Physical Activity Guidelines Advisory Committee Report
Part G. Section 4: Energy Balance
List of Figures
Introduction
Overweight and obesity are linked to increased risk of morbidity from
hypertension, dyslipidemia, type 2 diabetes, coronary heart disease, stroke,
gallbladder disease, osteoarthritis, sleep apnea and respiratory problems, and
endometrial, postmenopausal breast, prostate, and other cancers (1;2). In addition, obesity is associated
with excess overall mortality (3). Unfortunately, the
prevalence of overweight and obesity has increased dramatically over the past
20 years in the United States to 70.8% and 31.1% for adult men, and 61.8% and
33.2% for adult women, respectively (4). This increase has
been attributed to changes in environment and lifestyle factors because the
escalating prevalence has been occurring in a constant genetic milieu. The
focus in this chapter is on the role that physical activity plays in energy
balance.
Review of the Science
Overview of Questions Addressed
This chapter addresses 5 major questions related to physical activity
and energy balance.
- How much physical activity is needed for weight stability and weight
loss?
- How much physical activity is needed to prevent weight regain in
previously overweight individuals?
- What is the effect of physical activity on body composition
parameters (e.g., waist circumference, intra-abdominal fat, abdominal obesity,
total body fat) that are related specifically to metabolic disorders?
- What effects do sex and age have on the role of physical activity in
energy balance?
- How do the physical activity requirements for weight maintenance
differ across racial/ethnic and socioeconomic groups?
Data Sources and Process Used to Answer
Questions
The Energy Balance subcommittee used the Physical Activity
Guidelines for Americans Scientific Database as its primary source for
each question (see Part F. Scientific
Literature Search Methodology, for a complete description of
the Database). It also used other databases, reviews, and meta-analyses to
obtain evidence bearing on each question. Specific search strategies are
described for each question.
Caveats
Four points need to be mentioned at the outset of this chapter on
physical activity and energy balance. First, in contrast to outcomes addressed
in other chapters, in which physical activity can be discussed as the primary
variable affecting the outcome, achieving energy balance is dependent on both
energy intake and energy expenditure. With the availability of inexpensive and
easily accessed high-calorie, highly palatable foods, it is far easier to
increase energy intake than to increase energy expenditure in our society. In
support, the 2005 Dietary Guidelines Advisory Committee Report (5) indicated that most Americans are consuming energy in
excess of energy needs, and it is not likely to change in the near future.
Consequently, final recommendations related to the level of physical activity
needed for weight maintenance, weight loss, or prevention of weight regain
after weight loss must consider energy intake issues as well.
Second, when a caloric deficit induced by exercise is compared with an
equivalent caloric deficit created by a reduction in caloric intake, there is
little or no difference in weight loss (6). However, in
many weight loss studies, the proportion of the caloric deficit due to physical
activity is only a small fraction of the overall caloric deficit, and
consequently, the contribution that physical activity makes to weight loss is
relatively small. This must be remembered as we address the role of physical
activity alone on weight-related issues.
Third, secular trends have increased the use of automation and
labor-saving devices on the job, at home and in the community and increased
passive leisure-time physical activity (e.g., TV/VCR, computer use). These
trends influence the amount of physical activity needed to achieve energy
balance.
Finally, if we did not have an overweight and obesity problem in our
society, we would still need a physical activity recommendation to maintain
health and prevent disease. That simple message is lost on many who focus
solely on the role of physical activity in preventing overweight and obesity.
Consequently, the level of physical activity needed to maintain health and
prevent disease is the baseline for any physical activity recommendation for
energy balance.
Question 1: How Much Physical Activity Is Needed for
Weight Stability and Weight Loss?
Conclusions
All study designs provide clear evidence of a dose-response relation
between physical activity and weight loss. However, few data are available on
weight stability over the long term. Available data on weight stability are
from short-term clinical trials. Based on these trials, a dose of physical
activity in the range of 13 to 26 MET-hours per week resulted in a modest 1% to
3% weight loss, consistent with weight stability over time (7-9). Thirteen MET-hours per week is equivalent to walking at
a 4 mile per hour pace for 150 minutes per week or jogging at a 6 mile per hour
pace for 75 minutes per week. The magnitude of weight loss resulting from
studies of resistance exercise is typically less than 1 kilogram (2.2 pounds).
However, this result may be affected by the relatively short duration of these
studies and gains in fat-free mass that accompany such interventions. In
contrast, it is clear that if one wants to achieve weight loss (i.e., more than
5% decrease in body weight), a dietary intervention also is needed. The dietary
intervention could include either a maintenance of baseline caloric intake, or
a reduction in caloric intake to accompany the physical activity intervention.
The magnitude of change in weight due to physical activity is additive to that
associated with caloric restriction.
Definitions
To aid in the study of patterns of weight change, the scientific
literature has operationally defined the concept of weight stability. St. Jeor
and colleagues (10) define weight stability as a change of
2.3 kilograms (5 pounds) or less of initial body weight. In this study,
participants' weights were monitored over a period of time using this
criterion. It was determined that 62%, 52%, 49%, and 46% of participants were
classified as maintaining their body weight at 1, 2, 3, and 4 years of
follow-up, respectively. The Pound of Prevention Study also defined weight
maintenance as a change of 2.3 kilograms (5 pounds) or less (11) of initial body weight. When examined over a 3-year
period, 40% of men and 38% of women were classified as "maintainers," with a
mean weight change of 0.3 kilograms (0.7 pounds) and 0.2 kilograms (0.4
pounds), respectively. Moreover, across the entire sample of 957 individuals,
the mean weight gain over a 3-year period was 1.7 kilograms (3.7 pounds) for
men and 1.8 kilograms (4 pounds) for women. This would suggest that the mean
weight gain across the population may be approximately 0.6 kilograms (1.3
pounds) per year.
More recently, Stevens and colleagues (12) have
recommended that weight maintenance be defined as less than a 3% change in body
weight. Moreover, they recommended that a change in body weight of 3% to less
than 5% of initial weight be considered as small fluctuations in body weight,
and a change of 5% or more of body weight be considered clinically significant.
Considering these standards, an obese individual weighing 91 kilograms (200
pounds) would need to reduce body weight by 4.5 kilograms (10 pounds) to have a
significant weight loss, and a weight change of 2.7 kilograms (6 pounds) would
be considered weight stability. These standards should be considered when
evaluating the effect of physical activity on body weight change to determine
whether various doses and modes of physical activity result in weight stability
or clinically relevant weight loss.
Rationale
A search of the Physical Activity Guidelines for Americans
Scientific Database identified 126 research articles on the effect of physical
activity on weight loss and weight stability. Additionally, pertinent reviews
available through a MEDLINE search were considered.
Cross-Sectional Studies
Twenty-four cross-sectional studies were identified that examined the
association between physical activity and body weight. Of these 24 studies, 23
reported results suggesting an inverse relationship between physical activity
and body weight and/or body mass index (BMI) (13-35).
These studies tended to illustrate a dose-response relationship between
physical activity and body weight or BMI. For example, Giovannucci and
colleagues (14) reported that when 0.9, 4.8, 11.3, 22.6,
and 46.8 MET-hours per week were used to define quintiles of physical activity,
corresponding BMI values were 25.4, 25.3, 25.1, 24.7, and 24.4
kg/m2, respectively. More recently, Kavouras and colleagues (15)
reported that individuals participating in physical activity that is consistent
with the current consensus public health recommendations of at least 30 minutes
per day on 5 days a week had a significantly lower BMI (25.9 kg/m2)
when compared to the BMI (26.7 kg/m2) of less active individuals (Figure G4.1). Thus, based on these findings, it appears
that levels of physical activity that are consistent with a range of 30 to 60
minutes per day on at least 5 days per week (150 to 300 minutes per week) is
sufficient to maintain and/or significantly reduce body weight.
Prospective Studies
Nine prospective studies were identified that reported on the benefits
of physical activity to prevent weight gain and/or result in weight loss (36-44). Three studies, which had a follow up period of 1 to 3
years, all reported a favorable association between physical activity and
weight-related outcomes (36;37;39). The remaining 6 studies, which had a follow-up period of
6.5 years or greater, also reported a favorable association between physical
activity and weight-related outcomes (38;40-44). Berk and colleagues (43) found
that individuals who initially reported less than 60 minutes per week of
physical activity and increased to 134 minutes per week of physical activity
had an increase in BMI of 0.4 kg/m2 across a 16-year follow-up
period, but this was not significantly different from the 0.9kg/m2
increase observed for individuals who remained sedentary (less than 60 minutes
per week) at both assessment periods. These data suggest that less than 150
minutes per week of physical activity will result in a non-significant blunting
of weight gain compared to individuals who remain sedentary. However,
individuals who were classified as active at both assessment periods were
participating in 261 minutes per week of physical activity, and had a
significantly smaller change in BMI compared to individuals who were initially
active (more than 60 minutes per week) at baseline but became inactive at
follow-up (less than 60 minutes per week). This supports the need to maintain a
physically activity lifestyle to manage body weight long-term.
Figure G4.1. Differences in Body Mass Index Due
to Level of Physical Activity
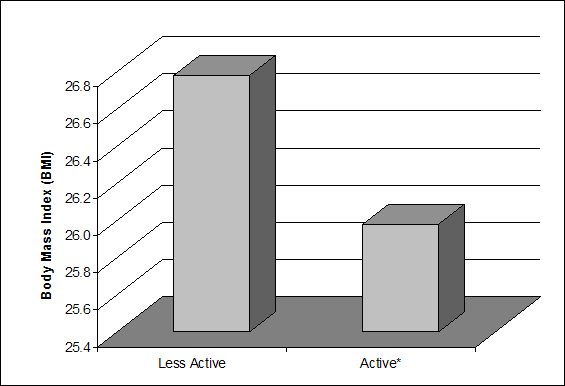
*Active is defined as the consensus public health
recommendation for physical activity (3 or more days per week of 20 minutes per
day at vigorous intensity or 5 or more days per week of 30 minutes per day at
moderate intensity). Source: Adapted from Kavouras and colleagues, 2007 (15).
Figure G4.1. Data Points
| |
Less Active |
Active* |
| BMI |
26.7 |
25.9 |
Randomized Trials
Endurance Exercise
Twenty studies were identified that examined the effect of endurance
exercise on body weight. However, 7 studies were not reviewed due to the intent
of the study to focus on marathon training, a dietary intervention to counter
or enhance the weight loss effects of exercise, the inclusion of only subjects
with serious psychiatric disabilities, the lack of a consistent training
paradigm across the observation period, or the exercise volume not expressed in
as minutes per week. The remaining 13 studies were reviewed in greater detail.
Twelve used a randomized design, although 3 of them did not have a control
group and/or the physical activity was in addition to a dietary intervention
(45-47), and 1 used a non randomized design to examine the
effect of physical activity but did not include a comparison group (48). In addition, the primary purpose of 5 of the studies was
on something other than weight loss (49-53). The remaining
4 studies (7-9;54) had sufficient
statistical power to evaluate the effect of physical activity on body weight
and body composition.
These studies ranged in duration from 8 to 16 months, and physical
activity level ranged from 180 minutes of moderate-intensity physical activity
per week to 360 minutes of moderate- to vigorous-intensity physical activity
per week. In addition, one study (9) evaluated 3 levels of
physical activity, and 2 (7;8)
established, post hoc, tertiles of physical activity participation (adherence)
based on activity logs and/or pedometer records to evaluate a dose-response
pattern. Typical weight losses were 1 to 3 kilograms (2.2 to 6.6 pounds), which
corresponded to less than 3% change in body weight, but evidence of a
dose-response relationship was clear, with those doing the greatest amount of
physical activity achieving weight losses of 4% to 6% (the latter associated
with an energy expenditure of 668 kcal per session, 5 days per week). A dose of
physical activity in the range of 13 to 26 MET-hours per week resulted in a
modest 1% to 3% weight loss, consistent with weight stability over time.
