
- Kelly AS, Wetzsteon RJ, Kaiser DR, Steinberger
J, Bank AJ, Dengel DR. Inflammation, insulin, and endothelial function in
overweight children and adolescents: the role of exercise. J.Pediatr. 2004
Dec;145(6):731-6.
- Vona M, Rossi A, Capodaglio P, Rizzo S, Servi P,
De MM, Cobelli F. Impact of physical training and detraining on
endothelium-dependent vasodilation in patients with recent acute myocardial
infarction. Am.Heart J. 2004 Jun;147(6):1039-46.
- Watts K, Beye P, Siafarikas A, O'Driscoll G,
Jones TW, Davis EA, Green DJ. Effects of exercise training on vascular function
in obese children. J Pediatr 2004 May;144(5):620-5.
- Watts K, Beye P, Siafarikas A, Davis EA, Jones
TW, O'Driscoll G, Green DJ. Exercise training normalizes vascular dysfunction
and improves central adiposity in obese adolescents. J.Am.Coll.Cardiol. 2004
May 19;43(10):1823-7.
- Belardinelli R, Lacalaprice F, Faccenda E,
Purcaro A, Perna G. Effects of short-term moderate exercise training on sexual
function in male patients with chronic stable heart failure. Int.J.Cardiol.
2005 May 11;101(1):83-90.
- Blumenthal JA, Sherwood A, Babyak MA, Watkins
LL, Waugh R, Georgiades A, Bacon SL, Hayano J, Coleman RE, Hinderliter A.
Effects of exercise and stress management training on markers of cardiovascular
risk in patients with ischemic heart disease: a randomized controlled trial.
JAMA 2005 Apr 6;293(13):1626-34.
- Moriguchi J, Itoh H, Harada S, Takeda K, Hatta
T, Nakata T, Sasaki S. Low frequency regular exercise improves flow-mediated
dilatation of subjects with mild hypertension. Hypertens.Res. 2005
Apr;28(4):315-21.
- Rakobowchuk M, McGowan CL, de Groot PC, Hartman
JW, Phillips SM, MacDonald MJ. Endothelial function of young healthy males
following whole body resistance training. J.Appl.Physiol 2005
Jun;98(6):2185-90.
- Laughlin MH. Endothelium-mediated control of
coronary vascular tone after chronic exercise training. Med.Sci.Sports Exerc.
1995 Aug;27(8):1135-44.
- Folsom AR, Eckfeldt JH, Weitzman S, Ma J,
Chambless LE, Barnes RW, Cram KB, Hutchinson RG. Relation of carotid artery
wall thickness to diabetes mellitus, fasting glucose and insulin, body size,
and physical activity. Atherosclerosis Risk in Communities (ARIC) Study
Investigators. Stroke 1994 Jan;25(1):66-73.
- Watarai T, Yamasaki Y, Ikeda M, Kubota M, Kodama
M, Tsujino T, Kishimoto M, Kawamori R, Hori M. Insulin resistance contributes
to carotid arterial wall thickness in patients with
non-insulin-dependent-diabetes mellitus. Endocr.J. 1999 Oct;46(5):629-38.
- Rauramaa R, Rankinen T, Tuomainen P, Vaisanen
S, Mercuri M. Inverse relationship between cardiorespiratory fitness and
carotid atherosclerosis. Atherosclerosis 1995 Jan 20;112(2):213-21.
- Hagg U, Wandt B, Bergstrom G, Volkmann R, Gan
LM. Physical exercise capacity is associated with coronary and peripheral
vascular function in healthy young adults. Am.J.Physiol Heart Circ.Physiol 2005
Oct;289(4):H1627-H1634.
- American College of Sports Medicine Position
Stand and American Heart Association. Recommendations for cardiovascular
screening, staffing, and emergency policies at health/fitness facilities.
Med.Sci.Sports Exerc. 1998 Jun;30(6):1009-18.
- Schmidt-Trucksass AS, Grathwohl D, Frey I,
Schmid A, Boragk R, Upmeier C, Keul J, Huonker M. Relation of leisure-time
physical activity to structural and functional arterial properties of the
common carotid artery in male subjects. Atherosclerosis 1999
Jul;145(1):107-14.
- Tanaka H, Seals DR, Monahan KD, Clevenger CM,
DeSouza CA, Dinenno FA. Regular aerobic exercise and the age-related increase
in carotid artery intima-media thickness in healthy men. J.Appl.Physiol 2002
Apr;92(4):1458-64.
