Physical Activity Guidelines Advisory Committee Report
Part G. Section 7: Cancer
List of Figures
Introduction
In the United States, an estimated 45% of men and 38% of women will
develop cancer in their lifetimes (1). Only 10% to 15% of
cancers are due to an inherited genetic predisposition, and the remainder is
thought to be due to lifestyle or environmental factors (2). The International Agency for Research on Cancer (IARC)
estimates that 25% of cancer cases worldwide are due to overweight and obesity
and a sedentary lifestyle (2). The most consistent
associations between increased physical activity and reduced cancer risk have
been observed for colon and breast cancers. Growing evidence supports a reduced
risk of endometrial and lung cancers in physically active versus sedentary
persons. A large number of studies have investigated the association between
physical activity and prostate cancer and, in summary, have found no
association between physical activity and risk of prostate cancer. Few data are
available to determine whether physical activity affects risk of cancer at
other sites.
A total of 1,437,180 new cancer cases and 565,650 deaths from cancers
were projected to occur in the United States in 2008, and cancer is the leading
cause of death for men and women younger than age 80 (1). A
growing body of literature supports a role for physical activity in improving
cancer prognosis and quality of life (3). Therefore, it is
imperative to identify lifestyle factors that could be modified to reduce the
impact of cancer.
Review of the Science
Overview of Questions Asked
This chapter addresses 3 specific questions:
- What are the associations between physical activity and incidence of
specific cancers? If an association exists, what is the dose-response
pattern?
- What are the effects of physical activity on cancer survivors,
including late and long-term effects of treatment, quality of life, and
prognosis?
- What mechanisms explain the associations between physical activity
and cancers?
Data Sources and Process Used to Answer
Questions
This chapter reviews the available epidemiologic data on associations
between physical activity and risks of specific cancers, the intervention study
data on physical activity in cancer survivors, and the human experimental data
on mechanisms that might explain the links between physical activity and
cancer. The Cancer subcommittee began its literature review with the
Physical Activity Guidelines for Americans Scientific Database, which
includes publications since 1995 (see Part F: Scientific Literature Search
Methodology, for a full description of the Database). This
search was augmented with MEDLINE searches of English-language articles using
the terms "physical activity," "exercise," and "cancer." In addition, the
subcommittee used several comprehensive literature reviews (2;4-8).
Because all of the studies that examined the association of physical
activity with risk of cancer are observational epidemiologic studies, causality
cannot be inferred. However, chance is unlikely as an alternate explanation
because many of the results were statistically significant, particularly for
colon and breast cancer.
Question 1. What Are the Associations
Between Physical Activity and Incidence of Specific Cancers? If an Association
Exists, What Is the Dose-Response Pattern?
Conclusions
A large body of epidemiologic data exists on the association between
physical activity and the risk of developing various types of cancer. Although
the direct evidence of these associations derives only from observational
studies, randomized controlled trials (RCTs) have provided indirect evidence by
examining the association of physical activity with markers of cancer risk,
such as circulating levels of sex hormones, insulin, and cytokines.
The observational data are clearest for colon and breast cancer, with
case-control and cohort studies supporting a moderate, inverse relation between
physical activity and the development of these cancers. Individuals engaging in
aerobic physical activity for approximately 3 to 4 hours per week at moderate
or greater levels of intensity have on average a 30% reduction in colon cancer
risk and a 20% to 40% lower risk of breast cancer, compared with those who are
sedentary. A dose-response relation also is apparent, with risk decreasing at
higher levels of physical activity. However, little information is available
regarding what additional amounts and intensity of physical activity are
associated with additional risk reductions; it also is unclear what the
magnitude of the additional decrements in risk may be. The available evidence
suggests that at least 30 to 60 minutes per day of moderate to vigorous
intensity physical activity is required to significantly lower the risk of
colon and breast cancer.
Compared with sedentary people, the available epidemiologic data suggest
that active people have approximate reductions in risk of lung, endometrial,
and ovarian cancers of 20%, 30%, and 20%, respectively. The data overall do not
support associations of physical activity with prostate or rectal cancers. Too
few data exist regarding the other site-specific cancers to make reasonable
conclusions.
Rationale
Breast Cancer
Overall Associations
More than two dozen prospective cohort studies (9-34), and an even greater number of population-based
case-control studies (35-71) have examined the relation
between physical activity and breast cancer risk. These studies have primarily
assessed the role of recreational physical activity on breast cancer risk.
Overall, most studies suggest that physically active women have a lower risk of
developing breast cancer than sedentary women. The majority of reported cohort
studies (10-15;17;18;20-22;24;27;29;31-34) have reported a reduction in risk with physical
activity ranging from 20% to 80%, and a number of population-based case-control
studies (35-37;41-49;51;53;54;56;69-71) have reported reduction in
risk ranging from 20% to 70%. In a meta-analysis of 23 studies focused on
physical activity in adolescence and young adulthood, a summary relative risk
(RR) estimate of breast cancer for highest versus lowest category of physical
activity was 0.81 (95% CI 0.73-0.89), and each 1-hour increase of recreational
physical activity per week was associated with a 3% (95% CI 0%-6%) risk
reduction (72). A more extensive systematic review of
recreational activity and breast cancer risk included 19 cohort and 29
case-control studies (73). The review concluded that
evidence was strong that physical activity reduced risk of postmenopausal
breast cancer by 20% to 80%, and that each additional hour of physical activity
per week reduced risk for breast cancer by 6% (95% CI 3%-8%). They further
concluded that the effect of physical activity on premenopausal breast cancer
risk was a more modest 15% to 20% reduction.
The relevant lifetime periods for the effects of physical activity on
breast cancer risk are not established. Lifetime recreational physical activity
(35;51;54;64), adolescent physical activity (15;17;24;35;42;45;47), and
physical activity at various life points (10-14;17;18;22;24;37;47;49;51;60) have been
associated with lower breast cancer risk in several studies. Studies examining
risk more broadly for specific decades of life also have observed an inverse
association with some or all examined time periods (24;45;58;59).
Furthermore, physical activity after menopause has been found to reduce breast
cancer risk (12;22;24). Other studies that specifically looked at physical
activity at various life periods have not found a reduced breast cancer risk
with physical activity at any time (25).
Associations for Specific Subgroups
Within the United States, associations between increased physical
activity and decreased breast cancer risk have been observed in multiethnic
populations (24;70;71). These observations also hold true for specific racial
and ethnic populations, including black (46;70), Hispanic (49;71), and Asian American women (56).
Results from some studies with sufficient numbers in subgroup
populations suggest that the association with physical activity may be stronger
in women without a family history of breast cancer than in those with a family
history (22;34;51;69), although other large studies
have found that women with as well as women without a family history of breast
cancer had reduced risk of breast cancer with increasing physical activity (24;32). Several previous studies
reported a greater reduction in risk with increasing physical activity among
parous compared to nulliparous women (34;35). However, other studies have either observed the opposite
finding with risk reduction greater in nulliparous women (32;44), or have found no effect
modification of parity (24). One study found that physical
activity may be more strongly associated with reduced risk of postmenopausal
breast cancer in women who do not use menopausal hormones (22;71), though other studies found that
physical activity effect did not change according to menopausal hormone use (16;24;32;40;64).
In 4 studies, researchers examined effect modification by adult weight
gain (14;17;45;74), and 1 study reported a greater
reduction in risk among women who had less than a 17% increase in adult weight
(74). Several studies documented a greater reduction in
risk among leaner women compared to heavier women (11;24;44;51), although
other studies found that physical activity reduced risk of breast cancer in
women with all levels of body mass index (BMI) (32;34).
Two cohort studies have addressed the effect of physical activity on
breast cancer within the context of other variables related to energy balance,
specifically adiposity and dietary energy intake. One study found evidence that
premenopausal women who did not participate in vigorous activity, were
overweight or obese (BMI greater than 25 kg/m2), and had a
relatively high calorie intake (more than 1,970 kilocalories per day, as
determined from a food frequency questionnaire), had a statistically
significant 60% increased risk of breast cancer compared with active, normal
weight women with lower calorie intake (26). Another
cohort study found that women with the highest quartile of energy intake, were
obese, and participated in less than 4 hours per week of vigorous physical
activity, had a RR of 2.1 (95% CI 1.27-3.45) compared with normal or
overweight, active women who had the lowest quartile of calorie intake (31).
In hypothesizing about the reasons for the effects of energy balance on
breast cancer risk, investigators speculate that, in addition to the potential
role of obesity as an effect modifier, obesity possibly may be on the causal
pathway between physical activity and postmenopausal breast cancer development.
Specifically, physical activity reduces levels of adiposity, and subsequently
reduces adipose tissue production of estrogen and testosterone, both of which
can promote breast carcinogenesis or progression. However, most studies of
physical activity and breast cancer have adjusted for BMI as a proxy for
adiposity, and an inverse association between physical activity and breast
cancer risk persisted. Thus, it is likely that physical activity may have some
effect on breast cancer risk independent of their mutual relation with
adiposity.
Dose-Response Pattern
Several studies have tried to quantify the level of physical activity
required for a decreased risk of breast cancer. Investigators have reported
statistically significantly lower rates of breast cancer among women exercising
at least 1 hour per week (14); exercising at least 3.8
hours per week (primarily vigorous exercise) (35);
exercising to keep fit at least 4 hours per week (11); and
exercising vigorously at least 7 hours per week (46).
Other investigators have observed significantly lower rates among women
expending at least 1,500 kilocalories per week (18)
(approximately 4 hours per week of moderate-intensity activity); at least 15.3
metabolic equivalent (MET)-hours per week (approximately 4 hours per week of
moderate-intensity activity) (41); and at least 17.6
MET-hours per week (4 to 5 hours per week of moderate-intensity activity) (74). Two reports from the same study found significantly
lower rates of breast cancer only among women who exercised vigorously on a
daily basis during the ages to 14 to 22 years (45;68). Finally, in a study where total physical activity over
the lifetime was assessed, significantly lower breast cancer rates were seen in
women who expended at least 47.5 MET-hours per week per year in total activity
(48). The impact of these various levels of activity on
reducing risk has varied from 20% to 40%. However, other studies that
specifically looked at dose-response have had not found an effect of any dose
of exercise on breast cancer risk (28;30). Overall, it appears that at least 4 to 7 hours per week
of moderate to vigorous intensity physical activity is required to produce a
statistically significant reduction in risk, although some evidence suggests
greater reduction in risk with greater amount of activity, such that 1 hour per
day of moderate or vigorous activity produces greater reduction in risk
compared with the Surgeon General recommendation of 30 minutes per day on most
days of the week (4).
Tumor Characteristics
The incidence of in situ breast cancer is largely influenced by
prevalence of screening, and therefore it is important to examine the effects
of physical activity on invasive breast cancer separately from that on in
situ breast cancer. Furthermore, breast cancers consist of distinct
biological subtypes that are strongly related to prognosis and that may differ
in etiology including tumor responsiveness to hormones (i.e., estrogen and
progesterone receptor [ER/PR] status positive or negative), and other tumor
characteristics (e.g., Her2neu receptor status, and other proliferative
indices). A few studies have separately examined the association between
physical activity and in situ versus invasive breast cancer, however,
very few have examined physical activity effects separately in hormone receptor
positive or negative tumors, and none has considered other tumor
characteristics. In one study, researchers examined the association between
physical activity and breast cancer stratified by stage of disease and found
risk reduction to be greater for localized invasive disease compared to either
in situ or regional/distant breast cancer (54). A
recent large cohort study found a greater risk reduction for in situ
(RR 0.69, P for trend 0.04) than for invasive breast cancer (RR 0.80,
P for trend 0.02) (34). One case-control study
focused specifically on in situ breast cancer, and found that risk of
this stage of breast cancer was approximately 35% lower in women reporting any
lifetime exercise activity compared with sedentary women (54). Two studies also reported risk ratios separately for
ER/PR positive and negative tumors, but neither found a difference in risk by
ER/PR subtype (18;75). A recent
cohort study found that women who reported high versus low levels of physical
activity at enrollment had a 13%, 33%, and 20% decreased risk of developing
ER+/PR+, ER+/PR−, and ER−/PR− breast cancer, respectively (29). Another large cohort study found the greatest risk
reduction from increased physical activity for ER−/PR− breast
cancer (34). Most other studies have included too few
women with hormone receptor negative disease to be able to assess the
association of physical activity with risk of this subtype of breast
cancer.
Type of Physical Activity
An association of sedentary occupations with increased risk of breast
cancer has been documented in reports from some (11;17;27) but not other (76) prospective cohort studies. Published reports from some
(43;47;48;53;61;77) but not
other (41;44;52;55;56)
population-based case-control studies also document an inverse association
between occupational physical activity and breast cancer risk.