Resistance Exercise
An alternative form of physical activity is resistance exercise. Ten
studies were reviewed that examined the impact of this form of exercise on
change in body weight, and all of these studies showed a modest reduction (less
than 1 kilogram) or a non-significant change in body weight (55-64). This finding of a modest impact of resistance
exercise on body weight was confirmed in a literature review (65). A potential explanation for this lack of a reduction in
body weight is that many of these studies reported an increase in fat-free mass
resulting from resistance exercise training, which resulted in a reduction in
percent body fat, but did not change absolute body weight or fat mass. Thus,
changes in body composition may be a desirable outcome to examine when
determining the effect of resistance exercise on body weight parameters.
However, the lack of a sufficient dose of physical activity to elicit a
significant energy deficit may also explain these findings, as many of these
studies were relatively short in duration and included only 2 to 3 days per
week of resistance exercise.
Five studies from the Physical Activity Guidelines for
Americans Scientific Database examined the combination of endurance and
resistance exercise on change in body weight. Two studies used randomized
designs to assign participants to a physical activity group or a control group
(66;67), 1 used a randomized
cross-over design involving 8 weeks of physical activity and 8 weeks of no
physical activity (68), and 2 examined the effect of
physical activity but did not include a control group (69;70). Four of these studies reported
no effect of combined endurance plus resistance exercise on change in body
weight (66-69), and 1 study that did not include a control
group (70) reported a significant effect. A potential
limitation of these studies is that they ranged from 8 to 10 weeks in duration,
which may have been too short a time to significantly affect body weight.
In general, regular participation in moderate-to-vigorous physical
activity is associated with weight maintenance over time. In contrast, it is
clear that if one wants to achieve clinically relevant weight loss (a decrease
of 5% or more in body weight), a dietary intervention is usually needed. This
is shown clearly in Figure G4.2, adapted from Wing, 1999 (71).
Figure G4.2. Weight Loss Related to a Diet
Intervention, an Exercise Intervention, and a Diet + Exercise Intervention

Source: Adapted from Wing, 1999 (71)
Figure G4.2. Data Points—Weight Loss in
kg
| |
0 Months |
6 Months |
| Diet |
0 |
-9.1 |
| Exercise |
0 |
-2.1 |
| Diet + Exercise |
0 |
-10.3 |
The magnitude of weight loss due to physical activity is additive to
caloric restriction, but physical activity is generally insufficient by itself
to bring about clinically significant weight loss. Consistent with this,
McTiernan and colleagues (8) estimated that the physical
activity intervention in their study should have produced a weight loss of 7.8
kilograms, rather than the 1.4 kilograms (women) and 1.8 kilograms (men)
observed, if caloric intake had remained stable. Further,
studies in which the caloric intake was held constant (by design) from baseline
showed that the weight loss associated with the physical activity intervention
was what one would predict from the physical activity energy expenditure (6). Consequently, the addition of a dietary restraint
to not increase caloric intake may have resulted in clinically
significant weight loss, rather than just weight stability with the physical
activity intervention mentioned above. The magnitude of weight loss reported in
these studies is consistent with earlier reviews on this topic by Wing (71)
and the Expert Panel of Clinical Guidelines for the Treatment of Obesity (1).
Question 2. How Much Physical Activity Is Needed to
Prevent Weight Regain in Previously Overweight Individuals?
Conclusions
Most of the available literature indicates that "more is better" when it
comes to the amount of physical activity needed to prevent weight regain
following weight loss. However, the literature has some considerable
shortcomings regarding the appropriate research design needed to directly
address this question. Given these limitations, the estimated gross energy
expenditure needed to achieve weight maintenance following substantial weight
loss is about 31 kilocalories per kilogram week or 4.4
kcal·kg-1·d-1, which is equivalent to
walking 54 minutes per day at a 4 mile per hour pace, walking 80 minutes per
day at a 3 mile per hour pace, or jogging 26 minutes per day at a 6 mile per
hour pace (72-74).
Rationale
Initial references were obtained with a search of the Physical
Activity Guidelines for Americans Scientific Database. Key words included
adults, exercise, physical activity, obesity, adiposity, weight, and BMI. Eight
systematic reviews or meta-analyses also were reviewed for pertinent
references. Studies that investigated special populations (e.g., physically
disabled), included individuals with a disease known to affect weight (e.g.,
cancer), or weight loss drugs, were excluded. To be included, studies had to
target a period of weight loss followed by a period of weight maintenance using
physical activity as the strategy for preventing weight regain.
Eight randomized trials met the above criteria and were used for this
review. Of the eight studies, only three had a design in which participants
were randomized after weight loss and only two used a control group. Three
observational or prospective cohort studies were identified that met the above
criteria and were used for this review. Four position papers or reports also
were used as references.
It is generally accepted that individuals can lose weight but most
cannot maintain significant weight loss. Because it has an energy equivalent,
physical activity is universally promoted as a necessary component of
strategies to maintain weight loss (1;75;76). Indeed, physical activity is
often cited as the best predictor of weight maintenance after weight loss (77;78). A systematic review of physical
activity to prevent weight regain subsequent to weight loss was completed by
Fogelholm and Kukkonen-Harjula (79). The majority of
studies included in this review were observational studies and studies of
individuals who were randomized at baseline to exercise or no exercise, or to
different levels of physical activity. Follow-up varied from several months to
several years and generally showed that individuals who engaged in exercise
experienced less regain than those individuals who did not, and those
individuals who engaged in greater amounts of physical activity experienced
less regain than those who did more moderate levels. Only 3 studies used a
design in which individuals were randomized to physical activity after weight
loss (80-82), and the results were inconsistent, showing
that physical activity had an indifferent, negative, or positive effect on
prevention of weight regain.
Despite the accepted concept that physical activity is necessary for
successful weight maintenance after weight loss, the amount that is needed
remains uncertain. The 1995 Centers for Disease Control/American College of
Sports Medicine (CDC/ACSM) recommendations for physical activity specified the
accumulation of 30 minutes of moderate-intensity physical activity for most
days of the week (83). These guidelines were provided for
health promotion and disease prevention. However, they were widely interpreted
to also be useful for weight management. Minimum levels of 150 minutes per week
(30 minutes per day, 5 days a week) of moderate-intensity physical activity
were also recommended by the ACSM Position Stand for "Appropriate Intervention
Strategies for Weight Loss and Prevention of Weight Regain for Adults"
(75). However, recent evidence suggests that greater
levels of physical activity may be necessary to prevent weight regain after
weight loss. For example, individuals in the National Weight Control Registry
who have maintained weight loss have shown levels of energy expenditure
equivalent to walking about 28 miles a week (77).
Schoeller and colleagues (74) used doubly-labeled water to
study women who recently lost 23±9 kilograms of weight in order to
estimate the energy expenditure needed to prevent weight regain. Retrospective
analyses of these data were performed to determine the level of physical
activity that provided maximum differentiation between gainers and maintainers.
Based on these analyses, it was determined that individuals would need to
expend about 4.4 kilocalories per kilogram per day in physical activity (which
is equivalent to about 80 minutes per day of moderate-intensity physical
activity or 35 minutes per day of vigorous physical activity) to prevent weight
regain.
Jakicic and colleagues (73;84) and Andersen and colleagues (85)
provided data from randomized trials showing that individuals who performed
large amounts of physical activity maintained weight loss better at follow-up
of 18 months, 12 months, and 12 months, respectively, than did those doing
smaller amounts of physical activity. In particular, Jakicic and colleagues (73;84) showed very little weight regain
in individuals who performed more than 200 minutes per week of
moderate-intensity physical activity. Ewbank and colleagues (72) also found similar results 2 years after weight loss by
very-low-energy diet. Retrospectively grouping participants by levels of
self-reported physical activity, individuals who reported greater levels (i.e.,
walking about 16 miles per week,) had significantly less weight regain than
individuals reporting less physical activity per week (4.8 to 9.1 miles per
week). However, it is important to note that individuals in all 3 studies above
were grouped into physical activity categories retrospectively and were not
randomly assigned to those groups after weight loss. Thus, the amount of
physical activity was self-selected and therefore does not provide clear
evidence for the amount needed to prevent weight regain.
To explore the effects of levels of physical activity greater than
those normally recommended in weight management programs, Jeffery and
colleagues (86), targeted energy expenditures of 1,000
kilocalories per week and 2,500 kilocalories week for 18 months in 2 groups of
participants; these levels were randomly assigned at baseline. The actual
reported energy expenditure at 18 months was 1,629 ± 1,483 and 2,317
± 1,854 kilocalories per week for the 1,000 and 2,500 kilocalories per
week groups, respectively. At 6 months, weight loss did not differ between the
groups, but there were significant differences at 12 and 18 months (weight
maintenance) follow-up, with the 2,500 kilocalories per week group showing
significantly greater weight losses (6.7 ± 8.1 kilograms versus 4.1
± 8.3 kilograms). The energy equivalent for walking for the 2,500
kilocalories per week group and 1,000 kilocalories per week group was about 3.3
miles per day and about 2.3 miles day, respectively. This study showed that
greater levels of physical activity resulted in significantly lower levels of
weight regain. However, the results must be interpreted with caution, as the
percentage of individuals meeting the targeted energy expenditure varied
greatly, and the behavioral interventions were not equal.
In general, large volumes of physical activity are needed to prevent
weight regain in those who have lost a great deal of weight. Studies by Ewbank
and colleagues (72), Jakicic and colleagues (73), and Schoeller and colleagues (74)
indicate that the volume of physical activity needed for that purpose is
approximately 31 MET-hours per week or 4.4 MET-hours per day.
Question 3. What Is the Effect of Physical Activity on
Body Composition Parameters (e.g., Waist Circumference, Intra-Abdominal Fat,
Abdominal Adiposity, Total Body Fat) That Are Specifically Related to Metabolic
Disorders?
Conclusions
A dose-response relation exists between volume of physical activity and
decreases in total and abdominal adiposity in overweight and obese individuals.
In the absence of coincident caloric restriction, aerobic physical activity in
the range of 13 to 26 MET-hours per week results in decreases in total and
abdominal adiposity that are consistent with improved metabolic function.
Thirteen MET-hours per week is equivalent to walking at a 4 mile per hour pace
for 150 minutes per week or jogging at a 6 mile per hour pace for 75 minutes
per week (7-9). However, larger volumes of physical
activity (e.g., 42 MET-hours per week) result in decreases in intra-abdominal
adipose tissue that are 3 to 4 times those seen with 13 to 26 MET-hours per
week, even without weight loss. The evidence thus far suggests that abdominal
fat loss with increased physical activity is proportional to overall fat
loss.
Definitions
The obesity phenotype that conveys the greatest health risk of
metabolic disorders, such as the metabolic syndrome and type 2 diabetes, is one
that favors an accumulation of adipose tissue in the abdominal region. Regular
physical activity is recognized as an effective method of preventing excessive
weight and fat gain throughout adulthood, and although physical activity is
commonly prescribed to reduce overall obesity, the influence of exercise
induced weight loss on abdominal adiposity is not clear. Abdominal adiposity is
characterized several ways in the scientific literature. Modern imaging
techniques such as MRI, DXA, and CT provide highly-precise quantification of
total body fat content (expressed in relative [%] or absolute [kilogram]
terms), as well as specific measures of abdominal fat, such as the subcutaneous
and visceral (intra-abdominal) fat areas (cm2). Although less
precise than the imaging measures, the waist circumference (measured in
centimeters and usually defined at the level of the lowest rib) is the most
widely-used anthropometric measure of abdominal adiposity and therefore has the
most clinical utility of all these measures.