- Markus RA, Mack WJ, Azen SP, Hodis HN. Influence
of lifestyle modification on atherosclerotic progression determined by
ultrasonographic change in the common carotid intima-media thickness.
Am.J.Clin.Nutr. 1997 Apr;65(4):1000-4.
- Okada K, Maeda N, Tatsukawa M, Shimizu C,
Sawayama Y, Hayashi J. The influence of lifestyle modification on carotid
artery intima-media thickness in a suburban Japanese population.
Atherosclerosis 2004 Apr;173(2):329-37.
- Rauramaa R, Halonen P, Vaisanen SB, Lakka TA,
Schmidt-Trucksass A, Berg A, Penttila IM, Rankinen T, Bouchard C. Effects of
aerobic physical exercise on inflammation and atherosclerosis in men: the
DNASCO Study: a six-year randomized, controlled trial. Ann.Intern.Med. 2004 Jun
15;140(12):1007-14.
- Wildman RP, Schott LL, Brockwell S, Kuller LH,
Sutton-Tyrrell K. A dietary and exercise intervention slows
menopause-associated progression of subclinical atherosclerosis as measured by
intima-media thickness of the carotid arteries. J.Am.Coll.Cardiol. 2004 Aug
4;44(3):579-85.
- Anderssen SA, Hjelstuen AK, Hjermann I, Bjerkan
K, Holme I. Fluvastatin and lifestyle modification for reduction of carotid
intima-media thickness and left ventricular mass progression in drug-treated
hypertensives. Atherosclerosis 2005 Feb;178(2):387-97.
- Chan SY, Mancini GB, Burns S, Johnson FF, Brozic
AP, Kingsbury K, Barr S, Kuramoto L, Schulzer M, Frohlich J, et al. Dietary
measures and exercise training contribute to improvement of endothelial
function and atherosclerosis even in patients given intensive pharmacologic
therapy. J.Cardiopulm.Rehabil. 2006 Sep;26(5):288-93.
- Meyer AA, Kundt G, Lenschow U, Schuff-Werner P,
Kienast W. Improvement of early vascular changes and cardiovascular risk
factors in obese children after a six-month exercise program.
J.Am.Coll.Cardiol. 2006 Nov 7;48(9):1865-70.
- Kadoglou NP, Iliadis F, Liapis CD. Exercise and
carotid atherosclerosis. Eur.J.Vasc.Endovasc.Surg. 2008 Mar;35(3):264-72.
- Bots ML. Response: Carotid intima-media
thickness measurements in intervention studies. Stroke 2004;35(5):e88.
- Vaitkevicius PV, Fleg JL, Engel JH, O'Connor FC,
Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity
on arterial stiffness in healthy adults. Circulation 1993 Oct;88(4 Pt
1):1456-62.
- O'Rourke MF, Staessen JA, Vlachopoulos C, Duprez
D, Plante GE. Clinical applications of arterial stiffness; definitions and
reference values. Am.J.Hypertens. 2002 May;15(5):426-44.
- Pannier BM, Avolio AP, Hoeks A, Mancia G,
Takazawa K. Methods and devices for measuring arterial compliance in humans.
Am.J.Hypertens. 2002 Aug;15(8):743-53.
- Van Bortel LM, Duprez D, Starmans-Kool MJ, Safar
ME, Giannattasio C, Cockcroft J, Kaiser DR, Thuillez C. Clinical applications
of arterial stiffness, Task Force III: recommendations for user procedures.
Am.J.Hypertens. 2002 May;15(5):445-52.
- Tanaka H, DeSouza CA, Seals DR. Absence of
age-related increase in central arterial stiffness in physically active women.
Arterioscler.Thromb.Vasc.Biol. 1998 Jan;18(1):127-32.
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM,
DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial
compliance. Circulation 2000 Sep 12;102(11):1270-5.
- Miyachi M, Donato AJ, Yamamoto K, Takahashi K,
Gates PE, Moreau KL, Tanaka H. Greater age-related reductions in central
arterial compliance in resistance-trained men. Hypertension 2003
Jan;41(1):130-5.
- Ikegami H, Satake M, et al. Effects of physical
training on body composition, respirocirculatory functions, blood constituents,
and physical abilities. J.Phys.Act.Jpn. 1983;32:302-9.