The effect of low-intensity activity (such as household activities,
gardening, dancing, leisurely walking, or other activities with a MET score
below 4) on breast cancer risk is still unclear, but may be of importance,
particularly for postmenopausal breast cancer, as a large portion of activity
among postmenopausal and elderly women is not vigorous. Although previous
studies have examined the association between leisure-time physical activity,
such as walking, biking, swimming, and aerobics, few studies have included the
effects of other low-intensity activities, such as gardening, housework, or
shopping, in their calculation of leisure-time physical activity. This may lead
to an underestimation of true energy expenditure, especially among groups of
women who do not have access to recreational or sports activity. In support of
the importance of household activity, a recent large European cohort study
found that for women in the highest versus lowest household activity quartile,
risks for postmenopausal and premenopausal breast cancer were reduced by 19%
(P for trend 0.0001) and 29% (P for trend 0.0003),
respectively (33).
Prostate Cancer
Overall Associations
A number of epidemiologic studies have examined physical activity and
prostate cancer risk (28;78-123),
including 25 cohort studies (28;78;80-82;87-89;91-102;117;119-121;123) and 14 case-control
studies (104-116;118). Several of
the cohort studies (78;80;81;94;117)
included prostate cancer mortality or advanced or metastatic prostate cancer as
at least one endpoint. One study (91) also examined the
association between cardiopulmonary fitness and risk of prostate cancer.
Of these studies, 19 found some suggestion for an inverse relation
between physical activity and prostate cancer (80;81;87;89;91;93;94;96;97;101;102;104;105;109;112;113; 117;120;123).
No overall association between physical activity and prostate cancer was found
in 14 studies (28;82;88;95;98-100;106;108;110;114;115;119;121), and an increased risk of prostate cancer among
physically active men was found in some studies (78;92;107;111). The
size of the association ranged from an 80% reduction in prostate cancer risk
for the highest physical activity levels (113) to a 220%
increased risk in one study (111).
Associations for Specific Subgroups
No consistent subgroup effects have been defined for demographic or
health factors such as age, race/ethnicity, or BMI. A recent study found that,
among men with a family history of prostate cancer, risk for those in the
highest quartile of physical activity was reduced by 52%, compared to that for
those in the lowest quartile of physical activity. Those without a family
history had no risk reduction (115).
Dose-Response Pattern
Several studies have attempted to quantitate a dose-response association
of prostate cancer risk with levels of physical activity (82;87;88;91-96;98;100-102;104-106;108-110;112-118;120;121). A statistically significant
trend toward decreasing prostate cancer risk with increasing physical activity
level was observed in several studies (87;93;94;102;104;105;113;118;120), although this was limited to
advanced disease in two studies (120;124). In one study, a 74% reduction in prostate cancer risk
was found in the highest compared to the lowest quartile of fitness level (91).
Type of Physical Activity
Occupational activity was associated with a decreased risk of prostate
cancer in several studies (80;81;93;97;105;113;116) and recreational activity
decreased risk of either overall or advanced prostate cancer in several
additional studies (91;93;94;96;101;104;115;117). In
one study, non-significant risk decreases were found for occupational and
recreational activity but an increased risk was observed for household activity
(115). No study differentiated between types of
recreational activity, such as aerobic or resistance exercise, or to their
subtypes such as jogging versus walking. Rather, activities were combined into
measures of MET-hours per week, or to measures of frequency or total duration
of activity per week.
Effect of Tumor Detection
One consideration for prostate cancer is the effect of screening.
Prostate-specific antigen (PSA) screening for early detection became widespread
in the United States in the 1990s. If physically active men also are more
health conscious (i.e., they are more likely to be screened for prostate
cancer), it may result in higher observed rates of prostate cancer among these
men because of increased detection. This notion is supported by several cohort
studies (94;117;120), which identified a reduction in risk of aggressive,
metastatic, or fatal prostate cancer with increased physical activity level. In
contrast, an investigation of physical activity and prostate cancer, diagnosed
in 1988 or earlier (before widespread PSA screening was available), among
Harvard University alumni found an almost halving of prostate cancer incidence
rates among men aged 70 years or older who expended at least 4,000 kilocalories
per week in physical activity, compared with those expending less than 1,000
kilocalories per week (84). However, an updated analysis
of these men, examining prostate cancer diagnosed after 1988, did not support
the earlier observations (100). These inconsistent
findings may have been due to bias arising from increased screening for
prostate cancer among the most active men.
Colon Cancer
To examine the association between physical activity and colon cancer,
the Cancer subcommittee searched the Physical Activity Guidelines for
Americans Scientific Database. Because the association of physical
activity with colon and rectal cancer appears different (see rectal cancer below), we did not include studies where
colorectal cancer was the outcome of interest, as the relation between physical
activity and colon cancer likely would be diluted. The search yielded 23
publications eligible for inclusion in the present report.
Overall Association
The 23 publications reviewed represented 12 prospective cohort studies
(28;99;124-133)
and 8 case-control studies (134-140). Four of these
publications (137-140) pertained to different aspects of
the same case-control study. The database represented by the 23 studies was
large, including a total of 9,747 cases of colon cancer with approximately
equal distribution between the sexes (4,933 in men and 4,814 in women). The
studies were conducted in the United States (124-126;129;131;132;137-142), Europe (Denmark (28), Finland
(99;127), Italy (143), Norway (133), Sweden (128) and Switzerland (136)), and Asia
(China (134), Japan (130;135), and Taiwan (144)).
Overall, the studies consistently show an inverse association between
physical activity and the risk of developing colon cancer, with 9 of the 12
cohort studies and 5 of the 8 case-control studies indicating significant,
inverse associations with at least one domain of physical activity (e.g.,
occupational versus leisure-time) and/or in one sex. Across all the studies,
the median RR, comparing most with least active subjects, was 0.7. More
specifically, results were similar across the cohort studies (median RR = 0.8)
and the case-control studies (median RR = 0.7), as well as for men and women
(median RR in both sexes = 0.7). These findings, encompassing studies published
in 1995 and later in the Physical Activity Guidelines for Americans
Scientific Database, are comparable with findings from a recent review of the
literature on physical activity and colon cancer risk that also included
studies published before 1995 (145). In this recent
review, the median RR, comparing most with least active subjects across all
studies, also was 0.7 (median RR for men, 0.7; for women, 0.6).
For the prospective cohort studies, bias due to recall of physical
activity is unlikely because physical activity was assessed before the
development of colon cancer. Thus, any misclassification of physical activity
is likely to be random, diluting associations rather than causing spurious
inverse relations. The results also do not appear to be confounded by other
factors associated with colon cancer risk because many studies adjusted their
findings for several factors, including BMI, smoking, alcohol, diet (e.g.,
energy intake, intake of calcium and folate intake of fiber, vegetables, and
meat), use of aspirin, screening, menopausal status and use of menopausal
hormone therapy, and family history of colon cancer. In particular, the
findings appear independent of BMI, with most cohort studies (9 of 12) (28;124;125;127-130;132;133)
adjusting for this factor, and continuing to observe significant inverse
associations. However, fewer than half the case-control studies (3 of 8)
adjusted for BMI. Finally, the inverse associations observed are supported by
plausible biologic mechanisms. Thus, although the data on physical activity and
risk of developing colon cancer are based on observational epidemiologic
studies, the inverse associations indicated by these studies are likely to be
real.
Associations for Specific Subgroups
Several studies have examined whether the association between physical
activity and decreased colon cancer risk varies, depending on use of menopausal
hormone therapy (125;131), various
aspects of diet (125;139), or BMI
(125;128;130;134;138;142). The findings have been inconsistent, with the most
consistent being the suggestion that the adverse impact of high BMI on colon
cancer risk may be ameliorated by higher levels of physical activity (134;138;142).
Several studies also have examined whether physical activity has a
different association with colon cancers occurring at different subsites of the
colon. The data have been equivocal, with some studies suggesting a larger
magnitude of association for cancers occurring in the proximal colon (130;136;142),
while others have reported greater associations for cancers of the distal colon
(128;132). Most studies, however,
have observed equivalent associations or unclear differences across proximal
and distal sites of the colon (125;126;131;133;135;140;143).
Dose-Response Pattern
All but one (99) of the studies classified subjects
according to at least 3 levels of physical activity, allowing investigators to
assess dose-response. In the cohort studies, 7 of 11 studies with at least 3
physical activity levels reported significant, inverse trends between physical
activity and colon cancer risk (124;126-128;130;132;133). For the case-control
studies, 4 of 8 also observed significant, inverse trends between activity
level and colon cancer risk (134;136;143;144). As
discussed above, because of the many different methods used to assess and
classify physical activity in these studies, it is difficult to ascertain the
shape of the dose-response curve, apart from noting that a dose-response
relation appears likely.
Types and Amount of Physical Activity
Most studies have assessed leisure-time and/or occupational physical
activity only, with one study also assessing active commuting, in the form of
walking or bicycling to work (134). Because of the
different questionnaires used to assess physical activity and the different
categories used to group subjects in the studies, it is difficult to integrate
the findings across the studies. Further, most studies presented their findings
according to overall volume of energy expended, and data are sparse on specific
kinds of activity associated with decreased colon cancer risk.
However, significantly lower risks of colon cancer have been observed
with leisure-time physical activity (ordered in approximately ascending doses
of physical activity) of at least twice a week for at least 10 minutes'
duration (142), at least 4 hours per week of
moderate-to-vigorous intensity recreational activity (131), at least 20 MET-hours per week of leisure-time
activity (144), more than 21 MET-hours per week of
leisure-time activity (132), 7 or more hours per week of
recreational activities including walking (126) a median
of 35.25 MET-hours per week of overall activity (130), a
median of 46.8 MET-hours per week of leisure-time activity (124), and more than 94.3 MET-hours per week of active
commuting (134). Additionally, the case-control study by
Slattery and colleagues suggests that physical activity needs to be vigorous in
intensity (137-140) to reduce colon cancer risk. Overall,
these data suggest that 30 to 60 minutes per day of moderate-to-vigorous
intensity physical activity may be needed to significantly lower the risk of
developing colon cancer.
Rectal Cancer
In contrast to the associations observed between physical activity and
colon cancer, the data on physical activity and risk of developing rectal
cancer are far more mixed. More than half of the studies have reported no
significant associations (99;126;130;133;143;144;146), with the remaining studies
observing significantly lower risks (or of borderline significance) with higher
levels of physical activity (127;128;135;136;140). In a recent review of the literature on physical
activity and rectal cancer risk, the median relative risk, comparing most with
least active subjects across all studies, was 1.0, indicating little
association (130).
Additional Cancer Sites
The available evidence for an association of physical activity with
reduced risk of lung, endometrial, ovarian, pancreatic, and other cancers is
less complete than that for breast, prostate, colon, and rectal cancers.
Therefore, the following sections present a general overview.
Lung Cancer
A review of the association of physical activity and lung cancer risk
was included within a recently published book chapter (145). At the time of that review, 15 cohort studies and 6
case-control studies had been published, overall indicating a median of 20%
reduced risk for lung cancer in the most versus the least active subjects. This
present review focused on studies published between 1996 and 2006. Results
indicate a 24% median reduction of lung cancer risk for the most versus least
active subjects (101;147-156). As
with the prior review, the reduction of risk was more obvious with case-control
(median RR over 2 studies = 0.61) (154;156) than with cohort studies (median RR over 8 studies =
0.77) (101;147-153). The inverse
relation of physical activity with lung cancer risk is similar for men (0.74, 8
studies since 1996) (101;147;149-153;156) and women (0.75, 6
studies since 1996) (147-149;152;154;156).
Most of the studies on the association of physical activity and lung
cancer adjust for cigarette smoking. However, even with this adjustment, the
potential for residual confounding is quite high. Three studies have reported
risk reductions specifically for current smokers, former smokers, or never
smokers (148;150;155). The risk reduction in these studies is more similar
for current and former smokers (median RR of 0.61 (148;150;155) and 0.59 (148;150), respectively) than for never
smokers (median RR of 1.03 for 2 studies reporting for this subgroup) (148;155). As yet, evidence is too
sparse to conclude that the reduction of lung cancer risk by physical activity
is isolated to current and former smokers.
The question of whether the association of physical activity with lung
cancer is due to residual confounding by smoking has been addressed in 2 ways:
examining consistency of association across histologic subtypes of lung cancer
and exploring the association in never smokers. Smoking is more clearly
established as a risk factor for some histologic subtypes of lung cancer than
others. Evidence links smoking more closely to small cell and squamous cell
lung cancers than to adenocarcinoma of the lung. Therefore, one indirect
approach to the question of whether the association of physical activity with
reduced risk for lung cancer is due to residual confounding by smoking status
is to evaluate whether the association is present for all histologic subtypes,
including adenocarcinoma. Three studies to date have examined whether the
association of physical activity is similar across most lung cancer histologic
subtypes, as well as within sex. In men, the median relative risks for lung
cancer for those who are most versus least active are 0.59, 0.96, 0.80, and
0.73 for small cell, squamous cell, adenocarcinoma, and other/nonspecified
histologic types, respectively (147;149;156). In women, the median RR
values for most versus least active among the same subtypes are 0.81, 0.77,
0.86, and 0.56, respectively (147;148;156). This evidence suggests that
the physical activity association is present across histologic subtypes,
including adenocarcinoma. Another approach to determining whether the overall
association of physical activity and lung cancer is due to residual confounding
by smoking is to study non-smokers. The RR (or odds radio) for non-smokers was
1.32 and 0.74 in the one cohort and one case-control study to report an
association specifically for non-smokers (148;155). It also should be noted that the 39% risk reduction
for most versus least active current smokers pales in comparison to the
reduction of risk from quitting smoking. Smoking cessation remains the most
important means to reduce lung cancer risk among smokers. That stated, it would
be of interest to understand better the potential mechanisms by which physical
activity may assist in marginally reducing lung cancer risk among current and
former smokers. To our knowledge, no research has directly addressed this
question.