Rationale
The Physical Activity Guidelines for Americans Scientific
Database was accessed using the following delineation terms: 1)
population sub-group: adults/older adults; 2) study
design: randomized controlled trials (RCTs); longitudinal experimental
studies (before/after); and prospective observational studies; and 3)
health outcomes: adiposity measures (e.g., total fat, percent
fat, abdominal fat area [visceral and subcutaneous], waist circumference)
related specifically to metabolic disorders. Evidence obtained using the
Scientific Database was supplemented with recently-published scientific papers
and review articles.
Favorable body composition changes (reduced fat mass and increased lean
mass) occur with the adoption of regular physical activity — even among
individuals aged 75 years and older, and evidence suggests that current
activity is more protective than past activity (87;88). What is not clear at this time
is the amount and type of activity necessary to result in meaningful
alterations in abdominal fat, which in turn can preserve or improve metabolic
function. Unfortunately, few large-scale RCTs have been directed toward this
question. The data that do exist are from relatively small RCTs and controlled
intervention studies. Nonetheless, these studies paint a consistent picture of
energy expenditure requirements for minimizing fat gain and/or reducing excess
total and abdominal fat.
Several RCTs and controlled interventions report the benefits of
moderate- to vigorous-intensity aerobic exercise to overall improvements in
body weight, body fat, and lean mass in middle-aged and older adults (7;8;89-93). The data
are equivocal, however, with regard to the ability to significantly alter
regional distribution of body fat with endurance training (7-9;54;91;93-95). In general, aerobic exercise, without dieting,
appears to have a beneficial effect on overall and abdominal adiposity.
However, the exercise dose necessary to result in these alterations is rather
high. In Irwin and colleagues (7), 176 minutes per week of
moderate- to vigorous-intensity physical activity performed over 12 months
resulted in a reduction in subcutaneous fat and intra-abdominal fat of 5.4% and
5.8%, respectively, with the impact being even larger when contrasted with the
control group. In addition, McTiernan and colleagues (8)
used a higher volume/longer duration aerobic exercise regimen (60 minutes or
more of moderate- to vigorous-intensity physical activity on 6 days per week)
over 12 months of training and also reported modest decreases in the
subcutaneous abdominal fat (5% in women and 11% in men) and intra-abdominal fat
(6% in women and 8% in men) depots and in the waist circumference (2% in women
and 3% in men). Data from the Studies of Targeted Risk Reduction Interventions
through Defined Exercise (STRRIDE) show that the highest amount of exercise
performed (equivalent of jogging approximately 20 miles per week) over 8 months
resulted in, at best, a 7% decrease in visceral and subcutaneous fat in men and
women aged 40 to 65 years (9). Ross and colleagues (93) report an
18% reduction in total fat and a 20% reduction in abdominal fat among
non-dieting abdominally-obese women who exercised every day for about 60
minutes (or 500 kilocalorie expenditure) for 14 weeks. Together, these findings
(7-9;93) and others (6) support the contention that in the absence of coincident
caloric restriction, aerobic physical activity in the range of 13 to 26
kilocalories per kilogram per week results in decreases in total and abdominal
adiposity that are consistent with improved metabolic function. However, as
mentioned above, when more physical activity is done per week (e.g., 42
MET-hours per week), decreases in intra-abdominal adipose tissue approach 3 to
4 times the level seen with 13 to 26 MET-hours per week, even without weight
loss (93).
A recent study employing 45 minutes of resistance exercise training
twice weekly also reports small, yet favorable changes in total and abdominal
fat (56) in middle-age and older adults, but not in
younger, non-obese women (96). In a 2-year study of
resistance training in overweight and obese premenopausal women, Schmitz and
colleagues (56) report a 4% decrease in total fat in the
exercise group versus a negligible change in the control group (P<0.01).
Interestingly, intra-abdominal fat increased over 2 years by 7% in the exercise
group and by 21% in the control group, underscoring the benefits of resistance
training (without caloric restriction) in at least minimizing intra-abdominal
fat gain in middle-aged women. The benefits of resistance training may be most
noticeable among obese and older populations, who typically have the greatest
amount of abdominal fat.
Generally, short-term (less than 6 months) exercise interventions will
have a positive effect on body composition. However, the magnitude of these
alterations in body fat or lean mass may be of limited biological significance
(48). Studies that employ moderate- to vigorous-intensity
aerobic exercise of at least 55-75% VO2peak (4.5-6 METs), on most
days of the week (i.e., 4 or more days), over intervention periods of at least
9 months, report the most significant changes in body composition (7-9;91;93). In
general, the amount of adiposity present in study subjects at baseline will
affect the amount of fat lost with a given intervention. Indeed, studies
employing overweight or obese subjects (7;8;56;92;93) report greater improvements in body composition than
those studies using subjects of normal weight (48;96). Also important is the dose-response relation highlighted
by Ross and colleagues (93) between exercise-induced
weight loss and fat loss — that is, greater total weight loss will result
in greater fat loss (7;93).
Nonetheless, Ross and colleagues (93) report that, even
without coincident weight loss, 60 minutes per day of vigorous-intensity
exercise (approximately 500 kilocalories per day) on 7 days per week still
resulted in statistically significant reductions in total (7%), abdominal
(10%), and intra-abdominal (18%) fat in abdominally-obese premenopausal
women.
Overall, regular participation in aerobic physical activity causes
decreases in both total and abdominal adiposity, changes that are consistent
with improved metabolic function. The greater the volume of physical activity,
the larger the change in adiposity.
Question 4: What Effects Do Sex and Age Have on the
Role of Physical Activity in Energy Balance?
Conclusions
Some evidence indicates that the amount of physical activity needed to
maintain a constant weight differs between men and women and increases with
age. This may be due to a number of physiologic and behavioral factors that
also vary by sex and by age. However, the evidence is not sufficient to
recommend differential physical activity prescriptions based on sex or on age
alone.
Rationale
The Physical Activity Guidelines for Americans Scientific
Database was accessed using the following delineation terms: 1)
population sub-group: adults/older adults; 2) study
design: randomized controlled trials, longitudinal experimental
studies (before/after), prospective observational studies, and cross-sectional
studies; 3) health outcomes: body weight; and 4)
search term: aging, age, gender, men, women. Studies
identified using the Scientific Database were supplemented by
recently-published or in press scientific papers and review articles. Findings
presented here were limited to studies having a forward study design (i.e.,
prospective observational and/or longitudinal experimental studies) with
adequate statistical power to distinguish moderate effect sizes from chance
alone.
Sex
The prevalence of obesity is higher among women compared with men,
particularly among women from ethnic minority groups (4;97). Although women report less physical activity than men,
it is not clear whether this is actually so, or whether it is a consequence of
measurement error resulting from the low sensitivity of traditional physical
activity surveys (83;98;99). In any case, potential sex differences in the influence
of physical activity on weight stability are important to consider in
maximizing the utility of future public health guidelines.
Cross-sectional and longitudinal epidemiologic studies generally have
demonstrated inverse associations between physical activity and weight gain in
both men and women (e.g., 100-105). Dose-response
relationships have been somewhat less consistent in women than in men. However,
as stated previously, this may be attributable to measurement error associated
with self-reported data (100;106).
Indeed, objective measurements of energy expenditure (e.g., doubly-labeled
water) have either stronger inverse associations in men than in women or no
biological sex differences in response to different amounts of physical
activity (107). The few intervention studies that
included both men and women (along with sex-specific analyses) report weight or
fat losses only in men (107), no change in either sex (67), or similar changes in both men and women (e.g.,
8;58;89;108;109).
It is likely that differences in findings among these intervention
studies reflect dissimilarities among study protocols. However, even within
particular study samples, observed sex differences in weight loss responses to
exercise can be attributed to a number of factors. For instance, several highly
controlled laboratory-based intervention studies have noted that women are more
resistant to weight loss or may require greater energy expenditure compared
with men to maintain a healthy body weight (54;100;107). Indeed, this suggests that a
similar absolute energy expenditure (e.g., 1,200 kilocalories per week) may not
yield the same results in men and women. This may be due to a greater
proportion of less lipolytically responsive gluteofemoral adipose tissue in
younger and middle-aged women than in men of the same age. Animal studies also
have observed a sex dimorphism in the control of energy homeostasis that might
be attributed to a differential interaction between adiposity hormones and food
intake control systems in the brain (110;111). These biological sex differences in responsiveness to
weight change may be difficult to discern in large community-based
interventions or at the population level, however, due to measured or
unmeasured sex differences in: 1) how a similar level of physical activity is
performed (walking vs. water aerobics vs. running); 2) adherence to a given
exercise prescription; or 3) dietary intake. Because a number of other
physiological (body mass, peak aerobic capacity) or behavioral factors
(cigarette smoking, drinking, hormone replacement therapy) also may vary
between men and women, studies that measure sex differences in weight loss
responses to exercise must be careful to control for these covariables either
by matching in experimental designs or by appropriate statistical adjustments
when feasible.
Age
Because the risk of chronic disease increases markedly with sedentary
lifestyles and with age, the public health burden associated with inactivity is
substantial among middle-aged and older adults (88). In
general, lower levels of physical activity are associated with higher body
weight and body fat in middle-aged and older adults (4;87;112-114). The epidemiologic studies
to date provide clear longitudinal evidence linking habitual physical activity
to the prevention of excess weight gain in both men and women (100-105;115) and this is true even in
older age. Although the effect sizes from these observational studies appear
small, over the lifespan these small savings in excess weight gain accumulate
into net savings that are quite meaningful with regard to minimizing the risk
of obesity-related disorders. Moreover, the longitudinal epidemiologic evidence
suggests that as people progress from young adulthood to old age, they require
increasing amounts of daily energy expenditure to maintain a constant body
weight (37;104;105;115). More than likely, this is
due to a combination of physiologic (e.g., sex hormone depletion, decline in
peak aerobic capacity) and lifestyle changes (e.g., retirement) that occur with
aging that make older people more susceptible to positive energy balance and
thus to weight gain.
An active lifestyle also is beneficial in preventing weight
loss, an increasingly important concern for the oldest sectors
of the population (those older than 85 years) because of its relation to
metabolic disorders and functional ability. Several observational studies have
demonstrated the longitudinal benefits of even modest levels of physical
activity on preventing excess weight loss in older age, presumably through the
maintenance and preservation of lean mass (116-118).
Among intervention studies, training protocols are too variable and
sample sizes are often too small to establish dose-response relations between
changes in weight and activity type, duration, and intensity for different age
subgroups. Nonetheless, some intervention studies have demonstrated
statistically significant improvements in various weight-related outcomes
(e.g., BMI, body fat distribution) with aerobic and resistance training in
older participants (e.g., 8;89;108), whereas others have not (104;105). The magnitude of improvement
observed in many of these intervention studies is similar, but is smaller than
what is often observed in younger populations given the same relative exercise
dose. A similar relative stimulus (say 75% of VO2peak) will
translate into a lower absolute exercise dose in older compared with younger
people (due to lower levels of lean mass and aerobic capacity) and therefore,
may not result in an adequate stimulus for fat loss in older people. This may
be especially true for older women.
Question 5: How Do the Physical Activity Requirements
for Weight Maintenance Differ Across Racial/Ethnic and Socioeconomic
Groups?