- Moreau KL, Donato AJ, Seals DR, DeSouza CA,
Tanaka H. Regular exercise, hormone replacement therapy and the age-related
decline in carotid arterial compliance in healthy women. Cardiovasc.Res. 2003
Mar;57(3):861-8.
- Mustata S, Chan C, Lai V, Miller JA. Impact of
an exercise program on arterial stiffness and insulin resistance in
hemodialysis patients. J.Am.Soc.Nephrol. 2004 Oct;15(10):2713-8.
- Ferrier KE, Waddell TK, Gatzka CD, Cameron JD,
Dart AM, Kingwell BA. Aerobic exercise training does not modify large-artery
compliance in isolated systolic hypertension. Hypertension 2001
Aug;38(2):222-6.
- Seals DR, Tanaka H, Clevenger CM, Monahan KD,
Reiling MJ, Hiatt WR, Davy KP, DeSouza CA. Blood pressure reductions with
exercise and sodium restriction in postmenopausal women with elevated systolic
pressure: role of arterial stiffness. J.Am.Coll.Cardiol. 2001
Aug;38(2):506-13.
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman
RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2
diabetes with lifestyle intervention or metformin. N.Engl.J.Med. 2002 Feb
7;346(6):393-403.
- Bertovic DA, Waddell TK, Gatzka CD, Cameron JD,
Dart AM, Kingwell BA. Muscular strength training is associated with low
arterial compliance and high pulse pressure. Hypertension 1999
Jun;33(6):1385-91.
- Miyachi M, Kawano H, Sugawara J, Takahashi K,
Hayashi K, Yamazaki K, Tabata I, Tanaka H. Unfavorable effects of resistance
training on central arterial compliance: a randomized intervention study.
Circulation 2004 Nov 2;110(18):2858-63.
- Cortez-Cooper MY, DeVan AE, Anton MM, Farrar RP,
Beckwith KA, Todd JS, Tanaka H. Effects of high intensity resistance training
on arterial stiffness and wave reflection in women. Am.J.Hypertens. 2005
Jul;18(7):930-4.
- Casey DP, Beck DT, Braith RW. Progressive
resistance training without volume increases does not alter arterial stiffness
and aortic wave reflection. Exp.Biol.Med.(Maywood.) 2007
Oct;232(9):1228-35.
- Casey DP, Pierce GL, Howe KS, Mering MC, Braith
RW. Effect of resistance training on arterial wave reflection and brachial
artery reactivity in normotensive postmenopausal women. Eur.J.Appl.Physiol 2007
Jul;100(4):403-8.
- Blair SN, Kohl HW, III, Paffenbarger RS, Jr.,
Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A
prospective study of healthy men and women. JAMA 1989 Nov
3;262(17):2395-401.
- Lee S, Kuk JL, Katzmarzyk PT, Blair SN, Church
TS, Ross R. Cardiorespiratory fitness attenuates metabolic risk independent of
abdominal subcutaneous and visceral fat in men. Diabetes Care 2005
Apr;28(4):895-901.
- Blair SN, Kampert JB, Kohl HW, III, Barlow CE,
Macera CA, Paffenbarger RS, Jr., Gibbons LW. Influences of cardiorespiratory
fitness and other precursors on cardiovascular disease and all-cause mortality
in men and women. JAMA 1996 Jul 17;276(3):205-10.
- Johnson JL, Slentz CA, Houmard JA, Samsa GP,
Duscha BD, Aiken LB, McCartney JS, Tanner CJ, Kraus WE. Exercise training
amount and intensity effects on metabolic syndrome (from Studies of a Targeted
Risk Reduction Intervention through Defined Exercise). Am.J.Cardiol. 2007 Dec
15;100(12):1759-66.
- Blomqvist CG, Saltin B. Cardiovascular
adaptations to physical training. Annu.Rev.Physiol 1983;45:169-89.
- Swain DP, Franklin BA. Comparison of
cardioprotective benefits of vigorous versus moderate intensity aerobic
exercise. Am.J.Cardiol. 2006 Jan 1;97(1):141-7.
- Seals DR, Hagberg JM, Hurley BF, Ehsani AA,
Holloszy JO. Endurance training in older men and women. I. Cardiovascular
responses to exercise. J.Appl.Physiol 1984 Oct;57(4):1024-9.
- Spina RJ. Cardiovascular adaptations to
endurance exercise training in older men and women. Exerc.Sport Sci.Rev.
1999;27:317-32.
- Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph
RE, Schwartz RS, Yukawa M, Aiello E, Potter JD, McTiernan A. Effect of exercise
on total and intra-abdominal body fat in postmenopausal women: a randomized
controlled trial. JAMA 2003 Jan 15;289(3):323-30.
- Duscha BD, Slentz CA, Johnson JL, Houmard JA,
Bensimhon DR, Knetzger KJ, Kraus WE. Effects of exercise training amount and
intensity on peak oxygen consumption in middle-age men and women at risk for
cardiovascular disease. Chest 2005 Oct;128(4):2788-93.
- Bouchard C, Rankinen T. Individual differences
in response to regular physical activity. Med.Sci.Sports Exerc. 2001 Jun;33(6
Suppl):S446-S451.
- Church TS, Earnest CP, Skinner JS, Blair SN.
Effects of different doses of physical activity on cardiorespiratory fitness
among sedentary, overweight or obese postmenopausal women with elevated blood
pressure: a randomized controlled trial. JAMA 2007 May 16;297(19):2081-91.
- DeBusk RF, Stenestrand U, Sheehan M, Haskell WL.
Training effects of long versus short bouts of exercise in healthy subjects.
Am.J.Cardiol. 1990 Apr 15;65(15):1010-3.
- Gill JM, Murphy MH, Hardman AE. Postprandial
lipemia: effects of intermittent versus continuous exercise. Med.Sci.Sports
Exerc. 1998 Oct;30(10):1515-20.
- Murphy MH, Hardman AE. Training effects of
short and long bouts of brisk walking in sedentary women. Med.Sci.Sports Exerc.
1998 Jan;30(1):152-7.
- Jakicic JM, Winters C, Lang W, Wing RR. Effects
of intermittent exercise and use of home exercise equipment on adherence,
weight loss, and fitness in overweight women: a randomized trial. JAMA 1999 Oct
27;282(16):1554-60.
- Woolf-May K, Kearney EM, Owen A, Jones DW,
Davison RC, Bird SR. The efficacy of accumulated short bouts versus single
daily bouts of brisk walking in improving aerobic fitness and blood lipid
profiles. Health Educ.Res. 1999 Dec;14(6):803-15.
- Murphy MH, Nevill AM, Hardman AE. Different
patterns of brisk walking are equally effective in decreasing postprandial
lipaemia. Int.J.Obes.Relat Metab Disord. 2000 Oct;24(10):1303-9.
- Schmidt WD, Biwer CJ, Kalscheuer LK. Effects of
long versus short bout exercise on fitness and weight loss in overweight
females. J.Am.Coll.Nutr. 2001 Oct;20(5):494-501.
- Thomas DQ, Lewis HL, McCaw ST, Adams MJ. The
effects of continuous and discontinuous walking on physiologic response in
college-age subjects. J.Strength.Cond.Res. 2001 May;15(2):264-5.
- Murtagh EM, Boreham CA, Nevill A, Hare LG,
Murphy MH. The effects of 60 minutes of brisk walking per week, accumulated in
two different patterns, on cardiovascular risk. Prev.Med. 2005
Jul;41(1):92-7.
- O'Donovan G, Owen A, Bird SR, Kearney EM, Nevill
AM, Jones DW, Woolf-May K. Changes in cardiorespiratory fitness and coronary
heart disease risk factors following 24 wk of moderate- or high-intensity
exercise of equal energy cost. J.Appl.Physiol 2005 May;98(5):1619-25.
- Osei-Tutu KB, Campagna PD. The effects of short-
vs. long-bout exercise on mood, VO2max, and percent body fat. Prev.Med. 2005
Jan;40(1):92-8.
- Macfarlane DJ, Taylor LH, Cuddihy
TF. Very short intermittent vs continuous bouts of activity in sedentary
adults. Prev.Med. 2006 Oct;43(4):332-6.
- Quinn TJ, Klooster JR, Kenefick RW.
Two short, daily activity bouts vs. one long bout: are health and fitness
improvements similar over twelve and twenty-four weeks? J.Strength.Cond.Res.
2006 Feb;20(1):130-5.
top of page
Note: Documents in PDF format require the
Adobe Acrobat Reader®.
If you experience problems with PDF documents, please
download the latest version of the Reader®.
Continue to G3. Metabolic Health
Back to G1. All-Cause Mortality
Back to
Physical Activity Guidelines Advisory Committee
Report
|