Endometrial Cancer
A review of the association of physical activity and endometrial cancer
risk was included within a recently published book chapter (145). At the time of that review, 4 cohort studies and 11
case control studies had been published, overall indicating a median relative
risk of 0.70 for the most versus the least active subjects. An update of that
review, focusing on studies published since 1996 reported a similar median RR
(0.70) for the 15 most recently published studies, which included 7 cohort
studies and 8 case-control studies (157-171). Of these
more recent studies, 5 include relative risks that are adjusted for multiple
variables but not for BMI (157-160;171). The median RR of 0.73 for these studies is similar to
the results after adjustment for BMI, which was 0.70. This is important because
the effect of physical activity on body weight has been hypothesized to mediate
the purported association of physical activity with reduction of risk of
endometrial cancer.
Another factor that should be accounted for in analyses is menopausal
hormone therapy, given the potential causal link between use of unopposed
estrogen therapy and increased risk of endometrial cancer. For example, the
median RR from the 3 case-control studies that adjusted for menopausal hormone
therapy was 0.70 (165;168;170) (no cohort studies published to date have adjusted for
this factor) compared to a median RR of 0.68 for the 10 case-control and cohort
studies that did not adjust for menopausal hormone therapy (157-164;169;171).
Overall, evidence indicates an inverse association between physical
activity and incidence of endometrial cancer. Further, the lack of change in
the median relative risks for studies that did versus did not adjust for BMI or
menopausal hormone therapy may indicate that the association is not mediated
through obesity or the generally healthy lifestyle commonly associated with
exogenous hormonal exposure.
Ovarian Cancer
The association of physical activity with incidence of ovarian cancer
was explored in a meta-analysis and systematic review published late in 2007
(172). This review concluded that a modest inverse
association exists, with a weighted pooled RR of 0.81 (95% confidence interval
was 0.72-0.92). Sensitivity analyses indicated no difference of findings when
summarized studies did versus did not adjust for BMI (which may be on the
causal pathway) and for exogenous hormone use (e.g., oral contraceptives).
Pancreatic Cancer
A total of 8 cohort studies (173-180) and 2
case-control studies (181;182) have
examined whether physical activity may reduce incidence of pancreatic cancer.
Case-control studies may be particularly biased for pancreatic cancer, given
that at diagnosis most patients have advanced disease, are symptomatic, and
often have recent weight loss. Four of the 10 cited case-control studies of
pancreatic cancer adjusted for BMI in multivariate models. In the 5 cohort
studies that did not adjust for BMI (173-177), the median
relative risk for the association of physical activity with pancreatic cancer
incidence was 1.21. One of the case-control studies provided an odds ratio (OR
= 0.78) for men that was not adjusted for BMI (182). Both
of the case-control studies provided odds ratios for women that were not
adjusted for BMI; the average of these was 0.82 (181;182). In spite of this inconsistent evidence, when taken in
combination with the observation of a weak positive association with BMI (177;183), it remains possible that a
level of physical activity sufficient for weight control would be associated
with reduced incidence of pancreatic cancer.
Other Cancers
The potential for physical activity to reduce incidence of other cancers
(e.g., thyroid, kidney, bladder, and hematopoietic) also has been studied.
Reviews of these cancers are not included here because the data are too sparse
to allow any conclusions regarding a potential relation with physical
inactivity. Readers are referred to other reviews for an overview of results
from these studies (2;145).
Question 2: What Are the Effects of
Physical Activity on Cancer Survivors, Including Late and Long-Term Effects of
Treatment, Quality of Life, and Prognosis?
Conclusions
A common definition of "cancer survivor" is any individual who has had a
diagnosis of cancer, from the point of diagnosis and for the balance of life.
Cancer survivors are a subset of the US adult population that is expected to
grow substantively in the coming decades. As such, the role of physical
activity in improving outcomes for cancer survivors is likely to increase in
importance as well. Recently, data have been published regarding the effects of
physical activity on health outcomes among persons who already have cancer.
These studies suggest that physically active individuals with breast or colon
cancer may have improved prognosis (i.e., fewer recurrences and deaths),
compared with sedentary survivors. In addition, physical activity may play an
important role in preventing, attenuating, or rehabilitating late and long-term
effects of cancer treatment. Walking is a commonly prescribed form of exercise
in the studies reviewed here and appears to have benefits on muscular strength
and endurance, as well as quality of life. Dose-response effects and long-term
outcomes are unknown for any outcomes from physical activity interventions in
cancer survivors at this time.
Introduction
More than 10 million people in the United States are cancer survivors,
and more than 16% of adults older than age 65 years are cancer survivors (184). The increasing success of cancer treatments has
required a shift in focus toward new outcomes, such as preventing recurrence
and mortality, and accommodating the unique medical and psychosocial needs of
cancer survivors.
Cancer treatment typically includes some combination of surgery,
radiation therapy, or chemotherapy, and may also include hormonal therapies,
steroid treatment, immunotherapies, or monoclonal antibody treatment. Each of
these therapies is associated with acute as well as late and long-term adverse
physiologic and psychological effects. The terms "late effects" or "long-term
effects" (185) are distinct in the timing of their onset.
Late effects are side effects or complications that are absent or subclinical
at the end of therapy but that emerge after compensatory systems fail or some
second insult (e.g., deconditioning) occurs that results in a clinically
significant diagnosis that can be traced back to effects of treatment. An
example of a late effect would be the diagnosis of a cardiac arrhythmia years
after treatment with a cardiotoxic chemotherapeutic agent such as adriamyacin
(186). Long-term effects are adverse effects or
complications that appear during treatment and persist long afterward, for
months, years, or the duration of life. Physical activity could be useful for
preventing or attenuating some late and long-term effects of cancer treatments
(Figure G7-1) (187), and may also be useful for
prevention of recurrence or cancer mortality among cancer survivors.
Figure G7.1. Late and Long-Term Effects of Cancer
Treatment That May Be Positively Affected by Physical Activity
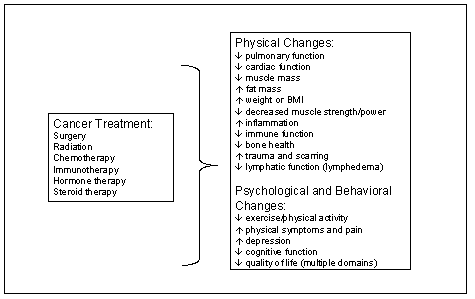
| Cancer
Treatment: |
Physical
Changes: |
Psychological and
Behavioral Changes: |
Surgery
Radiation
Chemotherapy
Immunotherapy
Hormone
therapy
Steroid therapy |
↓pulmonary
function
↓cardiac function
↓muscle mass
↑fat mass
↑weight or BMI
↓decreased muscle strength/power
↑inflammation
↓immune function
↓bone health
↑trauma and scarring
↓lymphatic function (lymphedema) |
↓exercise/physical
activity
↑physical symptoms and pain
↑depression
↓cognitive function
↓quality of life (multiple domains) |
The acute effects of treatment and the potential for positive effects of
physical activity during active cancer treatment are beyond the scope of this
review, but have been reviewed elsewhere (188;189). Also not reviewed here are the late or long-term
effects of childhood cancer treatment, as little research has been conducted in
this area.
Rationale
Effects of Physical Activity on Cancer Recurrence and Mortality
Though few studies have been conducted on the role of physical activity
in preventing cancer recurrence or reducing mortality, the consistent findings
of a preventive effect warrants comment. Data from the Nurses' Health Study
were used to explore the dose-response association of physical activity with
overall and breast cancer specific mortality, as well as recurrence, among
2,987 breast cancer survivors over a median of 96 months of follow-up (190). The results indicated a 29% decrease in overall
mortality among women who did at least 3 MET-hours per week of aerobic activity
after diagnosis, with minimal additional protection from greater levels of
physical activity. The decrease in breast cancer-specific mortality and
recurrence were 50% and 43%, respectively, in women who engaged in at least 9
MET-hours per week of physical activity compared with women who did less than
3. Additional benefits were small for activity levels greater than 9 MET-hours
per week, which can be translated to 3 hours per week of walking at 2.5 miles
per hour. Considerable evidence indicates that overweight, obesity, and weight
gain are associated with breast cancer recurrence (191-193). These results are consistent with the hypothesis
that physical activity reduces risk of mortality or recurrence among breast
cancer survivors through weight control.
Evidence for a role of physical activity in colon and colorectal cancer
survivorship comes from 2 recently published observational studies. One of
these, the Nurses' Health Study, observed an inverse dose-response association
of physical activity and overall and colorectal cancer-specific mortality in
554 women who had had a previous diagnosis of colorectal cancer. Women who
engaged in at least 18 MET-hours per week of physical activity after diagnosis
had a 61% and 57% reduced risk of colorectal cancer-specific and overall
mortality, respectively, compared to women who did less than 3 MET-hours per
week (194). A dose-response association of physical
activity and colon cancer disease-free survival also was seen in the cohort of
832 male and female patients who participated in the CALBG trial (195). In this latter cohort, 18 MET-hours per week, or 6
hours of walking per week at 2.5 miles per hour, was associated with a 49%
reduction in risk of recurrence (195).
Effects of Exercise on Prevention of Long-Term or Late Effects of
Cancer Treatment
A number of recent systematic reviews (188;196-205) as well as 2
meta-analyses (188;206) have
recently been conducted on the effects of physical activity interventions on a
variety of outcomes in cancer survivors. Readers interested in an in-depth
discussion of the effects of exercise on cancer survivors are guided to these
reviews. Below, the effects of exercise training on late or long-term effects
is reviewed for outcomes for which there is the greatest amount of evidence and
consensus and that may be most useful in guiding quantitative or qualitative
behavioral recommendations for cancer survivors. Of the 22 controlled clinical
trials published since 1995 and reviewed here, only 1 (207), examined a dose-response pattern of exercise training
on any outcome, and none was noted. For each of the outcomes reviewed below,
effects of walking programs are noted, if such data were available. Most of the
studies reviewed were relatively short in duration (6 months or less);
long-term effects are not yet known.
Physiologic Effects
Cardiorespiratory Fitness. Though the effect of
physical activity on cardiorespiratory fitness has been long established (see
Part G. Section 2: Cardiorespiratory
Health for a detailed discussion of this topic), it is of
particular relevance for cancer survivors given the cardiotoxic effects of
several commonly used cancer treatment drugs and radiation to the chest (186;208). A meta-analysis published in
2005 indicated a strong weighted mean effect size of 0.65 (P=0.003)
for cardiorespiratory fitness based on the 4 exercise interventions that had
assessed this outcome in cancer survivors after treatment (188). Since this meta-analysis, 9 additional RCTs have
assessed whether various of exercise interventions in cancer survivors improve
cardiorespiratory fitness after treatment (209-217). All
13 studies have shown positive effects, and 11 showed statistically significant
improvements on cardiorespiratory function tests ranging from a 6-minute walk
test to maximal treadmill or cycle ergometer tests. All of these studies
included breast cancer survivors, and most included only breast cancer
survivors. Fitness improvements have been demonstrated in a variety of
programs, including walking, yoga, tai chi chuan, exercise at home, and
exercise at fitness facilities. For most of the studies, exercise doses used
were 3 weekly sessions of 20 to 40 minutes in duration at moderate
intensity.
Muscular Strength and Endurance. Observational evidence
in small convenience-sample studies suggests that muscle mass may decrease and
fat mass may increase after some breast cancer chemotherapy regimens (218). Cancer treatment may result in a decrease in activity
(219), with subsequent deconditioning associated with
muscle disuse. Therefore, it is important to determine whether exercise
training improves muscular strength or endurance in cancer survivors. Six
studies have examined the effects of some form of resistance training, tai chi
chuan, or yoga on muscle strength or endurance (211-213;215;220;221). All of these studies were
conducted in women who had completed breast cancer treatment. Five observed
positive effects of training (211;213;215;220;221) and 4 reported statistically significant improvements
(211;213;215;220) in strength or endurance
tests.
Flexibility. Cancer surgeries may result in decreased
range of motion with scarring or tissue trauma, and these changes may result in
altered physical function. Six studies have examined the effects of exercise
training on flexibility. Three assessed effects of aerobic exercise or yoga on
lower body flexibility with the sit-and-reach test in breast and colon cancer
survivors (207;215;222). All 3 showed improvements in flexibility, but only one
(207) observed a statistically significant improvement
comparing changes between treatment and control participants. Three other
studies examined effects of tai chi chuan, dance and movement, or aerobic
exercise and stretching on shoulder range of motion in breast cancer survivors
(211;223;224). All 3 noted improvements, and 2 noted significant
between-group differences in shoulder range of motion (211;223).