Conclusions
Although some evidence suggests possible ethnic differences, the paucity
of data, particularly from the stronger longitudinal cohort or randomized,
controlled intervention study designs, makes it unwise to draw conclusions as
to whether physical activity requirements for weight stability or reduction
differ by racial/ethnic or socioeconomic groups.
Overview
Racial/ethnic disparities in obesity prevalence are robust and
persistent across socioeconomic groupings (e.g., 119-121). African Americans, American Indians/Alaska
Natives, Latinos and Pacific Islanders have substantially higher BMIs than do
whites and Asian Americans, and a significant interaction exists between
ethnicity and sex (122). For example, 54% of African
American women are obese, compared with 42% of Mexican American women and 30%
of white women (4). This contrasts with the similar obesity
rates among men: 34% of African Americans, 32% of Mexican Americans, and 31% of
whites (4).
Greater obesity implies a lesser ability to maintain weight and avoid
weight gain, which may be associated with less physical activity, more physical
inactivity, or both. However, racial/ethnic differences in the contribution of
physical activity to weight maintenance have been systematically examined only
infrequently. Therefore, in addition to the reasons to examine whether general
physical activity recommendations should differ between racial/ethnic groups
(See Part G. Section 11:
Understudied Populations, for a detailed discussion of this
topic), specifically exploring the possible need for different recommendations
to promote weight maintenance also is warranted. Available evidence suggests at
least 2 possible reasons for differential influences of physical activity on
weight maintenance by race/ethnicity:
- Differences in the energy cost of physical activity, such that some
ethnic groups would appear to derive lesser benefits for weight maintenance at
the same level of physical activity (e.g., 123).
- Differences in the relative contribution of physical activity and
excess calories (energy expenditure versus energy intake) to weight gain, such
that some ethnic groups would receive less benefit than others because physical
activity contributes less to the overall equation (124).
Experimental studies in exercise physiology have suggested that lower
resting energy expenditures and/or activity-related energy expenditures may
contribute to higher rates of obesity in Pima Indians and African Americans
than in whites (123;125;126). However, recent studies have demonstrated that these
physiological differences may, in fact, be explained by racial variations in
body morphology (e.g., trunk versus limb length, organ size) (127-129) that would not necessarily influence the ability to
maintain weight. The precise role in weight maintenance of racial/ethnic
differences in resting or activity-related energy metabolism (as opposed to age
or sex-related differences) in body composition is an important area for future
research.
Rationale
The Energy Balance subcommittee used a search strategy to generate 236
articles from the Physical Activity Guidelines for Americans
Scientific Database (all age group combinations except youth, with weight and
BMI as the outcome of interest, excluding studies focused on weight loss).
These articles were further screened to identify studies that linked physical
activity to weight-related outcomes and met the following criteria: 1)
targeting an ethnic minority group; or 2) including subgroup
analyses by ethnicity, not simply treating race/ethnicity as a co-variate and
adjusting for it; and 3) specifying the racial/ethnic minority
groups included in the analyses, not aggregating in the analyses as
"non-white;" and 4) having a sample size of 30 or more
participants or at least 30 participants per study arm; and 5)
having a "general audience" sample (i.e., not focusing on a specific subgroup
such as elite athletes or postpartum women). Even very recent studies in US
locations that have large ethnic minority populations, such as Baton Rouge, LA
(130) and St. Louis, MO (131), did
not characterize their samples by race/ethnicity. A MEDLINE search using
similar parameters to those of the Scientific Database (key words: ethnic
groups AND (body composition OR body weight OR obesity) AND (physical activity
OR exercise OR walking) yielded 399 articles, most of which were already
included in the Scientific Database. These articles were then further screened
by applying the above racial/ethnic minority inclusiveness and sample size
criteria, and eliminating those intervention studies in which physical activity
was not the dominant intervention component (i.e., nutrition was equally strong
or stronger). Reviews of relevant studies published after 1996 (132-135) and expert referral produced an additional in press
publication.
Of the 24 articles identified by this systematic review, half reported
on studies that were conducted outside the United States, including 9 in
Asia/Pacific Islands (China, Japan, Taiwan, India, New Zealand), 2 in Africa
(Nigeria, South Africa), and 1 in Central America (Mexico). Three were
longitudinal cohort studies, 7 were interventions, and 14 were cross sectional
studies.
Few of the 24 studies were population-based, and thus, findings may not
be representative even of subgroups with similar sociodemographic
characteristics to those studied. Relatively few studies included Latinos,
currently the largest minority group in the United States, and even fewer
studies included American Indians, with their tremendous intra-ethnic
heterogeneity from diverse tribal origins and affiliations. Most studies of
Asian Americans or Pacific Islanders took place outside of the United States,
introducing further complexity. International studies were included, however,
because so few domestic studies included substantive racial/ethnic diversity,
particularly among those with more rigorous designs. These studies may assist
in clarifying any influence of some biological or cultural differences which
may persist after migration to the United States, though they are likely to be
less applicable with regard to differences influenced by the specific
environmental or sociocultural context.
Of the 14 cross-sectional studies, which were conducted across a broad
variety of racial/ethnic minority groups, including African Americans,
Nigerians, South Africans, Pima Indians, Latinos, Asian Americans, Asians, and
East Indians, most found an inverse association between physical activity level
and weight/waist circumference/body fat percentage (29;103;113;136-146). This finding was consistent with studies in
predominantly white populations (147). Among elderly
Chinese, tai chi or swimming were associated with body fat distribution (lower
levels in the thigh and/or abdomen), but not with total body adiposity (145).
The exceptions were found in: (1) a study of 7,503 Mexican-American immigrants
in Harris County, Texas, in which physical inactivity was correlated with
obesity in women but not in men (103); (2) a study of 44
African American women (14% BMI less than 25, 25% to 30% Class II or III obese)
in rural areas and small cities in North Carolina, in which 3-day pedometer
step counts were not correlated with BMI or waist circumference (146); and (3) a study of 263 middle-aged Chinese in Hong
Kong (40% obese, 30% completely sedentary), in which low levels of physical
activity were not correlated with BMI or waist circumference (144). In these instances, it is likely that BMI and/or
physical activity was insufficiently variable to detect an effect.
Longitudinal studies in predominantly white populations generally
demonstrate associations between increases in physical activity and decreases
in the magnitude of weight gain (147). Of the 3
longitudinal studies identified in ethnic minority populations, however, only
one, a 4-city convenience sample across several US regions, The Study of
Women's Health Across the Nation or SWAN, replicated this association (113). SWAN study outcomes revealed associations between
increases in daily routine physical activity (active transportation and less TV
viewing) and exercise/sports, and less weight gain. On the other hand,
increases in physical activity, compared to baseline, were not associated with
smaller increases in weight, as reflected in findings of no change or decreases
in waist circumference (113). The findings of the two
nationally representative samples in the United States and Japan (114;148) were essentially null. He and
Baker (148) found that, between 1992 and 2000, regular
recreational physical activities, of any intensity, and work-related activities
were not associated with less weight gain. Race (Asian or white), education,
and income were not correlated with weight gain in multivariate analyses (148). However, although data were adjusted for
race/ethnicity, it is not clear whether differences in the physical
activity-weight gain association were analyzed by ethnicity. Lee and colleagues
(114) found no baseline association between physical
activity and weight, though the mean BMIs were 23.5–23.7 across activity
levels. This study apparently did not examine the relationship between changes
in physical activity and BMI changes. Thus, too few studies are available to
draw conclusions about the influence of race/ethnicity on the association
between physical activity and weight change over time.
Intervention studies selected for this review generally demonstrated
that resistance training, alone or in combination with moderate- to
vigorous-intensity aerobic physical activity, was necessary to produce changes
in BMI or body composition/distribution in ethnic minority populations (48;67;89;149-152),
despite the effectiveness of aerobic physical activity alone in improving
non-weight-related aspects of the metabolic profile, such as reducing blood
pressure (67;149). Wilmore and
colleagues (48) presented the only within-study "head to
head" inter-racial comparisons, with subgroup analyses after endurance training
using advancing intensity and duration on cycle ergometers. The magnitude of
weight loss for both whites and blacks was small; 0.2 kilogram (0.4 pound) mean
weight loss in both groups. The change was statistically significant in whites
but not blacks likely due to the larger sample size for whites (n=398) than
blacks (n=159). Changes in various measures of body fat followed a similar
pattern, with small but somewhat greater changes occurring in whites than
blacks (e.g., change in sum of skinfolds for whites = -7.1±0.8, blacks =
-4.1±1.5, P<0.05 for both). The ages (34.8 and 32.3 years for whites
and blacks, respectively) and BMI (25.0 and 26.6 kg/m2,
respectively) were similar. Adjustments for the subtle racial/ethnic variations
identified in experimental exercise physiology studies (e.g., 128) apparently were not performed (48). Wilmore and colleagues (48)
concluded that the magnitude of the changes in body composition was not
biologically significant in either blacks or whites and that a physical
activity intervention of greater volume or longer duration was needed to
produce meaningful changes in body weight and fat. In another study, in Japan,
even quantities/intensities of walking sufficient to increase VO2max
(13,500 to 14,500 steps per day in the experimental groups versus 5,800 in the
control group) did not alter BMI, although the participants in this study were
normal weight or minimally overweight (24.6 to 24.7 and 25.2 kg/m2,
respectively) (149). Participants were presumably
Japanese, although race/ethnicity of study samples is rarely specified in these
international studies. Contrasting findings were reported in another
international study. In this secondary analysis of data collected routinely on
government health and social services workers in Mexico, Lara and colleagues
(152) demonstrated a 0.32 kg/m2 BMI decrease,
a 1.0 kilogram (2.2 pound) weight loss, and a 1.6 centimeter (1.6 inch)
decrease in waist circumference at the end of 1 year after integrating
mandatory 10-minute structured group aerobic-calisthenic exercise breaks during
paid work time in this group of mostly middle-aged, overweight and abdominally
obese workers. Although the study had no control group, secular trends
documented in Mexico at that time were similar to the United States mean
increases of 1 to 2 pounds (0.45 to 0.9 kilograms) in body weight and 0.5
inches (1.27 centimeters) in waist circumference per year (113;152). The fact that the subjects
of the Mexican study were not volunteer participants, but rather a sample more
typical of the general population, and their overweight status, compared with
the mostly normal weight Japanese sample, may account for the discrepant
findings.
As noted in earlier reviews (e.g., 132-134;153) there is an extreme paucity of evidence on
racial/ethnic minority groups with regard to the effects of physical activity
on weight maintenance. In this review, no 2 studies examined the same
ethnic-sex samples — Japanese middle-aged men, Japanese elderly adults,
Japanese adults 30 to 69 years of age, Alaska Native women, African American
peri-menopausal women, African American and white young and middle-aged adults,
Mexican middle-aged adults — much less measures of activity duration or
intensity. Consequently, broad generalizations about the influence of
race/ethnicity on the physical activity requirements for weight stability or
reduction are premature.