Lymphedema. Surgical removal or irradiation of lymph
nodes results in damage to the lymphatic system that can result in an inability
of the affected body part to manage fluid balance and temperature regulation.
This damage may impair immune response and wound healing, as well as response
to trauma or injury. Swelling and pain in the affected body part can develop
immediately after surgery and/or radiation or years later, making lymphedema a
long-term risk among several types of survivors, including those with breast,
head and neck, melanoma, genital cancers, lower gastrointestinal tract and
bladder cancers. Lymphedema is considered a chronic condition, and occurs in 6%
to 50% of breast cancer survivors, depending on number of nodes removed and
intensity of radiation (225-227). Lower-limb lymphedema
also occurs in 20% to 30% of cancer patients who have had lymph node removal or
radiation in the groin or retroperitoneal lymph nodes (228-237). Four studies have examined the risk of lymphedema
onset or worsening among breast cancer survivors by measuring changes in arm
circumferences or symptoms resulting from exercise training (217;221;224;238). None of these studies has noted negative effects of
aerobic or resistance exercise on arm circumferences or symptoms; evidence of
possible benefit to the affected limb has not been examined. No studies of the
safety or efficacy of exercise for cancer survivors with or at risk for
lymphedema for cancer sites other than breast have been conducted.
Weight Change. Some breast cancer patients gain weight
after diagnosis, and the associated changes in body composition may include
decreased muscle mass and increased body fat, as suggested by a few
convenience-sample studies (218). These effects have not
been examined in population-based or clinical trial series of patients, nor in
patients with other cancers. However, it is important to determine the effects
of exercise training on body weight and body composition in survivors who have
had any type of cancer treatment. The results of the 13 identified controlled
trials conducted since 1995 indicate that, as for the general population,
exercise may decrease percent body fat to a small degree, but has little to no
effect on body weight in the absence of concurrent caloric restriction (188;207;210-215;217;222;239-241). (See Part G.
Section 4: Energy Balance, for a detailed discussion of the
association between physical activity, weight loss, and changes in body
composition.)
Psychosocial and Symptom Effects
Quality of Life. The effect of exercise on
health-related quality of life was examined in a systematic review and
meta-analysis published in 2005 (188). This review
concluded that evidence was strong for a positive effect of physical activity
on quality of life in cancer survivors, though the weighted mean effect size of
0.30 was not statistically significant. Since the publication of that
meta-analysis, an additional 10 studies have examined effects of physical
activity on cancer survivors after treatment (209;213-217;223;224;240;242). Of these, all showed positive effects, and 8 indicated
a statistically significant improvement in at least one quality of life
indicator after a physical activity intervention. Overall, 10 out of 13
identified studies showed statistically significant improvements in quality of
life resulting from a physical activity intervention after cancer
treatment.
Fatigue. Cancer-related fatigue is distinct from
ordinary types of fatigue in its persistence and severity (243). The effects of physical activity interventions on
fatigue in cancer survivors (primarily breast cancer) have been tested in 8
studies since 1995. Of these 8 trials (207;209;210;214;216;222;239;244), 3 reported statistically significant improvements in
fatigue after a program of aerobic exercise, walking, or cycling. Five other
studies, most of which focused on walking programs among breast cancer
survivors during the time period after treatment, observed improvements, but
not statistically significant improvements. The mechanisms through which
physical activity may improve cancer-related fatigue are not yet fully
understood (243).
Question 3: What Mechanisms Explain the
Associations Between Physical Activity and Cancer?
Conclusions
A number of plausible mechanisms might explain the associations between
physical activity and cancer risk and prognosis. Increased physical activity
reduces adiposity, which may explain reductions in cancers that are associated
with overweight and obesity, including postmenopausal breast, colon,
endometrial, and other cancers. Increased physical activity is associated with
reduced levels of sex hormones, which may explain a link between physical
activity and hormone-related cancers such as breast and endometrial cancers.
Another possible mechanism is through the effect of physical activity and
inflammation and immune function. Finally, increased physical activity reduces
insulin resistance, which could explain associations with risk for some
cancers, such as colon cancer, that may be increased in individuals with
insulin resistance or hyperinsulinemia.
Rationale
Physical activity could affect cancer risk or progression through
several plausible mechanisms (245). Many of these
mechanisms may act through the effects of physical activity on obesity, with
resulting changes to circulating levels of adipokines, cytokines, insulin, and
sex hormones. Other mechanisms may involve direct effects on target organs and
tissues. The effects of physical activity on carcinogenesis or prognosis are
likely to be multi-factorial and may be affected by many factors such as age,
sex, and adiposity, in addition to physical activity specific factors such as
type, duration, frequency, and intensity of physical activity.
Menstrual Factors and Sex Steroid Hormones
Several modifiable menstrual factors increase breast cancer risk,
including early age at menarche, frequent ovulation, regular cycles, and late
age at menopause (246). Women with elevated levels of
estrogens and androgens have increased risk of developing breast cancer. In a
combined analysis of 9 large cohort studies, postmenopausal women in the top
quintile for various estrogens or androgens had approximately double the risk
of developing breast cancer compared with women in the lowest quintile (247).
Elevated levels of estradiol or testosterone in premenopausal women increase
risk of breast cancer as well (248;249). Medications that block estrogen receptors or that
prevent estrogen production in peripheral tissues have been a mainstay of
treatment for women with estrogen receptor positive breast cancer (250). Women with elevated estrogen concentrations (unopposed
by progesterone) are at an increased risk for endometrial cancer (251). In men, anti-androgen therapy improves prostate cancer
survival (252) and reduces overall incidence of the
disease when tested as a preventive agent (253). However,
recent evidence suggests that blood levels of androgens or estrogens are not
related to prostate cancer risk (247), suggesting that
only prostatic levels of sex hormones are relevant for prostate carcinogenesis
or progression.
Premenopausal Women
The effect of physical activity on age at menarche, menstrual cycle
function, and level of ovarian-produced sex steroid hormone levels in girls and
young women are potential mechanisms for reduced breast cancer risk (254). Moderate-intensity physical activity may cause small
changes in reproductive hormones in premenopausal women, but high intensity or
volume of exercise sufficient to cause a negative energy balance may be
required to induce menstrual dysfunction (amenorrhea, anovular cycles and
luteal phase deficiency) with significantly decreased production of ovarian
estradiol and progesterone (255-263).
Postmenopausal Women
In postmenopausal women, increased physical activity has been associated
with decreased serum concentrations of estradiol, estrone, and androgens (264-266). The positive effect of physical activity is
closely linked to body composition because the primary source of estrogen in
postmenopausal women is from aromatization of androgen precursors in
peripheral, mainly adipose, tissue. In a sub-sample from the Women's Health
Initiative (WHI) Dietary Modification Trial, women with low self-reported
physical activity had higher levels of estrone, estradiol, and free estradiol,
and lower levels of sex-hormone binding globulin (which binds estradiol, making
less available to target tissue) than did active women (265). The highest levels of estrogen were observed in women
who were both below the median level for physical activity (i.e., less than 6.5
MET-hours per week, approximately less than 1.5 hours per week of brisk
walking) and above the median BMI (i.e., at least 29 kg/m2).
In an RCT, 173 overweight, sedentary postmenopausal women were assigned
to a moderate-intensity aerobic exercise, 45 minutes per day, 5 days per week
for 12 months or to a control group. A significant decrease in estradiol,
estrone, and free estradiol was seen from baseline to 3 months in exercisers
versus controls, with an attenuation of the effect at 12 months (267). However, in those women who lost body fat, the
exercise intervention resulted in a statistically significant reduction in
these estrogens at both 3 and 12 months. Similarly, in women who lost body fat,
a statistically significant decrease in testosterone and free testosterone
occurred in exercisers compared with controls (268).
These intervention and observational studies results suggest that both
increased physical activity and reduced body fat will produce the greatest
protection against breast cancer by producing the greatest decrease in serum
sex hormones.
Men
Chronically lowered testosterone concentrations have been reported in
athletes, but this finding may require a threshold amount or intensity of
physical activity to occur (269), and the effects of
moderate-intensity aerobic exercise on sex steroid hormones in men is not
known. In a recent trial, 102 men aged 40 to 75 years were randomly assigned to
a 12-month moderate to vigorous intensity aerobic exercise intervention (60
minutes per day, 6 days per week) or a control group (no change in activity)
(270). Dihydrotestosterone (DHT) increased 14.5% in
exercisers compared to 1.7% in controls at 3 months (P=0.04). At 12
months, DHT remained 8.6% above baseline in exercisers versus a 3.1% decrease
in controls (P=0.03). Sex hormone binding globulin increased 14.3% in
exercisers versus 5.7% in controls at 3 months (P=0.04), while at 12
months it remained 8.9% above baseline in exercisers compared to 4.0% in
controls (P=0.13). No statistically significant differences were
observed for testosterone, free testosterone, 3α-Diol-G, estradiol, or
free estradiol in exercisers versus controls. Therefore, the association of
physical activity with circulating hormone levels in men is still unclear.
Metabolic and Other Hormones
Insulin resistance has been linked to increased risk of breast, colon,
pancreas, endometrial and stomach cancers (271). Higher
cancer incidence and mortality also have been noted in those with type 2
diabetes or impaired glucose tolerance (271;272). Insulin can enhance tumor development by stimulating
cell proliferation or inhibiting apoptosis (271). It also
can regulate the synthesis and biological availability of sex steroid hormones,
and inhibit hepatic synthesis of sex hormone binding globulin (271). Acute bouts of physical activity improve insulin
sensitivity and increase glucose uptake by skeletal muscle for up to 12 hours
(273), and chronic exercise training results in prolonged
improvements in insulin sensitivity (274-276). Although
body composition has been strongly associated with insulin sensitivity,
exercise-induced changes in insulin sensitivity can occur from physical
activity, independent of the changes in weight or body composition (273;274;277). An
additive effect of resistance training to improve insulin sensitivity and
glycemic control also has been proposed because skeletal muscle is a primary
site of insulin resistance (273;278).
Women with elevated levels of prolactin are at increased risk of breast
cancer (279), and a recent clinical trial found that
increased physical activity levels as measured by increased VO2max
over 1 year in a moderate-intensity exercise intervention was associated with
statistically significant reductions in prolactin levels in postmenopausal,
overweight women (280).
Inflammation
Elevated levels of pro-inflammatory factors, such as C-reactive protein
(CRP), interleukin (IL)-6, tumor-necrosis factor-α (TNF-α), and
decreased levels of anti-inflammatory factors, such as adiponectin, have been
linked with increased cancer risk (281). Physical
activity may reduce systemic inflammation alone or in combination with body
weight or composition through reducing macrophage or adipose cell production of
inflammatory cytokines in adipose tissue, although exact mechanisms are unknown
(278;282).
Although cross-sectional studies support an association between chronic
physical activity and lower levels of the inflammatory markers CRP, serum
amyloid A (SAA), IL-6 and TNF-α in both men and women, intervention
studies of exercise alone or exercise and diet combined have had inconsistent
results, with some studies but not others showing reductions in these
inflammatory markers (282).
Increases in adiponectin have been seen with physical activity
interventions in the presence of significant weight loss (283). Shorter duration prospective physical activity and
weight loss interventions have failed to alter adiponectin levels despite
modest changes in body weight and body composition (278).
Immune Function
The immune system is thought to play a role in reducing cancer risk by
recognizing and eliminating abnormal cells or through acquired and/or innate
immune system components (284). The role of physical
activity on immune factors related to cancer has been largely untested, but one
hypothesis is that physical activity could improve the number or function of
natural killer cells, which play a role in tumor suppression (282).
Bouts of physical activity have been shown to result in acute increases
in a number of components of immune function (e.g., neutrophils, monocytes,
eosinophils, and lymphocytes), followed by a dip below pre-exercise levels
lasting up to 1 to 3 hours (285). For chronic physical
activity, an inverted J-shaped dose-response relation between intensity of
physical activity and immune function has been shown. Moderate physical
activity results in enhanced immune function, but exhaustive exercise,
overtraining, or high-intensity exercise may lead to immunosuppression, which
may result in increased susceptibility to ailments such as upper respiratory
infections (282). However, the current evidence on
moderate-intensity physical activity from randomized controlled trials is
inconclusive, with differences in components of the innate immune system noted
in some, but not all, cross sectional studies that compare exercisers to
non-exercisers. Randomized controlled trials of moderate physical activity show
little effect on immune function (282).
Other Mechanisms
Physical activity also could mediate cancer risk through additional
mechanisms, such as its effects on other body structures. For example, physical
activity affects colon motility, leading to decreased transit time and,
perhaps, reduced carcinogen exposure in the colon (254).
In addition, physical activity has been hypothesized to affect various tissues
leading to reduced carcinogenic prostaglandin (PG) production (286), although an RCT found no effect of a 12-month aerobic
exercise intervention (1 hour per day, 6 day per week) on colon mucosal
prostaglandins PGE2 or PGF2alpha in 202 men or women aged 40 to 75 years (287).