Overall Summary and Conclusions
The overall conclusions of this chapter on physical activity and energy
balance can be summarized as follows:
Physical Activity, Weight Stability, and Weight
Loss
Regular participation in physical activity provides benefits for weight
stability, but with few data on this topic from long-term studies, the optimal
amount is not known. Available data from short-term clinical trials indicate
that a dose of physical activity in the range of 13 to 26 MET-hours per week
results in a modest 1% to 3% weight loss, consistent with weight stability over
time (7-9). Thirteen MET-hours per week is equivalent to
walking at a 4 mile per hour pace for 150 minutes per week or jogging at a 6
mile per hour pace for 75 minutes per week. Aerobic physical activity done at
this level would reduce upward migration of individuals from one BMI category
to the next. The wide range of physical activity levels (13 to 26 MET-hours per
week) needed for weight stability probably reflects individual variation in the
inherent (non-structured) level of physical activity and the degree to which
caloric intake is increased over time when a physical activity intervention is
initiated. The magnitude of weight loss resulting from resistance exercise in
this review was typically less than 1 kilogram (2.2 pounds). However, this may
have been affected by the relatively short duration of the study period and the
increase in fat-free mass associated with this type of intervention. Although a
weight loss of 5% or more of body weight can be achieved with large volumes of
physical activity, a coincident dietary intervention is typically needed to
achieve this goal. The dietary intervention could include maintenance of (at
pre-intervention levels) or an actual reduction in caloric intake.
Physical Activity and Weight Regain
Most of the available literature indicates that "more is better" when
it comes to the amount of physical activity needed to prevent weight regain
following weight loss. However, as indicated above, the literature has some
considerable shortcomings regarding the appropriate research design needed to
directly address this question. Studies by Ewbank and colleagues (72), Jakicic and colleagues (73) and
Schoeller and colleagues (74) indicate that the volume of
physical activity needed to prevent weight regain following weight loss is
approximately 31 MET-hours per week or 4.4 MET-hours per day. This is
equivalent to walking 54 minutes per day at 4 miles per hour or 80 minutes per
day at 3 miles per hour, or jogging for 26 minutes per day at 6 miles per
hour.
Physical Activity and Body Composition
Parameters
Ample evidence exists for a positive dose-response relation between the
volume (frequency, intensity, and duration) of endurance and/or resistance
exercise, the training duration, and the amount of total and regional fat loss.
Moreover, the evidence suggests that regional fat loss is greater with greater
amounts of exercise-induced total weight loss and among those with the greatest
levels of adiposity. In the absence of coincident caloric restriction, aerobic
physical activity in the range of 13 to 26 MET-hours per week results in
decreases in total and abdominal adiposity that are consistent with improved
metabolic function (7-9). Thirteen MET-hours per week is
equivalent to walking at a 4 mile per hour pace for 150 minutes per week or
jogging at a 6 mile per hour pace for 75 minutes per week. However, when more
physical activity is done (e.g., 42 MET-hours per week), decreases in
intra-abdominal adipose tissue approach 3 to 4 times the level seen with this
range of physical activity (93).
The Effect of Sex and Age on Physical Activity
and Energy Balance
Some evidence suggests that the amount of exercise necessary to
maintain a constant body weight differs between men and women and increases
with age due to a variety of physiological and lifestyle factors. Moreover,
even within a given sex- or age-group, weight loss responses to exercise vary
substantially. Thus, it is quite difficult to make a standard daily activity
recommendation that relates to optimal weight maintenance for everyone. On the
other hand, the evidence base is too sparse at this time to recommend
differential physical activity prescriptions based on sex or on age alone.
Physical Activity Requirements Across
Race/Ethnicity and Socioeconomic Groups
Although some evidence suggests possible ethnic differences, the
paucity of data, particularly from longitudinal cohort or randomized,
controlled intervention study designs, makes it unwise to draw conclusions as
to whether the effects of physical activity on weight maintenance or loss
differ by race/ethnicity or socioeconomic groups. Some of the questions
outlined in this section have yet to be fully addressed, although evidence is
suggestive, for example, that socioeconomic constraints, cultural preferences,
and baseline levels of sedentariness or obesity make low-intensity,
social-environmental interventions feasible, sustainable, and effective in many
racial/ethnic minority groups (152;154-160). However, simply conducting studies that include
representative sample populations will not suffice, because there likely will
be too few members of any one group to disaggregate findings by socioeconomic
status, race/ethnicity, and sex, or to examine interactions between these
critical sociodemographic factors.
Research Needs
This review of physical activity and energy balance identified a number
of research needs in each of the topic areas covered in the chapter.
Physical Activity, Weight Stability, and
Weight Loss
Studies that are appropriately designed, with sufficient statistical
power, and of sufficient length are needed to specifically examine the effects
of varying doses of physical activity on weight loss and weight stability
across a variety of population groups, especially for those in the normal BMI
range. Further examination of effects of physical activity mode, intensity,
duration, and frequency on weight loss and/or weight stability also would make
a valuable contribution to this area. Finally, research is needed to further
examine intervention strategies that are most effective at promoting and
maintaining sufficient doses of physical activity that will facilitate weight
loss and/or weight stability.
Physical Activity and Weight Regain
Most available literature is observational or has relied on
retrospective analysis of self selected and self-reported levels of physical
activity. Use of state-of-the art technology and complete energy balance
designs are absent from the literature. Specifically, adequately powered
studies of sufficient duration with randomization to different levels of
physical activity after weight loss appear to be lacking. This limitation needs
to be addressed to adequately explore the question of how much physical
activity is needed to prevent weight regain following weight loss.
Physical Activity and Body Composition
Parameters
There remains a need for more RCTs to distinguish exercise effects on
total and regional fat loss from those of weight loss per se. In addition, the
large-scale use of imaging techniques is necessary to distinguish the
responsiveness of subcutaneous and visceral fat depots to endurance and/or
resistance training. The ability of studies to translate imaging findings into
simple anthropometric measures, such as waist or abdominal circumference, would
increase the clinical and personal utility of the research. Finally, there is a
need to identify and to study people who are very susceptible to weight gain in
the current social environment and who thus may be most resistant to weight or
fat loss with exercise.
The Effect of Sex and Age on Physical Activity
and Energy Balance
Journal requirements stipulating that sex- and age-specific analyses be
conducted with sufficient statistical power would help to address the dearth of
information pertaining to individual and population differences in body weight
response to physical activity. In addition, it would be helpful to identify and
study people in the current social environment who are very susceptible to
weight gain and who thus may be most resistant to weight or fat loss with
exercise. Studies of how susceptibility to weight gain or resistance to
weight/fat loss may vary by sex and age would contribute substantially to the
obesity literature.
Physical Activity Requirements Across
Race/Ethnicity and Socioeconomic Groups
Two clear mandates emerge from this research synthesis. The first is to
increase attention and resources for studies that focus on diverse
race/ethnicity groups and lower socioeconomic status populations, or that
include sufficient numbers to permit subgroup analyses by race/ethnicity or
socioeconomic status. The second is to establish standards for peer-review
journals that require investigators to report race/ethnicity of samples. These
standards also should require investigators to conduct subgroup analyses by
race/ethnicity and/or socioeconomic status if sample sizes are sufficient,
rather than simply treating these as co-variates and adjusting for them.
Reference List
- National Heart, Lung, and Blood Institute,
National Institute of Diabetes and Digestive and Kidney Diseases. Clinical
guidelines on the identification, evaluation, and treatment of overweight and
obesity in adults: the evidence report. [Bethesda, Md.]: National Institutes of
Health, National Heart, Lung, and Blood Institute; 1998.
- Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen
M. Body-mass index and incidence of cancer: a systematic review and
meta-analysis of prospective observational studies. Lancet 2008 Feb
16;371(9612):569-78.
- Flegal KM, Graubard BI, Williamson DF, Gail MH.
Cause-specific excess deaths associated with underweight, overweight, and
obesity. JAMA 2007 Nov 7;298(17):2028-37.
- Ogden CL, Carroll MD, Curtin LR, McDowell MA,
Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States,
1999-2004. JAMA 2006 Apr 5;295(13):1549-55.
- United States.Agricultural Research Service.
Report of the Dietary Guidelines Advisory Committee on Dietary Guidelines for
Americans, 2005 2005 Available from
http://www.health.gov/dietaryguidelines/dga2005/report/default.htm.
- Ross R, Dagnone D, Jones PJ, Smith H, Paddags A,
Hudson R, Janssen I. Reduction in obesity and related comorbid conditions after
diet-induced weight loss or exercise-induced weight loss in men. A randomized,
controlled trial. Ann.Intern.Med. 2000 Jul 18;133(2):92-103.
- Irwin ML, Yasui Y, Ulrich CM, Bowen D, Rudolph
RE, Schwartz RS, Yukawa M, Aiello E, Potter JD, McTiernan A. Effect of exercise
on total and intra-abdominal body fat in postmenopausal women: a randomized
controlled trial. JAMA 2003 Jan 15;289(3):323-30.
- McTiernan A, Sorensen B, Irwin ML, Morgan A,
Yasui Y, Rudolph RE, Surawicz C, Lampe JW, Lampe PD, Ayub K, et al. Exercise
effect on weight and body fat in men and women. Obesity.(Silver.Spring) 2007
Jun;15(6):1496-512.
- Slentz CA, Aiken LB, Houmard JA, Bales CW,
Johnson JL, Tanner CJ, Duscha BD, Kraus WE. Inactivity, exercise, and visceral
fat. STRRIDE: a randomized, controlled study of exercise intensity and amount.
J.Appl.Physiol 2005 Oct;99(4):1613-8.
- St Jeor ST, Brunner RL, Harrington ME, Scott BJ,
Daugherty SA, Cutter GR, Brownell KD, Dyer AR, Foreyt JP. A classification
system to evaluate weight maintainers, gainers, and losers. J.Am.Diet.Assoc.
1997 May;97(5):481-8.
- Sherwood NE, Jeffery RW, French SA, Hannan PJ,
Murray DM. Predictors of weight gain in the Pound of Prevention study.
Int.J.Obes.Relat Metab Disord. 2000 Apr;24(4):395-403.
- Stevens J, Truesdale KP, McClain JE, Cai J. The
definition of weight maintenance. Int.J.Obes.(Lond) 2006 Mar;30(3):391-9.
- Folsom AR, Arnett DK, Hutchinson RG, Liao F,
Clegg LX, Cooper LS. Physical activity and incidence of coronary heart disease
in middle-aged women and men. Med.Sci.Sports Exerc. 1997 Jul;29(7):901-9.
- Giovannucci E, Ascherio A, Rimm EB, Colditz GA,
Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer
and adenoma in men. Ann.Intern.Med. 1995 Mar 1;122(5):327-34.
- Kavouras SA, Panagiotakos DB, Pitsavos C,
Chrysohoou C, Anastasiou CA, Lentzas Y, Stefanadis C. Physical activity,
obesity status, and glycemic control: The ATTICA study. Med.Sci.Sports Exerc.
2007 Apr;39(4):606-11.
- Katzel LI, Fleg JL, Busby-Whitehead MJ, Sorkin
JD, Becker LC, Lakatta EG, Goldberg AP. Exercise-induced silent myocardial
ischemia in master athletes. Am.J.Cardiol. 1998 Feb 1;81(3):261-5.
- Williams PT. Coronary heart disease risk factors
of vigorously active sexagenarians and septuagenarians. J.Am.Geriatr.Soc. 1998
Feb;46(2):134-42.
- Van Pelt RE, Davy KP, Stevenson ET, Wilson TM,
Jones PP, Desouza CA, Seals DR. Smaller differences in total and regional
adiposity with age in women who regularly perform endurance exercise.
Am.J.Physiol 1998 Oct;275(4 Pt 1):E626-E634.
- Rimbert V, Montaurier C, Bedu M, Boirie Y, Morio
B. Behavioral and physiological regulation of body fatness: a cross-sectional
study in elderly men. Int.J.Obes.(Lond) 2006 Feb;30(2):322-30.
- Brien SE, Katzmarzyk PT. Physical activity and
the metabolic syndrome in Canada. Appl.Physiol Nutr.Metab 2006 Feb;31(1):40-7.