Overall Summary and Conclusions
Increased physical activity is associated with reduced risk of several
cancers. Most evidence for this association is available for breast and colon
cancers, although growing evidence suggests an association between increased
physical activity and reduced risk of endometrial and lung cancers. Overall,
data suggest that 30 to 60 minutes per day of moderate-to-vigorous intensity
physical activity may be needed to significantly lower the risk of developing
breast and colon cancers. The effect of physical activity is larger for colon
cancer (median reduction in risk of 30% across reviewed studies) compared with
breast cancer (median reduction in risk of 20% across studies). A large part of
the effect of physical activity on cancer is likely mediated through obesity
and other hormonal and metabolic mechanisms. Randomized controlled trials have
demonstrated effects of physical activity interventions on cancer risk factors,
which further support a role of physical activity in reducing risk for
cancer.
Strong evidence links increased physical activity in cancer survivors
with improved quality of life and increased fitness. Less evidence is available
regarding the effect of physical activity on cancer recurrence and survival. A
2006 publication from the American Cancer Society (3)
states that although the current public health guidelines of 30 to 60 minutes
of moderate-intensity aerobic exercise 5 times per week have not been studied
systematically in cancer survivors, there is no reason to think that this would
not also benefit survivors. Overall, results indicate that guidelines for
cardiovascular exercise for cancer survivors who have completed treatment need
not be different from those of the general population, and that particular
physiologic and psychosocial effects of cancer and its treatments are
positively affected by cardiovascular exercise, resistance training, and
flexibility training.
Research Needs
Knowledge about the role of physical activity in reducing the risk of
common cancers would benefit from additional evidence gathered from clinical
trials. In the survivorship setting, clinical trials showing a benefit of
physical activity interventions on reducing deaths, recurrences, and reducing
the impact of late or long-term treatment effects, also would make a valuable
contribution to our understanding of the needs of this growing population.
Other research needs include studies to clarify biological mechanisms
linking physical activity to specific cancers in order to identify associations
with less commonly studied cancers, define the shape of dose-response curve of
the physical activity-cancer relation, determine the effect of low-intensity
activities and accumulated bouts, and assess the effect of physical activity
within specific population subgroups.
Additional observational epidemiologic research to identify the dose,
type, and frequency of physical activity on risk of various cancer sites and
subtypes is needed, in addition to identifying the effect of physical activity
on risk of specific cancers within particular population subgroups including
various race/ethnic, age, sex, and groups at elevated risk of cancer.
Reference List
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T,
Thun MJ. Cancer statistics, 2008. CA Cancer J.Clin. 2008 Mar;58(2):71-96.
- Vainio H, Bianchini F, International Agency for
Research on Cancer. Weight control and physical activity. Lyon: IARC Press;
2002.
- Doyle C, Kushi LH, Byers T, Courneya KS,
mark-Wahnefried W, Grant B, McTiernan A, Rock CL, Thompson C, Gansler T, et al.
Nutrition and physical activity during and after cancer treatment: an American
Cancer Society guide for informed choices. CA Cancer J.Clin. 2006
Nov;56(6):323-53.
- Thune I, Furberg AS. Physical activity and cancer
risk: dose-response and cancer, all sites and site-specific. Med.Sci.Sports
Exerc. 2001 Jun;33(6 Suppl):S530-S550.
- Lee IM. Physical activity and cancer
prevention--data from epidemiologic studies. Med.Sci.Sports Exerc. 2003
Nov;35(11):1823-7.
- Friedenreich CM. Physical activity and prostate
cancer risk. In: McTiernan A, editor. Cancer prevention and management through
exercise and weight control. Boca Raton: CRC Press; 2006. p. 91-120.
- Slattery ML. Physical activity and colorectal
cancer. In: McTiernan A, editor. Cancer prevention and management through
exercise and weight control. Boca Raton: CRC Press; 2006. p. 75-90.
- Patel AV, Bernstein L. Physical activity and
cancer incidence: breast cancer. In: McTiernan A, editor. Cancer prevention and
management through exercise and weight control. Boca Raton: CRC Press; 2006. p.
49-74.
- Dorgan JF, Brown C, Barrett M, Splansky GL,
Kreger BE, D'Agostino RB, Albanes D, Schatzkin A. Physical activity and risk of
breast cancer in the Framingham Heart Study. Am.J.Epidemiol. 1994 Apr
1;139(7):662-9.
- Fraser GE, Shavlik D. Risk factors, lifetime
risk, and age at onset of breast cancer. Ann.Epidemiol. 1997
Aug;7(6):375-82.
- Thune I, Brenn T, Lund E, Gaard M. Physical
activity and the risk of breast cancer. N.Engl.J.Med. 1997 May
1;336(18):1269-75.
- Cerhan JR, Chiu BC-H, Wallace RB, Lemke JH,
Lynch CF, Torner JC, Rubenstein LM. Physical activity, physical function, and
the risk of breast cancer in a prospective study among elderly women.
J.Gerontol.A.Biol.Sci.Med.Sci. 1998 Jul;53(4):M251-m256.
- Sesso HD, Paffenbarger RS, Jr., Lee IM. Physical
activity and breast cancer risk in the College Alumni Health Study (United
States). Cancer Causes Control 1998 Aug;9(4):433-9.
- Rockhill B, Willett WC, Hunter DJ, Manson JE,
Hankinson SE, Colditz GA. A prospective study of recreational physical activity
and breast cancer risk. Arch.Intern.Med. 1999 Oct 25;159(19):2290-6.
- Wyshak G, Frisch RE. Breast cancer among former
college athletes compared to non-athletes: a 15-year follow-up. Br.J.Cancer
2000 Feb;82(3):726-30.
- Moore DB, Folsom AR, Mink PJ, Hong CP, Anderson
KE, Kushi LH. Physical activity and incidence of postmenopausal breast cancer.
Epidemiology 2000 May;11(3):292-6.
- Dirx MJ, Voorrips LE, Goldbohm RA, van den
Brandt PA. Baseline recreational physical activity, history of sports
participation, and postmenopausal breast carcinoma risk in the Netherlands
Cohort Study. Cancer 2001 Sep 15;92(6):1638-49.
- Lee IM, Rexrode KM, Cook NR, Hennekens CH, Burin
JE. Physical activity and breast cancer risk: the Women's Health Study (United
States). Cancer Causes Control 2001 Feb;12(2):137-45.
- Luoto R, Latikka P, Pukkala E, Hakulinen T,
Vihko V. The effect of physical activity on breast cancer risk: a cohort study
of 30,548 women. Eur.J.Epidemiol. 2000;16(10):973-80.
- Breslow RA, Ballard-Barbash R, Munoz K, Graubard
BI. Long-term recreational physical activity and breast cancer in the National
Health and Nutrition Examination Survey I epidemiologic follow-up study. Cancer
Epidemiol.Biomarkers Prev. 2001 Jul;10(7):805-8.
- Moradi T, Adami HO, Ekbom A, Wedren S, Terry P,
Floderus B, Lichtenstein P. Physical activity and risk for breast cancer a
prospective cohort study among Swedish twins. Int.J.Cancer 2002 Jul
1;100(1):76-81.
- Patel AV, Callel EE, Bernstein L, Wu AH, Thun
MJ. Recreational physical activity and risk of postmenopausal breast cancer in
a large cohort of US women. Cancer Causes Control 2003 Aug;14(6):519-29.
- Colditz GA, Feskanich D, Chen WY, Hunter DJ,
Willett WC. Physical activity and risk of breast cancer in premenopausal women.
Br.J.Cancer 2003 Sep 1;89(5):847-51.
- McTiernan A, Kooperberg C, White E, Wilcox S,
Coates R, ms-Campbell LL, Woods N, Ockene J. Recreational physical activity and
the risk of breast cancer in postmenopausal women: the Women's Health
Initiative Cohort Study. JAMA 2003 Sep 10;290(10):1331-6.
- Margolis KL, Mucci L, Braaten T, Kumle M, Trolle
LY, Adami HO, Lund E, Weiderpass E. Physical activity in different periods of
life and the risk of breast cancer: the Norwegian-Swedish Women's Lifestyle and
Health cohort study. Cancer Epidemiol.Biomarkers Prev. 2005
Jan;14(1):27-32.
- Silvera SA, Jain M, Howe GR, Miller AB, Rohan
TE. Energy balance and breast cancer risk: a prospective cohort study. Breast
Cancer Res.Treat. 2006 May;97(1):97-106.
- Rintala P, Pukkala E, Laara E, Vihko V. Physical
activity and breast cancer risk among female physical education and language
teachers: a 34-year follow-up. Int.J.Cancer 2003 Nov 1;107(2):268-70.
- Schnohr P, Gronbaek M, Petersen L, Hein HO,
Sorensen TI. Physical activity in leisure-time and risk of cancer: 14-year
follow-up of 28,000 Danish men and women. Scand.J.Public Health
2005;33(4):244-9.
- Bardia A, Hartmann LC, Vachon CM, Vierkant RA,
Wang AH, Olson JE, Sellers TA, Cerhan JR. Recreational physical activity and
risk of postmenopausal breast cancer based on hormone receptor status.
Arch.Intern.Med. 2006 Dec 11;166(22):2478-83.
- Mertens AJ, Sweeney C, Shahar E, Rosamond WD,
Folsom AR. Physical activity and breast cancer incidence in middle-aged women:
a prospective cohort study. Breast Cancer Res.Treat. 2006
May;97(2):209-14.
- Chang SC, Ziegler RG, Dunn B,
Stolzenberg-Solomon R, Lacey JV, Jr., Huang WY, Schatzkin A, Reding D, Hoover
RN, Hartge P, et al. Association of energy intake and energy balance with
postmenopausal breast cancer in the prostate, lung, colorectal, and ovarian
cancer screening trial. Cancer Epidemiol.Biomarkers Prev. 2006
Feb;15(2):334-41.
- Tehard B, Friedenreich CM, Oppert JM,
Clavel-Chapelon F. Effect of physical activity on women at increased risk of
breast cancer: results from the E3N cohort study. Cancer Epidemiol.Biomarkers
Prev. 2006 Jan;15(1):57-64.
- Lahmann PH, Friedenreich C, Schuit AJ, Salvini
S, Allen NE, Key TJ, Khaw KT, Bingham S, Peeters PH, Monninkhof E, et al.
Physical activity and breast cancer risk: the European Prospective
Investigation into Cancer and Nutrition. Cancer Epidemiol.Biomarkers Prev. 2007
Jan;16(1):36-42.
- Dallal CM, Sullivan-Halley J, Ross RK, Wang Y,
Deapen D, Horn-Ross PL, Reynolds P, Stram DO, Clarke CA, nton-Culver H, et al.
Long-term recreational physical activity and risk of invasive and in situ
breast cancer: the California teachers study. Arch.Intern.Med. 2007 Feb
26;167(4):408-15.
- Bernstein L, Henderson BE, Hanisch R,
Sullivan-Halley J, Ross RK. Physical exercise and reduced risk of breast cancer
in young women. J.Natl.Cancer Inst. 1994 Sep 21;86(18):1403-8.
- Friedenreich CM, Rohan TE. Physical activity and
risk of breast cancer. Eur.J.Cancer Prev. 1995 Apr;4(2):145-51.
- McTiernan A, Stanford JL, Weiss NS, Daling JR,
Voigt LF. Occurrence of breast cancer in relation to recreational exercise in
women age 50-64 years. Epidemiology 1996 Nov;7(6):598-604.
- Chen CL, White E, Malone KE, Daling JR.
Leisure-time physical activity in relation to breast cancer among young women
(Washington, United States). Cancer Causes Control 1997 Jan;8(1):77-84.
- Hu YH, Nagata C, Shimizu H, Kaneda N, Kashiki Y.
Association of body mass index, physical activity, and reproductive histories
with breast cancer: a case-control study in Gifu, Japan. Breast Cancer
Res.Treat. 1997 Mar;43(1):65-72.
- Gammon MD, Schoenberg JB, Britton JA, Kelsey JL,
Coates RJ, Brogan D, Potischman N, Swanson CA, Daling JR, Stanford JL, et al.
Recreational physical activity and breast cancer risk among women under age 45
years. Am.J.Epidemiol. 1998 Feb 1;147(3):273-80.
- Ueji M, Ueno E, Osei-Hyiaman D, Takahashi H,
Kano K. Physical activity and the risk of breast cancer: a case-control study
of Japanese women. J.Epidemiol. 1998 Jun;8(2):116-22.
- Marcus PM, Newman B, Moorman PG, Millikan RC,
Baird DD, Qaqish B, Sternfeld B. Physical activity at age 12 and adult breast
cancer risk (United States). Cancer Causes Control 1999 Aug;10(4):293-302.
- Verloop J, Rookus MA, van der KK, van Leeuwen
FE. Physical activity and breast cancer risk in women aged 20-54 years.
J.Natl.Cancer Inst. 2000 Jan 19;92(2):128-35.
- Moradi T, Nyren O, Zack M, Magnusson C, Persson
I, Adami HO. Breast cancer risk and lifetime leisure-time and occupational
physical activity (Sweden). Cancer Causes Control 2000 Jul;11(6):523-31.