- Oppert JM, Thomas F, Charles MA, Benetos A,
Basdevant A, Simon C. Leisure-time and occupational physical activity in
relation to cardiovascular risk factors and eating habits in French adults.
Public Health Nutr. 2006 Sep;9(6):746-54.
- Williams PT. Nonlinear relationships between
weekly walking distance and adiposity in 27,596 women. Med.Sci.Sports Exerc.
2005 Nov;37(11):1893-901.
- Kyle UG, Genton L, Gremion G, Slosman DO,
Pichard C. Aging, physical activity and height-normalized body composition
parameters. Clin.Nutr. 2004 Feb;23(1):79-88.
- Schroder H, Marrugat J, Elosua R, Covas MI.
Relationship between body mass index, serum cholesterol, leisure-time physical
activity, and diet in a Mediterranean Southern-Europe population. Br.J.Nutr.
2003 Aug;90(2):431-9.
- Rennie KL, McCarthy N, Yazdgerdi S, Marmot M,
Brunner E. Association of the metabolic syndrome with both vigorous and
moderate physical activity. Int.J.Epidemiol. 2003 Aug;32(4):600-6.
- Jakes RW, Day NE, Khaw KT, Luben R, Oakes S,
Welch A, Bingham S, Wareham NJ. Television viewing and low participation in
vigorous recreation are independently associated with obesity and markers of
cardiovascular disease risk: EPIC-Norfolk population-based study.
Eur.J.Clin.Nutr. 2003 Sep;57(9):1089-96.
- Lawlor DA, Taylor M, Bedford C, Ebrahim S. Is
housework good for health? Levels of physical activity and factors associated
with activity in elderly women. Results from the British Women's Heart and
Health Study. J.Epidemiol.Community Health 2002 Jun;56(6):473-8.
- Ball K, Owen N, Salmon J, Bauman A, Gore CJ.
Associations of physical activity with body weight and fat in men and women.
Int.J.Obes.Relat Metab Disord. 2001 Jun;25(6):914-9.
- Forrest KY, Bunker CH, Kriska AM, Ukoli FA,
Huston SL, Markovic N. Physical activity and cardiovascular risk factors in a
developing population. Med.Sci.Sports Exerc. 2001 Sep;33(9):1598-604.
- Trichopoulou A, Gnardellis C, Lagiou A, Benetou
V, Trichopoulos D. Body mass index in relation to energy intake and expenditure
among adults in Greece. Epidemiology 2000 May;11(3):333-6.
- Vaz de Almeida MD, Graca P, Afonso C, D'Amicis
A, Lappalainen R, Damkjaer S. Physical activity levels and body weight in a
nationally representative sample in the European Union. Public Health Nutr.
1999 Mar;2(1A):105-13.
- Martinez JA, Kearney JM, Kafatos A, Paquet S,
Martinez-Gonzalez MA. Variables independently associated with self-reported
obesity in the European Union. Public Health Nutr. 1999 Mar;2(1A):125-33.
- Slawta JN, McCubbin JA, Wilcox AR, Fox SD, Nalle
DJ, Anderson G. Coronary heart disease risk between active and inactive women
with multiple sclerosis. Med.Sci.Sports Exerc. 2002 Jun;34(6):905-12.
- Slattery ML, Potter J, Caan B, Edwards S, Coates
A, Ma KN, Berry TD. Energy balance and colon cancer--beyond physical activity.
Cancer Res. 1997 Jan 1;57(1):75-80.
- Schnohr P, Scharling H, Jensen JS. Intensity
versus duration of walking, impact on mortality: the Copenhagen City Heart
Study. Eur.J.Cardiovasc.Prev.Rehabil. 2007 Feb;14(1):72-8.
- Balkau B, Vierron E, Vernay M, Born C, Arondel
D, Petrella A, Ducimetiere P. The impact of 3-year changes in lifestyle habits
on metabolic syndrome parameters: the D.E.S.I.R study.
Eur.J.Cardiovasc.Prev.Rehabil. 2006 Jun;13(3):334-40.
- Williams PT, Wood PD. The effects of changing
exercise levels on weight and age-related weight gain. Int.J.Obes.(Lond) 2006
Mar;30(3):543-51.
- Kyle UG, Melzer K, Kayser B, Picard-Kossovsky M,
Gremion G, Pichard C. Eight-year longitudinal changes in body composition in
healthy Swiss adults. J.Am.Coll.Nutr. 2006 Dec;25(6):493-501.
- Kyle UG, Zhang FF, Morabia A, Pichard C.
Longitudinal study of body composition changes associated with weight change
and physical activity. Nutrition 2006 Nov;22(11-12):1103-11.
- Schroeder TE, Hawkins SA, Hyslop D, Vallejo AF,
Jensky NE, Wiswell RA. Longitudinal change in coronary heart disease risk
factors in older runners. Age Ageing 2007 Jan;36(1):57-62.
- Droyvold WB, Holmen J, Midthjell K, Lydersen S.
BMI change and leisure time physical activity (LTPA): an 11-y follow-up study
in apparently healthy men aged 20-69 y with normal weight at baseline.
Int.J.Obes.Relat Metab Disord. 2004 Mar;28(3):410-7.
- Wang BW, Ramey DR, Schettler JD, Hubert HB,
Fries JF. Postponed development of disability in elderly runners: a 13-year
longitudinal study. Arch.Intern.Med. 2002 Nov 11;162(20):2285-94.
- Berk DR, Hubert HB, Fries JF. Associations of
changes in exercise level with subsequent disability among seniors: a 16-year
longitudinal study. J.Gerontol.A Biol.Sci.Med.Sci. 2006 Jan;61(1):97-102.
- Murray LA, Reilly JJ, Choudhry M, Durnin JV. A
longitudinal study of changes in body composition and basal metabolism in
physically active elderly men. Eur.J.Appl.Physiol Occup.Physiol
1996;72(3):215-8.
- Donnelly JE, Jacobsen DJ, Heelan KS, Seip R,
Smith S. The effects of 18 months of intermittent vs. continuous exercise on
aerobic capacity, body weight and composition, and metabolic fitness in
previously sedentary, moderately obese females. Int.J.Obes.Relat Metab Disord.
2000 May;24(5):566-72.
- Ross R, Pedwell H, Rissanen J. Effects of energy
restriction and exercise on skeletal muscle and adipose tissue in women as
measured by magnetic resonance imaging. Am.J.Clin.Nutr. 1995 Jun;61(6):1179-85.
- Sykes K, Choo LL, Cotterrell M. Accumulating
aerobic exercise for effective weight control. J.R.Soc.Health 2004
Jan;124(1):24-8.
- Wilmore JH, Despres JP, Stanforth PR, Mandel S,
Rice T, Gagnon J, Leon AS, Rao D, Skinner JS, Bouchard C. Alterations in body
weight and composition consequent to 20 wk of endurance training: the HERITAGE
Family Study. Am.J.Clin.Nutr. 1999 Sep;70(3):346-52.
- Boudou P, Sobngwi E, Mauvais-Jarvis F, Vexiau P,
Gautier JF. Absence of exercise-induced variations in adiponectin levels
despite decreased abdominal adiposity and improved insulin sensitivity in type
2 diabetic men. Eur.J.Endocrinol. 2003 Nov;149(5):421-4.
- Campbell KL, Westerlind KC, Harber VJ, Bell GJ,
Mackey JR, Courneya KS. Effects of aerobic exercise training on estrogen
metabolism in premenopausal women: a randomized controlled trial. Cancer
Epidemiol.Biomarkers Prev. 2007 Apr;16(4):731-9.
- Dengel DR, Galecki AT, Hagberg JM, Pratley RE.
The independent and combined effects of weight loss and aerobic exercise on
blood pressure and oral glucose tolerance in older men. Am.J.Hypertens. 1998
Dec;11(12):1405-12.
- Murphy M, Nevill A, Neville C, Biddle S, Hardman
A. Accumulating brisk walking for fitness, cardiovascular risk, and
psychological health. Med.Sci.Sports Exerc. 2002 Sep;34(9):1468-74.
- Nakamura Y, Tanaka K, Yabushita N, Sakai T,
Shigematsu R. Effects of exercise frequency on functional fitness in older
adult women. Arch.Gerontol.Geriatr. 2007 Mar;44(2):163-73.
- Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J,
Sullivan DK, Johnson SL, Heelan K, Hise M, Fennessey PV, Sonko B, et al.
Effects of a 16-month randomized controlled exercise trial on body weight and
composition in young, overweight men and women: the Midwest Exercise Trial.
Arch.Intern.Med. 2003 Jun 9;163(11):1343-50.
- Schmitz KH, Jensen MD, Kugler KC, Jeffery RW,
Leon AS. Strength training for obesity prevention in midlife women.
Int.J.Obes.Relat Metab Disord. 2003 Mar;27(3):326-33.
- Schmitz KH, Hannan PJ, Stovitz SD, Bryan CJ,
Warren M, Jensen MD. Strength training and adiposity in premenopausal women:
strong, healthy, and empowered study. Am.J.Clin.Nutr. 2007 Sep;86(3):566-72.
- Kraemer WJ, Hakkinen K, Triplett-Mcbride NT, Fry
AC, Koziris LP, Ratamess NA, Bauer JE, Volek JS, McConnell T, Newton RU, et al.
Physiological changes with periodized resistance training in women tennis
players. Med.Sci.Sports Exerc. 2003 Jan;35(1):157-68.
- Maddalozzo GF, Snow CM. High intensity
resistance training: effects on bone in older men and women. Calcif.Tissue Int.
2000 Jun;66(6):399-404.
- Dionne IJ, Melancon MO, Brochu M, Ades PA,
Poelhman ET. Age-related differences in metabolic adaptations following
resistance training in women. Exp.Gerontol. 2004 Jan;39(1):133-8.
- Joseph LJ, Davey SL, Evans WJ, Campbell WW.
Differential effect of resistance training on the body composition and
lipoprotein-lipid profile in older men and women. Metabolism 1999
Nov;48(11):1474-80.
- Shaw I, Shaw BS. Consequence of resistance
training on body composition and coronary artery disease risk.
Cardiovasc.J.S.Afr. 2006 May;17(3):111-6.
- Teixeira PJ, Going SB, Houtkooper LB, Metcalfe
LL, Blew RM, Flint-Wagner HG, Cussler EC, Sardinha LB, Lohman TG. Resistance
training in postmenopausal women with and without hormone therapy.
Med.Sci.Sports Exerc. 2003 Apr;35(4):555-62.
- Taaffe DR, Duret C, Wheeler S, Marcus R.
Once-weekly resistance exercise improves muscle strength and neuromuscular
performance in older adults. J.Am.Geriatr.Soc. 1999 Oct;47(10):1208-14.
- Keeler LK, Finkelstein LH, Miller W, Fernhall B.
Early-phase adaptations of traditional-speed vs. superslow resistance training
on strength and aerobic capacity in sedentary individuals. J.Strength.Cond.Res.
2001 Aug;15(3):309-14.
- Donnelly JE, Jakicic JM, Pronk MP, Smith BK,
Kirk EP, Jacobsen DJ, Washburn R. Is resistance exercise effective for weight
management? Evid.Based Prev.Med. 2004;1(2):21-9.
- Engels HJ, Drouin J, Zhu W, Kazmierski JF.
Effects of low-impact, moderate-intensity exercise training with and without
wrist weights on functional capacities and mood states in older adults.
Gerontology 1998;44(4):239-44.