- Shoff SM, Newcomb PA, Trentham-Dietz A,
Remington PL, Mittendorf R, Greenberg ER, Willett WC. Early-life physical
activity and postmenopausal breast cancer: effect of body size and weight
change. Cancer Epidemiol.Biomarkers Prev. 2000 Jun;9(6):591-5.
- Adams-Campbell LL, Rosenberg L, Rao RS, Palmer
JR. Strenuous physical activity and breast cancer risk in African-American
women. J.Natl.Med.Assoc. 2001 Jul;93(7-8):267-75.
- Matthews CE, Shu XO, Jin F, Dai Q, Hebert JR,
Ruan ZX, Gao YT, Zheng W. Lifetime physical activity and breast cancer risk in
the Shanghai Breast Cancer Study. Br.J.Cancer 2001 Apr 6;84(7):994-1001.
- Friedenreich CM, Bryant HE, Courneya KS.
Case-control study of lifetime physical activity and breast cancer risk.
Am.J.Epidemiol. 2001 Aug 15;154(4):336-47.
- Gilliland FD, Li YF, Baumgartner K, Crumley D,
Samet JM. Physical activity and breast cancer risk in hispanic and non-hispanic
white women. Am.J.Epidemiol. 2001 Sep 1;154(5):442-50.
- Lee IM, Cook NR, Rexrode KM, Buring JE. Lifetime
physical activity and risk of breast cancer. Br.J.Cancer 2001 Sep
28;85(7):962-5.
- Carpenter CL, Ross RK, Paganini-Hill A,
Bernstein L. Effect of family history, obesity and exercise on breast cancer
risk among postmenopausal women. Int.J.Cancer 2003 Aug 10;106(1):96-102.
- Dorn J, Vena J, Brasure J, Freudenheim J, Graham
S. Lifetime physical activity and breast cancer risk in pre- and
postmenopaus">al women. Med.Sci.Sports Exerc. 2003 Feb;35(2):278-85.
- John EM, Horn-Ross PL, Koo J. Lifetime physical
activity and breast cancer risk in a multiethnic population: the San Francisco
Bay area breast cancer study. Cancer Epidemiol.Biomarkers Prev. 2003 Nov;12(11
Pt 1):1143-52.
- Patel AV, Press MF, Meeske K, Calle EE,
Bernstein L. Lifetime recreational exercise activity and risk of breast
carcinoma in situ. Cancer 2003 Nov 15;98(10):2161-9.
- Steindorf K, Schmidt M, Kropp S, Chang-Claude J.
Case-control study of physical activity and breast cancer risk among
premenopausal women in Germany. Am.J.Epidemiol. 2003 Jan 15;157(2):121-30.
- Yang D, Bernstein L, Wu AH. Physical activity
and breast cancer risk among Asian-American women in Los Angeles: a
case-control study. Cancer 2003 May 15;97(10):2565-75.
- Taioli E, Barone J, Wynder EL. A case-control
study on breast cancer and body mass. The American Health Foundation--Division
of Epidemiology. Eur.J.Cancer 1995;31A(5):723-8.
- Mezzetti M, La VC, Decarli A, Boyle P, Talamini
R, Franceschi S. Population attributable risk for breast cancer: diet,
nutrition, and physical exercise. J.Natl.Cancer Inst. 1998 Mar
4;90(5):389-94.
- Levi F, Pasche C, Lucchini F, La VC.
Occupational and leisure time physical activity and the risk of breast cancer.
Eur.J.Cancer 1999 May;35(5):775-8.
- Hirose K, Hamajima N, Takezaki T, Miura S,
Tajima K. Physical exercise reduces risk of breast cancer in Japanese women.
Cancer Sci. 2003 Feb;94(2):193-9.
- Coogan PF, Newcomb PA, Clapp RW, Trentham-Dietz
A, Baron JA, Longnecker MP. Physical activity in usual occupation and risk of
breast cancer (United States). Cancer Causes Control 1997 Jul;8(4):626-31.
- Kumar NB, Riccardi D, Cantor A, Dalton K, Allen
K. A case-control study evaluating the association of purposeful physical
activity, body fat distribution, and steroid hormones on premenopausal breast
cancer risk. Breast J. 2005 Jul;11(4):266-72.
- Malin A, Matthews CE, Shu XO, Cai H, Dai Q, Jin
F, Gao YT, Zheng W. Energy balance and breast cancer risk. Cancer
Epidemiol.Biomarkers Prev. 2005 Jun;14(6):1496-501.
- Friedenreich CM, Courneya KS, Bryant HE.
Influence of physical activity in different age and life periods on the risk of
breast cancer. Epidemiology 2001 Nov;12(6):604-12.
- Friedenreich CM, Courneya KS, Bryant HE.
Relation between intensity of physical activity and breast cancer risk
reduction. Med.Sci.Sports Exerc. 2001 Sep;33(9):1538-45.
- Magnusson CM, Roddam AW, Pike MC, Chilvers C,
Crossley B, Hermon C, McPherson K, Peto J, Vessey M, Beral V. Body fatness and
physical activity at young ages and the risk of breast cancer in premenopausal
women. Br.J.Cancer 2005 Oct 3;93(7):817-24.
- Adams SA, Matthews CE, Hebert JR, Moore CG,
Cunningham JE, Shu XO, Fulton J, Gao Y, Zheng W. Association of physical
activity with hormone receptor status: the Shanghai Breast Cancer Study. Cancer
Epidemiol.Biomarkers Prev. 2006 Jun;15(6):1170-8.
- Mittendorf R, Longnecker MP, Newcomb PA, Dietz
AT, Greenberg ER, Bogdan GF, Clapp RW, Willett WC. Strenuous physical activity
in young adulthood and risk of breast cancer (United States). Cancer Causes
Control 1995 Jul;6(4):347-53.
- Sprague BL, Trentham-Dietz A, Newcomb PA,
Titus-Ernstoff L, Hampton JM, Egan KM. Lifetime recreational and occupational
physical activity and risk of in situ and invasive breast cancer. Cancer
Epidemiol.Biomarkers Prev. 2007 Feb;16(2):236-43.
- Bernstein L, Patel AV, Ursin G, Sullivan-Halley
J, Press MF, Deapen D, Berlin JA, Daling JR, McDonald JA, Norman SA, et al.
Lifetime recreational exercise activity and breast cancer risk among black
women and white women. J.Natl.Cancer Inst. 2005 Nov 16;97(22):1671-9.
- Slattery ML, Edwards S, Murtaugh MA, Sweeney C,
Herrick J, Byers T, Giuliano AR, Baumgartner KB. Physical activity and breast
cancer risk among women in the southwestern United States. Ann.Epidemiol. 2007
May;17(5):342-53.
- Lagerros YT, Hsieh SF, Hsieh CC. Physical
activity in adolescence and young adulthood and breast cancer risk: a
quantitative review. Eur.J.Cancer Prev. 2004 Feb;13(1):5-12.
- Monninkhof EM, Elias SG, Vlems FA, van dT, I,
Schuit AJ, Voskuil DW, van Leeuwen FE. Physical activity and breast cancer: a
systematic review. Epidemiology 2007 Jan;18(1):137-57.
- Carpenter CL, Ross RK, Paganini-Hill A,
Bernstein L. Lifetime exercise activity and breast cancer risk among
post-menopausal women. Br.J.Cancer 1999 Aug;80(11):1852-8.
- Enger SM, Ross RK, Paganini-Hill A, Carpenter
CL, Bernstein L. Body size, physical activity, and breast cancer hormone
receptor status: results from two case-control studies. Cancer
Epidemiol.Biomarkers Prev. 2000 Jul;9(7):681-7.
- Moradi T, Adami HO, Bergstrom R, Gridley G, Wolk
A, Gerhardsson M, Dosemeci M, Nyren O. Occupational physical activity and risk
for breast cancer in a nationwide cohort study in Sweden. Cancer Causes Control
1999 Oct;10(5):423-30.
- Coogan PF, Aschengrau A. Occupational physical
activity and breast cancer risk in the upper Cape Cod cancer incidence study.
Am.J.Ind.Med. 1999 Aug;36(2):279-85.
- Polednak AP. College athletics, body size, and
cancer mortality. Cancer 1976 Jul;38(1):382-7.
- Whittemore AS, Paffenbarger RS, Jr., Anderson K,
Lee JE. Early precursors of site-specific cancers in college men and women.
J.Natl.Cancer Inst. 1985 Jan;74(1):43-51.
- Paffenbarger RS, Jr., Hyde RT, Wing AL. Physical
activity and incidence of cancer in diverse populations: a preliminary report.
Am.J.Clin.Nutr. 1987 Jan;45(1 Suppl):312-7.
- Vena JE, Graham S, Zielezny M, Brasure J,
Swanson MK. Occupational exercise and risk of cancer. Am.J.Clin.Nutr. 1987
Jan;45(1 Suppl):318-27.
- Severson RK, Nomura AM, Grove JS, Stemmermann
GN. A prospective analysis of physical activity and cancer. Am.J.Epidemiol.
1989 Sep;130(3):522-9.
- Albanes D, Blair A, Taylor PR. Physical activity
and risk of cancer in the NHANES I population. Am.J.Public Health 1989
Jun;79(6):744-50.
- Lee IM, Paffenbarger RS, Jr., Hsieh CC. Physical
activity and risk of prostatic cancer among college alumni. Am.J.Epidemiol.
1992 Jan 15;135(2):169-79.
- Paffenbarger RS, Lee IM, Wong AL. The influence
of phsyical activity on the incidence of site-specific cancers in college
alumni. In: Jacobs MM, editor. Exercise, calories, fat, and cancer. New York:
Plenum Press; 1992. p. 7-15.
- Lee IM, Paffenbarger RS, Jr. Physical activity
and its relation to cancer risk: a prospective study of college alumni.
Med.Sci.Sports Exerc. 1994 Jul;26(7):831-7.
- Thune I, Lund E. Physical activity and the risk
of prostate and testicular cancer: a cohort study of 53,000 Norwegian men.
Cancer Causes Control 1994 Nov;5(6):549-56.
- Hsing AW, McLaughlin JK, Zheng W, Gao YT, Blot
WJ. Occupation, physical activity, and risk of prostate cancer in Shanghai,
People's Republic of China. Cancer Causes Control 1994 Mar;5(2):136-40.
- Steenland K, Nowlin S, Palu S. Cancer incidence
in the National Health and Nutrition Survey I. Follow-up data: diabetes,
cholesterol, pulse and physical activity. Cancer Epidemiol.Biomarkers Prev.
1995 Dec;4(8):807-11.
- Ilic M, Vlajinac H, Marinkovic J. Case-control
study of risk factors for prostate cancer. Br.J.Cancer 1996
Nov;74(10):1682-6.
- Oliveria SA, Kohl HW, III, Trichopoulos D, Blair
SN. The association between cardiorespiratory fitness and prostate cancer.
Med.Sci.Sports Exerc. 1996 Jan;28(1):97-104.
- Cerhan JR, Torner JC, Lynch CF, Rubenstein LM,
Lemke JH, Cohen MB, Lubaroff DM, Wallace RB. Association of smoking, body mass,
and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health
Study (United States). Cancer Causes Control 1997 Mar;8(2):229-38.
- Hartman TJ, Albanes D, Rautalahti M, Tangrea JA,
Virtamo J, Stolzenberg R, Taylor PR. Physical activity and prostate cancer in
the Alpha-Tocopherol, Beta-Carotene (ATBC) Cancer Prevention Study (Finland).
Cancer Causes Control 1998 Jan;9(1):11-8.
- Giovannucci EL, Liu Y, Leitzmann MF, Stampfer
MJ, Willett WC. A prospective study of physical activity and incident and fatal
prostate cancer. Arch.Intern.Med. 2005 May 9;165(9):1005-10.
- Liu S, Lee IM, Linson P, Ajani U, Buring JE,
Hennekens CH. A prospective study of physical activity and risk of prostate
cancer in US physicians. Int.J.Epidemiol. 2000 Feb;29(1):29-35.
- Lund Nilsen TI, Johnsen R, Vatten LJ.
Socio-economic and lifestyle factors associated with the risk of prostate
cancer. Br.J.Cancer 2000 Apr;82(7):1358-63.
- Clarke G, Whittemore AS. Prostate cancer risk in
relation to anthropometry and physical activity: the National Health and
Nutrition Examination Survey I Epidemiological Follow-Up Study. Cancer
Epidemiol.Biomarkers Prev. 2000 Sep;9(9):875-81.
- Putnam SD, Cerhan JR, Parker AS, Bianchi GD,
Wallace RB, Cantor KP, Lynch CF. Lifestyle and anthropometric risk factors for
prostate cancer in a cohort of Iowa men. Ann.Epidemiol. 2000
Aug;10(6):361-9.
- Pukkala E, Kaprio J, Koskenvuo M, Kujala U,
Sarna S. Cancer incidence among Finnish world class male athletes. Int.J.Sports
Med. 2000 Apr;21(3):216-20.
- Lee IM, Sesso HD, Paffenbarger RS, Jr. A
prospective cohort study of physical activity and body size in relation to
prostate cancer risk (United States). Cancer Causes Control 2001
Feb;12(2):187-93.
- Wannamethee SG, Shaper AG, Walker M. Physical
activity and risk of cancer in middle-aged men. Br.J.Cancer 2001 Nov
2;85(9):1311-6.
- Norman A, Moradi T, Gridley G, Dosemeci M, Rydh
B, Nyren O, Wolk A. Occupational physical activity and risk for prostate cancer
in a nationwide cohort study in Sweden. Br.J.Cancer 2002 Jan 7;86(1):70-5.
- Platz EA, Leitzmann MF, Michaud DS, Willett WC,
Giovannucci E. Interrelation of energy intake, body size, and physical activity
with prostate cancer in a large prospective cohort study. Cancer Res. 2003 Dec
1;63(23):8542-8.
- Yu H, Harris RE, Wynder EL. Case-control study
of prostate cancer and socioeconomic factors. Prostate 1988;13(4):317-25.
- Brownson RC, Chang JC, Davis JR, Smith CA.
Physical activity on the job and cancer in Missouri. Am.J.Public Health 1991
May;81(5):639-42.
- Le Marchand L, Kolonel LN, Yoshizawa CN.
Lifetime occupational physical activity and prostate cancer risk.
Am.J.Epidemiol. 1991 Jan 15;133(2):103-11.
- West DW, Slattery ML, Robison LM, French TK,
Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a
case-control study with special emphasis on aggressive tumors. Cancer Causes
Control 1991 Mar;2(2):85-94.
- Dosemeci M, Hayes RB, Vetter R, Hoover RN,
Tucker M, Engin K, Unsal M, Blair A. Occupational physical activity,
socioeconomic status, and risks of 15 cancer sites in Turkey. Cancer Causes
Control 1993 Jul;4(4):313-21.
- Andersson SO, Baron J, Wolk A, Lindgren C,
Bergstrom R, Adami HO. Early life risk factors for prostate cancer: a
population-based case-control study in Sweden. Cancer Epidemiol.Biomarkers
Prev. 1995 Apr;4(3):187-92.
- Whittemore AS, Kolonel LN, Wu AH, John EM,
Gallagher RP, Howe GR, Burch JD, Hankin J, Dreon DM, West DW, et al. Prostate
cancer in relation to diet, physical activity, and body size in blacks, whites,
and Asians in the United States and Canada. J.Natl.Cancer Inst. 1995 May
3;87(9):652-61.
- Sung JF, Lin RS, Pu YS, Chen YC, Chang HC, Lai
MK. Risk factors for prostate carcinoma in Taiwan: a case-control study in a
Chinese population. Cancer 1999 Aug 1;86(3):484-91.
- Villeneuve PJ, Johnson KC, Kreiger N, Mao Y.
Risk factors for prostate cancer: results from the Canadian National Enhanced
Cancer Surveillance System. The Canadian Cancer Registries Epidemiology
Research Group. Cancer Causes Control 1999 Oct;10(5):355-67.
- Bairati I, Larouche R, Meyer F, Moore L, Fradet
Y. Lifetime occupational physical activity and incidental prostate cancer
(Canada). Cancer Causes Control 2000 Sep;11(8):759-64.
- Lacey JV, Jr., Deng J, Dosemeci M, Gao YT,
Mostofi FK, Sesterhenn IA, Xie T, Hsing AW. Prostate cancer, benign prostatic
hyperplasia and physical activity in Shanghai, China. Int.J.Epidemiol. 2001
Apr;30(2):341-9.
- Friedenreich CM, McGregor SE, Courneya KS,
Angyalfi SJ, Elliott FG. Case-control study of lifetime total physical activity
and prostate cancer risk. Am.J.Epidemiol. 2004 Apr 15;159(8):740-9.
- Pierotti B, Altieri A, Talamini R, Montella M,
Tavani A, Negri E, Franceschi S, La VC. Lifetime physical activity and prostate
cancer risk. Int.J.Cancer 2005 Apr 20;114(4):639-42.
- Patel AV, Rodriguez C, Jacobs EJ, Solomon L,
Thun MJ, Calle EE. Recreational physical activity and risk of prostate cancer
in a large cohort of U.S. men. Cancer Epidemiol.Biomarkers Prev. 2005
Jan;14(1):275-9.
- Jian L, Shen ZJ, Lee AH, Binns CW. Moderate
physical activity and prostate cancer risk: a case-control study in China.
Eur.J.Epidemiol. 2005;20(2):155-60.
- Zeegers MP, Dirx MJ, van den Brandt PA.
Physical activity and the risk of prostate cancer in the Netherlands cohort
study, results after 9.3 years of follow-up. Cancer Epidemiol.Biomarkers Prev.
2005 Jun;14(6):1490-5.
- Nilsen TI, Romundstad PR, Vatten LJ.
Recreational physical activity and risk of prostate cancer: A prospective
population-based study in Norway (the HUNT study). Int.J.Cancer 2006 Dec
15;119(12):2943-7.
- Littman AJ, Kristal AR, White E. Recreational
physical activity and prostate cancer risk (United States). Cancer Causes
Control 2006 Aug;17(6):831-41.
- Giovannucci E, Liu Y, Platz EA, Stampfer MJ,
Willett WC. Risk factors for prostate cancer incidence and progression in the
health professionals follow-up study. Int.J.Cancer 2007 Oct
1;121(7):1571-8.
- Darlington GA, Kreiger N, Lightfoot N, Purdham
J, Sass-Kortsak A. Prostate cancer risk and diet, recreational physical
activity and cigarette smoking. Chronic.Dis.Can. 2007;27(4):145-53.
- Giovannucci E, Ascherio A, Rimm EB, Colditz GA,
Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer
and adenoma in men. Ann.Intern.Med. 1995 Mar 1;122(5):327-34.
- Calton BA, Lacey JV, Jr., Schatzkin A, Schairer
C, Colbert LH, Albanes D, Leitzmann MF. Physical activity and the risk of colon
cancer among women: a prospective cohort study (United States). Int.J.Cancer
2006 Jul 15;119(2):385-91.
- Chao A, Connell CJ, Jacobs EJ, McCullough ML,
Patel AV, Calle EE, Cokkinides VE, Thun MJ. Amount, type, and timing of
recreational physical activity in relation to colon and rectal cancer in older
adults: the Cancer Prevention Study II Nutrition Cohort. Cancer
Epidemiol.Biomarkers Prev. 2004 Dec;13(12):2187-95.
- Colbert LH, Hartman TJ, Malila N, Limburg PJ,
Pietinen P, Virtamo J, Taylor PR, Albanes D. Physical activity in relation to
cancer of the colon and rectum in a cohort of male smokers. Cancer
Epidemiol.Biomarkers Prev. 2001 Mar;10(3):265-8.
- Larsson SC, Rutegard J, Bergkvist L, Wolk A.
Physical activity, obesity, and risk of colon and rectal cancer in a cohort of
Swedish men. Eur.J.Cancer 2006 Oct;42(15):2590-7.
- Lee IM, Manson JE, Ajani U, Paffenbarger RS,
Jr., Hennekens CH, Buring JE. Physical activity and risk of colon cancer: the
Physicians' Health Study (United States). Cancer Causes Control 1997
Jul;8(4):568-74.
- Lee KJ, Inoue M, Otani T, Iwasaki M, Sasazuki
S, Tsugane S. Physical activity and risk of colorectal cancer in Japanese men
and women: the Japan Public Health Center-based prospective study. Cancer
Causes Control 2007 Mar;18(2):199-209.
- Mai PL, Sullivan-Halley J, Ursin G, Stram DO,
Deapen D, Villaluna D, Horn-Ross PL, Clarke CA, Reynolds P, Ross RK, et al.
Physical activity and colon cancer risk among women in the California Teachers
Study. Cancer Epidemiol.Biomarkers Prev. 2007 Mar;16(3):517-25.
- Martinez ME, Giovannucci E, Spiegelman D,
Hunter DJ, Willett WC, Colditz GA. Leisure-time physical activity, body size,
and colon cancer in women. Nurses' Health Study Research Group. J.Natl.Cancer
Inst. 1997 Jul 2;89(13):948-55.
- Thune I, Lund E. Physical activity and risk of
colorectal cancer in men and women. Br.J.Cancer 1996 May;73(9):1134-40.
- Hou L, Ji BT, Blair A, Dai Q, Gao YT, Chow WH.
Commuting physical activity and risk of colon cancer in Shanghai, China.
Am.J.Epidemiol. 2004 Nov 1;160(9):860-7.
- Isomura K, Kono S, Moore MA, Toyomura K, Nagano
J, Mizoue T, Mibu R, Tanaka M, Kakeji Y, Maehara Y, et al. Physical activity
and colorectal cancer: the Fukuoka Colorectal Cancer Study. Cancer Sci. 2006
Oct;97(10):1099-104.
- Levi F, Pasche C, Lucchini F, Tavani A, La VC.
Occupational and leisure-time physical activity and the risk of colorectal
cancer. Eur.J.Cancer Prev. 1999 Dec;8(6):487-93.
- Slattery ML, Edwards SL, Ma KN, Friedman GD,
Potter JD. Physical activity and colon cancer: a public health perspective.
Ann.Epidemiol. 1997 Feb;7(2):137-45.
- Slattery ML, Potter J, Caan B, Edwards S,
Coates A, Ma KN, Berry TD. Energy balance and colon cancer--beyond physical
activity. Cancer Res. 1997 Jan 1;57(1):75-80.
- Slattery ML, Potter JD. Physical activity and
colon cancer: confounding or interaction? Med.Sci.Sports Exerc. 2002
Jun;34(6):913-9.
- Slattery ML, Edwards S, Curtin K, Ma K, Edwards
R, Holubkov R, Schaffer D. Physical activity and colorectal cancer.
Am.J.Epidemiol. 2003 Aug 1;158(3):214-24.
- White E, Jacobs EJ, Daling JR. Physical
activity in relation to colon cancer in middle-aged men and women.
Am.J.Epidemiol. 1996 Jul 1;144(1):42-50.
- Zhang Y, Cantor KP, Dosemeci M, Lynch CF, Zhu
Y, Zheng T. Occupational and leisure-time physical activity and risk of colon
cancer by subsite. J.Occup.Environ.Med. 2006 Mar;48(3):236-43.
- Tavani A, Braga C, La VC, Conti E, Filiberti R,
Montella M, Amadori D, Russo A, Franceschi S. Physical activity and risk of
cancers of the colon and rectum: an Italian case-control study. Br.J.Cancer
1999 Apr;79(11-12):1912-6.
- Tang R, Wang JY, Lo SK, Hsieh LL. Physical
activity, water intake and risk of colorectal cancer in Taiwan: a
hospital-based case-control study. Int.J.Cancer 1999 Aug 12;82(4):484-9.
- Lee IM, Oguma Y. Physical activity. In:
Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and prevention. 3rd
ed. New York: Oxford University Press; 2006. p. 449-67.
- Mao Y, Pan S, Wen SW, Johnson KC. Physical
inactivity, energy intake, obesity and the risk of rectal cancer in Canada.
Int.J.Cancer 2003 Jul 20;105(6):831-7.
- Steindorf K, Friedenreich C, Linseisen J,
Rohrmann S, Rundle A, Veglia F, Vineis P, Johnsen NF, Tjonneland A, Overvad K,
et al. Physical activity and lung cancer risk in the European Prospective
Investigation into Cancer and Nutrition Cohort. Int.J.Cancer 2006 Nov
15;119(10):2389-97.
- Sinner P, Folsom AR, Harnack L, Eberly LE,
Schmitz KH. The association of physical activity with lung cancer incidence in
a cohort of older women: the Iowa Women's Health Study. Cancer
Epidemiol.Biomarkers Prev. 2006 Dec;15(12):2359-63.
- Thune I, Lund E. The influence of physical
activity on lung-cancer risk: A prospective study of 81,516 men and women.
Int.J.Cancer 1997 Jan 6;70(1):57-62.
- Lee IM, Sesso HD, Paffenbarger RS, Jr. Physical
activity and risk of lung cancer. Int.J.Epidemiol. 1999 Aug;28(4):620-5.
- Colbert LH, Hartman TJ, Tangrea JA, Pietinen P,
Virtamo J, Taylor PR, Albanes D. Physical activity and lung cancer risk in male
smokers. Int.J.Cancer 2002 Apr 10;98(5):770-3.
- Bak H, Christensen J, Thomsen BL, Tjonneland A,
Overvad K, Loft S, Raaschou-Nielsen O. Physical activity and risk for lung
cancer in a Danish cohort. Int.J.Cancer 2005 Sep 1;116(3):439-44.
- Alfano CM, Klesges RC, Murray DM, Bowen DJ,
McTiernan A, Vander Weg MW, Robinson LA, Cartmel B, Thornquist MD, Barnett M,
et al. Physical activity in relation to all-site and lung cancer incidence and
mortality in current and former smokers. Cancer Epidemiol.Biomarkers Prev. 2004
Dec;13(12):2233-41.
- Kubik A, Zatloukal P, Tomasek L, Pauk N,
Petruzelka L, Plesko I. Lung cancer risk among nonsmoking women in relation to
diet and physical activity. Neoplasma 2004;51(2):136-43.