- Ishikawa K, Ohta T, Zhang J, Hashimoto S, Tanaka
H. Influence of age and gender on exercise training-induced blood pressure
reduction in systemic hypertension. Am.J.Cardiol. 1999 Jul 15;84(2):192-6.
- Maiorana A, O'Driscoll G, Dembo L, Goodman C,
Taylor R, Green D. Exercise training, vascular function, and functional
capacity in middle-aged subjects. Med.Sci.Sports Exerc. 2001 Dec;33(12):2022-8.
- Cheema BS, Gaul CA. Full-body exercise training
improves fitness and quality of life in survivors of breast cancer.
J.Strength.Cond.Res. 2006 Feb;20(1):14-21.
- Annesi JJ, Gann S, Westcott WW. Preliminary
evaluation of a 10-wk. resistance and cardiovascular exercise protocol on
physiological and psychological measures for a sample of older women.
Percept.Mot.Skills 2004 Feb;98(1):163-70.
- Wing RR. Physical activity in the treatment of
the adulthood overweight and obesity: current evidence and research issues.
Med.Sci.Sports Exerc. 1999 Nov;31(11 Suppl):S547-S552.
- Ewbank PP, Darga LL, Lucas CP. Physical activity
as a predictor of weight maintenance in previously obese subjects. Obes.Res.
1995 May;3(3):257-63.
- Jakicic JM, Winters C, Lang W, Wing RR. Effects
of intermittent exercise and use of home exercise equipment on adherence,
weight loss, and fitness in overweight women: a randomized trial. JAMA 1999 Oct
27;282(16):1554-60.
- Schoeller DA, Shay K, Kushner RF. How much
physical activity is needed to minimize weight gain in previously obese women?
Am.J.Clin.Nutr. 1997 Sep;66(3):551-6.
- Jakicic JM, Clark K, Coleman E, Donnelly JE,
Foreyt J, Melanson E, Volek J, Volpe SL. American College of Sports Medicine
position stand. Appropriate intervention strategies for weight loss and
prevention of weight regain for adults. Med.Sci.Sports Exerc. 2001
Dec;33(12):2145-56.
- Trumbo P, Schlicker S, Yates AA, Poos M. Dietary
reference intakes for energy, carbohydrate, fiber, fat, fatty acids,
cholesterol, protein and amino acids. J.Am.Diet.Assoc. 2002
Nov;102(11):1621-30.
- Klem ML, Wing RR, McGuire MT, Seagle HM, Hill
JO. A descriptive study of individuals successful at long-term maintenance of
substantial weight loss. Am.J.Clin.Nutr. 1997 Aug;66(2):239-46.
- Tate DF, Jeffery RW, Sherwood NE, Wing RR.
Long-term weight losses associated with prescription of higher physical
activity goals. Are higher levels of physical activity protective against
weight regain? Am.J.Clin.Nutr. 2007 Apr;85(4):954-9.
- Fogelholm M, Kukkonen-Harjula K. Does physical
activity prevent weight gain--a systematic review. Obes.Rev. 2000
Oct;1(2):95-111.
- Perri MG, McAllister DA, Gange JJ, Jordan RC,
McAdoo G, Nezu AM. Effects of four maintenance programs on the long-term
management of obesity. J.Consult Clin.Psychol. 1988 Aug;56(4):529-34.
- Leermakers EA, Perri MG, Shigaki CL, Fuller PR.
Effects of exercise-focused versus weight-focused maintenance programs on the
management of obesity. Addict.Behav. 1999 Mar;24(2):219-27.
- Fogelholm M, Kukkonen-Harjula K, Nenonen A,
Pasanen M. Effects of walking training on weight maintenance after a
very-low-energy diet in premenopausal obese women: a randomized controlled
trial. Arch.Intern.Med. 2000 Jul 24;160(14):2177-84.
- Pate RR, Pratt M, Blair SN, Haskell WL, Macera
CA, Bouchard C, Buchner D, Ettinger W, Heath GW, King AC, et al. Physical
activity and public health. A recommendation from the Centers for Disease
Control and Prevention and the American College of Sports Medicine. JAMA 1995
Feb 1;273(5):402-7.
- Jakicic JM, Marcus BH, Gallagher KI, Napolitano
M, Lang W. Effect of exercise duration and intensity on weight loss in
overweight, sedentary women: a randomized trial. JAMA 2003 Sep
10;290(10):1323-30.
- Andersen RE, Wadden TA, Bartlett SJ, Zemel B,
Verde TJ, Franckowiak SC. Effects of lifestyle activity vs structured aerobic
exercise in obese women: a randomized trial. JAMA 1999 Jan 27;281(4):335-40.
- Jeffery RW, Wing RR, Sherwood NE, Tate DF.
Physical activity and weight loss: does prescribing higher physical activity
goals improve outcome? Am.J.Clin.Nutr. 2003 Oct;78(4):684-9.
- DiPietro L. Physical activity in the prevention
of obesity: current evidence and research issues. Med.Sci.Sports Exerc. 1999
Nov;31(11 Suppl):S542-S546.
- DiPietro L. Physical activity in aging: changes
in patterns and their relationship to health and function. J.Gerontol.A
Biol.Sci.Med.Sci. 2001 Oct;56 Spec No 2:13-22.
- Tsuzuku S, Kajioka T, Endo H, Abbott RD, Curb
JD, Yano K. Favorable effects of non-instrumental resistance training on fat
distribution and metabolic profiles in healthy elderly people.
Eur.J.Appl.Physiol 2007 Mar;99(5):549-55.
- Dunstan DW, Daly RM, Owen N, Jolley D, De Court,
Shaw J, Zimmet P. High-intensity resistance training improves glycemic control
in older patients with type 2 diabetes. Diabetes Care 2002 Oct;25(10):1729-36.
- Evans EM, Van Pelt RE, Binder EF, Williams DB,
Ehsani AA, Kohrt WM. Effects of HRT and exercise training on insulin action,
glucose tolerance, and body composition in older women. J.Appl.Physiol 2001
Jun;90(6):2033-40.
- Gan SK, Kriketos AD, Ellis BA, Thompson CH,
Kraegen EW, Chisholm DJ. Changes in aerobic capacity and visceral fat but not
myocyte lipid levels predict increased insulin action after exercise in
overweight and obese men. Diabetes Care 2003 Jun;26(6):1706-13.
- Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL,
Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R. Exercise-induced
reduction in obesity and insulin resistance in women: a randomized controlled
trial. Obes.Res. 2004 May;12(5):789-98.
- DiPietro L, Dziura J, Yeckel CW, Neufer PD.
Exercise and improved insulin sensitivity in older women: evidence of the
enduring benefits of higher intensity training. J.Appl.Physiol 2006
Jan;100(1):142-9.
- Ryan AS, Hurlbut DE, Lott ME, Ivey FM, Fleg J,
Hurley BF, Goldberg AP. Insulin action after resistive training in insulin
resistant older men and women. J.Am.Geriatr.Soc. 2001 Mar;49(3):247-53.
- Poehlman ET, Dvorak RV, DeNino WF, Brochu M,
Ades PA. Effects of resistance training and endurance training on insulin
sensitivity in nonobese, young women: a controlled randomized trial.
J.Clin.Endocrinol.Metab 2000 Jul;85(7):2463-8.
- United States.Public Health Service.Office of
the Surgeon General., United States.Office of Disease Prevention and Health
Promotion., Centers for Disease Control and Prevention (, National Institutes
of Health (. The Surgeon General's call to action to prevent and decrease
overweight and obesity. Rockville, MD; Washington, DC: U.S. Dept. of Health and
Human Services, Public Health Service, Office of the Surgeon General ; For sale
by the Supt. of Docs., U.S. G.P.O.; 2001.
- DiPietro L. Physical activity, body weight, and
adiposity: an epidemiologic perspective. Exerc.Sport Sci.Rev. 1995;23:275-303.
- Prevalence of fruit and vegetable consumption
and physical activity by race/ethnicity--United States, 2005. MMWR
Morb.Mortal.Wkly.Rep. 2007 Apr 6;56(13):301-4.
- Haapanen N, Miilunpalo S, Pasanen M, Oja P,
Vuori I. Association between leisure time physical activity and 10-year body
mass change among working-aged men and women. Int.J.Obes.Relat Metab Disord.
1997 Apr;21(4):288-96.
- Thune I, Njolstad I, Lochen ML, Forde OH.
Physical activity improves the metabolic risk profiles in men and women: the
Tromso Study. Arch.Intern.Med. 1998 Aug 10;158(15):1633-40.
- Wier LT, Ayers GW, Jackson AS, Rossum AC,
Poston WS, Foreyt JP. Determining the amount of physical activity needed for
long-term weight control. Int.J.Obes.Relat Metab Disord. 2001 May;25(5):613-21.
- Barcenas CH, Wilkinson AV, Strom SS, Cao Y,
Saunders KC, Mahabir S, Hernandez-Valero MA, Forman MR, Spitz MR, Bondy ML.
Birthplace, years of residence in the United States, and obesity among
Mexican-American adults. Obesity.(Silver.Spring) 2007 Apr;15(4):1043-52.
- DiPietro L, Kohl HW, III, Barlow CE, Blair SN.
Improvements in cardiorespiratory fitness attenuate age-related weight gain in
healthy men and women: the Aerobics Center Longitudinal Study. Int.J.Obes.Relat
Metab Disord. 1998 Jan;22(1):55-62.
- DiPietro L., Dziura J, Blair SN. Estimated
change in physical activity level (PAL) and prediction of 5-year weight change
in men: the Aerobics Center Longitudinal Study. Int.J.Obes.Relat Metab Disord.
2004 Dec;28(12):1541-7.
- Lamonte MJ, Ainsworth BE. Quantifying energy
expenditure and physical activity in the context of dose response.
Med.Sci.Sports Exerc. 2001 Jun;33(6 Suppl):S370-S378.
- Saris WH, Blair SN, van Baak MA, Eaton SB,
Davies PS, Di PL, Fogelholm M, Rissanen A, Schoeller D, Swinburn B, et al. How
much physical activity is enough to prevent unhealthy weight gain? Outcome of
the IASO 1st Stock Conference and consensus statement. Obes.Rev. 2003
May;4(2):101-14.
- Singh MA. Exercise comes of age: rationale and
recommendations for a geriatric exercise prescription. J.Gerontol.A
Biol.Sci.Med.Sci. 2002 May;57(5):M262-M282.
- Wieser M, Haber P. The effects of systematic
resistance training in the elderly. Int.J.Sports Med. 2007 Jan;28(1):59-65.
- Woods SC, Gotoh K, Clegg DJ. Gender differences
in the control of energy homeostasis. Exp.Biol.Med.(Maywood.) 2003
Nov;228(10):1175-80.
- Sainsbury A, Schwarzer C, Couzens M, Fetissov
S, Furtinger S, Jenkins A, Cox HM, Sperk G, Hokfelt T, Herzog H. Important role
of hypothalamic Y2 receptors in body weight regulation revealed in conditional
knockout mice. Proc.Natl.Acad.Sci.U.S.A 2002 Jun 25;99(13):8938-43.
- Guo SS, Zeller C, Chumlea WC, Siervogel RM.
Aging, body composition, and lifestyle: the Fels Longitudinal Study.
Am.J.Clin.Nutr. 1999 Sep;70(3):405-11.
- Sternfeld B, Wang H, Quesenberry CP, Jr.,
Abrams B, Everson-Rose SA, Greendale GA, Matthews KA, Torrens JI, Sowers M.
Physical activity and changes in weight and waist circumference in midlife
women: findings from the Study of Women's Health Across the Nation.
Am.J.Epidemiol. 2004 Nov 1;160(9):912-22.
- Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki
S, Tsugane S. Physical activity and risk of colorectal cancer in Japanese men
and women: the Japan Public Health Center-based prospective study. Cancer
Causes Control 2007 Mar;18(2):199-209.
- Lewis CE, Smith DE, Wallace DD, Williams OD,
Bild DE, Jacobs DR, Jr. Seven-year trends in body weight and associations with
lifestyle and behavioral characteristics in black and white young adults: the
CARDIA study. Am.J.Public Health 1997 Apr;87(4):635-42.
- Suominen H. Changes in physical characteristics
and body composition during 5-year follow-up in 75- and 80-year-old men and
women. Scand.J.Soc.Med.Suppl 1997;53:19-24.
- Dziura J, Mendes de LC, Kasl S, DiPietro L. Can
physical activity attenuate aging-related weight loss in older people? The Yale
Health and Aging Study, 1982-1994. Am.J.Epidemiol. 2004 Apr 15;159(8):759-67.
- Ekelund U, Brage S, Franks PW, Hennings S, Emms
S, Wong MY, Wareham NJ. Physical activity energy expenditure predicts changes
in body composition in middle-aged healthy whites: effect modification by age.
Am.J.Clin.Nutr. 2005 May;81(5):964-9.
- Kumanyika S. Obesity in black women.
Epidemiol.Rev. 1987;9:31-50.
- Williams DR. Racial/ethnic variations in
women's health: the social embeddedness of health. Am.J.Public Health 2002
Apr;92(4):588-97.
- Chang VW, Lauderdale DS. Income disparities in
body mass index and obesity in the United States, 1971-2002. Arch.Intern.Med.
2005 Oct 10;165(18):2122-8.
- Adams PF, Schoenborn CA. Health behaviors of
adults: United States, 2002-04. Vital Health Stat.10 2006 Sep;(230):1-140.
- Ravussin E, Lillioja S, Knowler WC, Christin L,
Freymond D, Abbott WG, Boyce V, Howard BV, Bogardus C. Reduced rate of energy
expenditure as a risk factor for body-weight gain. N.Engl.J.Med. 1988 Feb
25;318(8):467-72.
- Kumanyika SK, Obarzanek E, Stevens VJ, Hebert
PR, Whelton PK. Weight-loss experience of black and white participants in
NHLBI-sponsored clinical trials. Am.J.Clin.Nutr. 1991 Jun;53(6 Suppl):1631S-8S.
- Chitwood LF, Brown SP, Lundy MJ, Dupper MA.
Metabolic propensity toward obesity in black vs white females: responses during
rest, exercise and recovery. Int.J.Obes.Relat Metab Disord. 1996
May;20(5):455-62.
- Allison DB, Edlen-Nezin L, Clay-Williams G.
Obesity among African American women: prevalence, consequences, causes, and
developing research. Womens Health 1997;3(3-4):243-74.
- Heimburger DC, Allison DB, Goran MI, Heini AF,
Hensrud DD, Hunter GR, Klein S, Kumanyika SK, Kushner RF, Rolls BJ, et al. A
festschrift for Roland L. Weinsier: nutrition scientist, educator, and
clinician. Obes.Res. 2003 Oct;11(10):1246-62.
- Byrne NM, Weinsier RL, Hunter GR, Desmond R,
Patterson MA, Darnell BE, Zuckerman PA. Influence of distribution of lean body
mass on resting metabolic rate after weight loss and weight regain: comparison
of responses in white and black women. Am.J.Clin.Nutr. 2003 Jun;77(6):1368-73.
- Luke A, Dugas L, Kramer H. Ethnicity, energy
expenditure and obesity: are the observed black/white differences meaningful?
Curr.Opin.Endocrinol.Diabetes Obes. 2007 Oct;14(5):370-3.
- Redman LM, Heilbronn LK, Martin CK, Alfonso A,
Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on
body composition and fat distribution. J.Clin.Endocrinol.Metab 2007
Mar;92(3):865-72.
- Weiss EP, Holloszy JO. Improvements in body
composition, glucose tolerance, and insulin action induced by increasing energy
expenditure or decreasing energy intake. J.Nutr. 2007 Apr;137(4):1087-90.
- Banks-Wallace J, Conn V. Interventions to
promote physical activity among African American women. Public Health Nurs.
2002 Sep;19(5):321-35.
- Kumanyika S. Obesity treatment in minorities.
In: Wadden TA, Stunkard AJ, editors. Obesity: theory and therapy. 3rd ed. New
York: Guilford Publications Inc.; 2002. p. xiii,377.
- Yancey AK, Kumanyika SK, Ponce NA, McCarthy WJ,
Fielding JE, Leslie JP, Akbar J. Population-based interventions engaging
communities of color in healthy eating and active living: a review.
Prev.Chronic.Dis. 2004 Jan;1(1):A09.
- Gibson CA, Kirk EP, LeCheminant JD, Bailey BW,
Jr., Huang G, Donnelly JE. Reporting quality of randomized trials in the diet
and exercise literature for weight loss. BMC.Med.Res.Methodol. 2005 Feb
23;5(1):9.
- Ghosh A, Das Chaudhuri AB. Explaining body
composition by some covariate factors among the elderly Bengalee Hindu women of
Calcutta, India. J.Nutr.Health Aging 2005 Nov;9(6):403-6.
- Slattery ML, Sweeney C, Edwards S, Herrick J,
Murtaugh M, Baumgartner K, Guiliano A, Byers T. Physical activity patterns and
obesity in Hispanic and non-Hispanic white women. Med.Sci.Sports Exerc. 2006
Jan;38(1):33-41.
- Yancey AK, Wold CM, McCarthy WJ, Weber MD, Lee
B, Simon PA, Fielding JE. Physical inactivity and overweight among Los Angeles
County adults. Am.J.Prev.Med. 2004 Aug;27(2):146-52.
- Hornbuckle LM, Bassett DR, Jr., Thompson DL.
Pedometer-determined walking and body composition variables in African-American
women. Med.Sci.Sports Exerc. 2005 Jun;37(6):1069-74.
- Mack KA, Anderson L, Galuska D, Zablotsky D,
Holtzman D, Ahluwalia I. Health and sociodemographic factors associated with
body weight and weight objectives for women: 2000 behavioral risk factor
surveillance system. J.Womens Health (Larchmt.) 2004 Nov;13(9):1019-32.
- Kruger HS, Venter CS, Vorster HH, Margetts BM.
Physical inactivity is the major determinant of obesity in black women in the
North West Province, South Africa: the THUSA study. Transition and Health
During Urbanisation of South Africa. Nutrition 2002 May;18(5):422-7.
- Fitzgerald SJ, Kriska AM, Pereira MA, De Court.
Associations among physical activity, television watching, and obesity in adult
Pima Indians. Med.Sci.Sports Exerc. 1997 Jul;29(7):910-5.
- Sternfeld B, Cauley J, Harlow S, Liu G, Lee M.
Assessment of physical activity with a single global question in a large,
multiethnic sample of midlife women. Am.J.Epidemiol. 2000 Oct 1;152(7):678-87.
- Hui SSC, Thomas N, Tomlinson B. Relationship
between physical activity, fitness and CHD risk factors in middle-age Chinese.
J.Phys.Act.Health 2005;2(3):307-23.
- Yu TY, Pei YC, Lau YC, Chen CK, Hsu HC, Wong
AM. Comparison of the effects of swimming and Tai Chi Chuan on body fat
composition in elderly people. Chang Gung.Med.J. 2007 Mar;30(2):128-34.
- Crane PB, Wallace DC. Cardiovascular risks and
physical activity in middle-aged and elderly African American women.
J.Cardiovasc.Nurs. 2007 Jul;22(4):297-303.
- Phelan S, Butryn M, Wing RR. Obesity prevention
during adulthood. In: Kumanyika S, Brownson RC, editors. Handbook of Obesity
Prevention. New York: Springer; 2007. p. 485-514.
- He XZ, Baker DW. Changes in weight among a
nationally representative cohort of adults aged 51 to 61, 1992 to 2000.
Am.J.Prev.Med. 2004 Jul;27(1):8-15.
- Iwane M, Arita M, Tomimoto S, Satani O,
Matsumoto M, Miyashita K, Nishio I. Walking 10,000 steps/day or more reduces
blood pressure and sympathetic nerve activity in mild essential hypertension.
Hypertens.Res. 2000 Nov;23(6):573-80.
- Witmer JM, Hensel MR, Holck PS, Ammerman AS,
Will JC. Heart disease prevention for Alaska Native women: a review of pilot
study findings. J.Womens Health (Larchmt.) 2004 Jun;13(5):569-78.
- Yancey AK, Ortega AN, Kumanyika SK. Effective
recruitment and retention of minority research participants. Annu.Rev.Public
Health 2006;27:1-28.
- Lara A, Yancey AK, Tapia-Conye R, Flores Y,
Kuri-Morales P, Mistry R, Subirats E, McCarthy WJ. Pausa para tu Salud:
reduction of weight and waistlines by integrating exercise breaks into
workplace organizational routine. Prev.Chronic.Dis. 2008 Jan;5(1):A12.
- Wilcox S, Parra-Medina D, Thompson-Robinson M,
Will J. Nutrition and physical activity interventions to reduce cardiovascular
disease risk in health care settings: a quantitative review with a focus on
women. Nutr.Rev. 2001 Jul;59(7):197-214.
- Kumanyika SK. Minisymposium on obesity:
overview and some strategic considerations. Annu.Rev.Public Health
2001;22:293-308.
- McNeill LH, Kreuter MW, Subramanian SV. Social
environment and physical activity: a review of concepts and evidence.
Soc.Sci.Med. 2006 Aug;63(4):1011-22.
- Marcus BH, Williams DM, Dubbert PM, Sallis JF,
King AC, Yancey AK, Franklin BA, Buchner D, Daniels SR, Claytor RP. Physical
activity intervention studies: what we know and what we need to know: a
scientific statement from the American Heart Association Council on Nutrition,
Physical Activity, and Metabolism (Subcommittee on Physical Activity); Council
on Cardiovascular Disease in the Young; and the Interdisciplinary Working Group
on Quality of Care and Outcomes Research. Circulation 2006 Dec
12;114(24):2739-52.
- Yancey AK, McCarthy WJ, Harrison GG, Wong WK,
Siegel JM, Leslie J. Challenges in improving fitness: results of a
community-based, randomized, controlled lifestyle change intervention. J.Womens
Health (Larchmt.) 2006 May;15(4):412-29.
- Yancey AK, Ory MG, Davis SM. Dissemination of
physical activity promotion interventions in underserved populations.
Am.J.Prev.Med. 2006 Oct;31(4 Suppl):S82-S91.
- Van Duyn MA, McCrae T, Wingrove BK, Henderson
KM, Boyd JK, Kagawa-Singer M, Ramirez AG, Scarinci-Searles I, Wolff LS,
Penalosa TL, et al. Adapting evidence-based strategies to increase physical
activity among African Americans, Hispanics, Hmong, and Native Hawaiians: a
social marketing approach. Prev.Chronic.Dis. 2007 Oct;4(4):A102.
- Kumanyika S, Bell R, Field A, Fortmann S,
Franklin B, Gillman M. Population based prevention of obesity: comprehensive
promotion of healthful eating, physical activity and energy balance - AHA
scientific statement. Circulation.In Press 2008.
top of page
Continue to G5. Musculoskeletal Health
Back to G3. Metabolic Health
Back to Physical Activity Guidelines Advisory Committee Report
|