- Kubik AK, Zatloukal P, Tomasek L, Pauk N, Havel
L, Krepela E, Petruzelka L. Dietary habits and lung cancer risk among
non-smoking women. Eur.J.Cancer Prev. 2004 Dec;13(6):471-80.
- Mao Y, Pan S, Wen SW, Johnson KC. Physical
activity and the risk of lung cancer in Canada. Am.J.Epidemiol. 2003 Sep
15;158(6):564-75.
- Moradi T, Nyren O, Bergstrom R, Gridley G,
Linet M, Wolk A, Dosemeci M, Adami HO. Risk for endometrial cancer in relation
to occupational physical activity: a nationwide cohort study in Sweden.
Int.J.Cancer 1998 May 29;76(5):665-70.
- Terry P, Baron JA, Weiderpass E, Yuen J,
Lichtenstein P, Nyren O. Lifestyle and endometrial cancer risk: a cohort study
from the Swedish Twin Registry. Int.J.Cancer 1999 Jul 2;82(1):38-42.
- Colbert LH, Lacey JV, Jr., Schairer C, Albert
P, Schatzkin A, Albanes D. Physical activity and risk of endometrial cancer in
a prospective cohort study (United States). Cancer Causes Control 2003
Aug;14(6):559-67.
- Friberg E, Mantzoros CS, Wolk A. Physical
activity and risk of endometrial cancer: a population-based prospective cohort
study. Cancer Epidemiol.Biomarkers Prev. 2006 Nov;15(11):2136-40.
- Friedenreich C, Cust A, Lahmann PH, Steindorf
K, Boutron-Ruault MC, Clavel-Chapelon F, Mesrine S, Linseisen J, Rohrmann S,
Pischon T, et al. Physical activity and risk of endometrial cancer: the
European prospective investigation into cancer and nutrition. Int.J.Cancer 2007
Jul 15;121(2):347-55.
- Furberg AS, Thune I. Metabolic abnormalities
(hypertension, hyperglycemia and overweight), lifestyle (high energy intake and
physical inactivity) and endometrial cancer risk in a Norwegian cohort.
Int.J.Cancer 2003 May 10;104(6):669-76.
- Schouten LJ, Goldbohm RA, van den Brandt PA.
Anthropometry, physical activity, and endometrial cancer risk: results from the
Netherlands Cohort Study. J.Natl.Cancer Inst. 2004 Nov 3;96(21):1635-8.
- Hirose K, Tajima K, Hamajima N, Takezaki T,
Inoue M, Kuroishi T, Kuzuya K, Nakamura S, Tokudome S. Subsite
(cervix/endometrium)-specific risk and protective factors in uterus cancer.
Jpn.J.Cancer Res. 1996 Sep;87(9):1001-9.
- Kalandidi A, Tzonou A, Lipworth L, Gamatsi I,
Filippa D, Trichopoulos D. A case-control study of endometrial cancer in
relation to reproductive, somatometric, and life-style variables. Oncology 1996
Sep;53(5):354-9.
- Goodman MT, Hankin JH, Wilkens LR, Lyu LC,
McDuffie K, Liu LQ, Kolonel LN. Diet, body size, physical activity, and the
risk of endometrial cancer. Cancer Res. 1997 Nov 15;57(22):5077-85.
- Olson SH, Vena JE, Dorn JP, Marshall JR,
Zielezny M, Laughlin R, Graham S. Exercise, occupational activity, and risk of
endometrial cancer. Ann.Epidemiol. 1997 Jan;7(1):46-53.
- Moradi T, Weiderpass E, Signorello LB, Persson
I, Nyren O, Adami HO. Physical activity and postmenopausal endometrial cancer
risk (Sweden). Cancer Causes Control 2000 Oct;11(9):829-37.
- Salazar-Martinez E, Lazcano-Ponce EC, Lira-Lira
GG, Escudero-De los RP, Salmeron-Castro J, Larrea F, Hernandez-Avila M.
Case-control study of diabetes, obesity, physical activity and risk of
endometrial cancer among Mexican women. Cancer Causes Control 2000
Sep;11(8):707-11.
- Littman AJ, Voigt LF, Beresford SA, Weiss NS.
Recreational physical activity and endometrial cancer risk. Am.J.Epidemiol.
2001 Nov 15;154(10):924-33.
- Matthews CE, Xu WH, Zheng W, Gao YT, Ruan ZX,
Cheng JR, Xiang YB, Shu XO. Physical activity and risk of endometrial cancer: a
report from the Shanghai endometrial cancer study. Cancer Epidemiol.Biomarkers
Prev. 2005 Apr;14(4):779-85.
- Olsen CM, Green AC, Whiteman DC, Sadeghi S,
Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: a
systematic review and meta-analysis. Eur.J.Cancer 2007 Mar;43(4):690-709.
- Sinner PJ, Schmitz KH, Anderson KE, Folsom AR.
Lack of association of physical activity and obesity with incident pancreatic
cancer in elderly women. Cancer Epidemiol.Biomarkers Prev. 2005
Jun;14(6):1571-3.
- Lee IM, Sesso HD, Oguma Y, Paffenbarger RS, Jr.
Physical activity, body weight, and pancreatic cancer mortality. Br.J.Cancer
2003 Mar 10;88(5):679-83.
- Michaud DS, Giovannucci E, Willett WC, Colditz
GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of
pancreatic cancer. JAMA 2001 Aug 22;286(8):921-9.
- Patel AV, Rodriguez C, Bernstein L, Chao A,
Thun MJ, Calle EE. Obesity, recreational physical activity, and risk of
pancreatic cancer in a large U.S. Cohort. Cancer Epidemiol.Biomarkers Prev.
2005 Feb;14(2):459-66.
- Berrington de Gonzalez A, Spencer EA,
Bueno-de-Mesquita HB, Roddam A, Stolzenberg-Solomon R, Halkjaer J, Tjonneland
A, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, et al. Anthropometry,
physical activity, and the risk of pancreatic cancer in the European
prospective investigation into cancer and nutrition. Cancer
Epidemiol.Biomarkers Prev. 2006 May;15(5):879-85.
- Luo J, Iwasaki M, Inoue M, Sasazuki S, Otani T,
Ye W, Tsugane S. Body mass index, physical activity and the risk of pancreatic
cancer in relation to smoking status and history of diabetes: a large-scale
population-based cohort study in Japan--the JPHC study. Cancer Causes Control
2007 Aug;18(6):603-12.
- Lin Y, Kikuchi S, Tamakoshi A, Yagyu K, Obata
Y, Inaba Y, Kurosawa M, Kawamura T, Motohashi Y, Ishibashi T. Obesity, physical
activity and the risk of pancreatic cancer in a large Japanese cohort.
Int.J.Cancer 2007 Jun 15;120(12):2665-71.
- Nothlings U, Wilkens LR, Murphy SP, Hankin JH,
Henderson BE, Kolonel LN. Body mass index and physical activity as risk factors
for pancreatic cancer: the Multiethnic Cohort Study. Cancer Causes Control 2007
Mar;18(2):165-75.
- Hanley AJ, Johnson KC, Villeneuve PJ, Mao Y.
Physical activity, anthropometric factors and risk of pancreatic cancer:
results from the Canadian enhanced cancer surveillance system. Int.J.Cancer
2001 Oct 1;94(1):140-7.
- Eberle CA, Bracci PM, Holly EA. Anthropometric
factors and pancreatic cancer in a population-based case-control study in the
San Francisco Bay area. Cancer Causes Control 2005 Dec;16(10):1235-44.
- Giovannucci E, Michaud D. The role of obesity
and related metabolic disturbances in cancers of the colon, prostate, and
pancreas. Gastroenterology 2007 May;132(6):2208-25.
- Hewitt ME, Greenfield S, Stovall E, National
Cancer Policy Board (U.S.). From cancer patient to cancer survivor : lost in
transition. Washington, D.C.: National Academies Press; 2006.
- Aziz NM, Rowland JH. Trends and advances in
cancer survivorship research: challenge and opportunity. Semin.Radiat.Oncol.
2003 Jul;13(3):248-66.
- Carver JR, Shapiro CL, Ng A, Jacobs L, Schwartz
C, Virgo KS, Hagerty KL, Somerfield MR, Vaughn DJ. American Society of Clinical
Oncology clinical evidence review on the ongoing care of adult cancer
survivors: cardiac and pulmonary late effects. J.Clin.Oncol. 2007 Sep
1;25(25):3991-4008.
- Schmitz KH, Cappola AR, Stricker CT, Sweeney C,
Norman SA. The intersection of cancer and aging: establishing the need for
breast cancer rehabilitation. Cancer Epidemiol.Biomarkers Prev. 2007
May;16(5):866-72.
- Schmitz KH, Holtzman J, Courneya KS, Masse LC,
Duval S, Kane R. Controlled physical activity trials in cancer survivors: a
systematic review and meta-analysis. Cancer Epidemiol.Biomarkers Prev. 2005
Jul;14(7):1588-95.
- Markes M, Brockow T, Resch KL. Exercise for
women receiving adjuvant therapy for breast cancer. Cochrane.Database.Syst.Rev.
2006;(4):CD005001.
- Holmes MD, Chen WY, Feskanich D, Kroenke CH,
Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA
2005 May 25;293(20):2479-86.
- Chlebowski RT, Aiello E, McTiernan A. Weight
loss in breast cancer patient management. J.Clin.Oncol. 2002 Feb
15;20(4):1128-43.
- Kroenke CH, Rosner B, Chen WY, Kawachi I,
Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis.
J.Clin.Oncol. 2004 May 15;22(10):1849-56.
- Trentham-Dietz A, Newcomb PA, Nichols HB,
Hampton JM. Breast cancer risk factors and second primary malignancies among
women with breast cancer. Breast Cancer Res.Treat. 2007
Oct;105(2):195-207.
- Meyerhardt JA, Giovannucci EL, Holmes MD, Chan
AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after
colorectal cancer diagnosis. J.Clin.Oncol. 2006 Aug 1;24(22):3527-34.
- Meyerhardt JA, Heseltine D, Niedzwiecki D,
Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, et al.
Impact of physical activity on cancer recurrence and survival in patients with
stage III colon cancer: findings from CALGB 89803. J.Clin.Oncol. 2006 Aug
1;24(22):3535-41.
- Bicego D, Brown K, Ruddick M, Storey D, Wong C,
Harris SR. Exercise for women with or at risk for breast cancer-related
lymphedema. Phys.Ther. 2006 Oct;86(10):1398-405.
- Ingram C, Courneya KS, Kingston D. The effects
of exercise on body weight and composition in breast cancer survivors: an
integrative systematic review. Oncol.Nurs.Forum 2006 Sep;33(5):937-47.
- Hewitt JA, Mokbel K, van Someren KA, Jewell AP,
Garrod R. Exercise for breast cancer survival: the effect on cancer risk and
cancer-related fatigue (CRF). Int.J.Fertil.Womens Med. 2005 Sep;50(5 Pt
1):231-9.
- Knols R, Aaronson NK, Uebelhart D, Fransen J,
Aufdemkampe G. Physical exercise in cancer patients during and after medical
treatment: a systematic review of randomized and controlled clinical trials.
J.Clin.Oncol. 2005 Jun 1;23(16):3830-42.
- Galvao DA, Newton RU. Review of exercise
intervention studies in cancer patients. J.Clin.Oncol. 2005 Feb
1;23(4):899-909.
- Stevinson C, Lawlor DA, Fox KR. Exercise
interventions for cancer patients: systematic review of controlled trials.
Cancer Causes Control 2004 Dec;15(10):1035-56.
- Stricker CT, Drake D, Hoyer KA, Mock V.
Evidence-based practice for fatigue management in adults with cancer: exercise
as an intervention. Oncol.Nurs.Forum 2004 Sep;31(5):963-76.
- Courneya KS, Friedenreich CM, Quinney HA,
Fields AL, Jones LW, Fairey AS. Predictors of adherence and contamination in a
randomized trial of exercise in colorectal cancer survivors. Psychooncology.
2004 Dec;13(12):857-66.
- Oldervoll LM, Kaasa S, Hjermstad MJ, Lund JA,
Loge JH. Physical exercise results in the improved subjective well-being of a
few or is effective rehabilitation for all cancer patients? Eur.J.Cancer 2004
May;40(7):951-62.
- Irwin ML, Ainsworth BE. Physical activity
interventions following cancer diagnosis: methodologic challenges to delivery
and assessment. Cancer Invest 2004;22(1):30-50.
- Shamley DR, Barker K, Simonite V, Beardshaw A.
Delayed versus immediate exercises following surgery for breast cancer: a
systematic review. Breast Cancer Res.Treat. 2005 Apr;90(3):263-71.
- Burnham TR, Wilcox A. Effects of exercise on
physiological and psychological variables in cancer survivors. Med.Sci.Sports
Exerc. 2002 Dec;34(12):1863-7.
- Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety
and efficacy of weight training in recent breast cancer survivors to alter body
composition, insulin, and insulin-like growth factor axis proteins. Cancer
Epidemiol.Biomarkers Prev. 2005 Jul;14(7):1672-80.
- Thorsen L, Skovlund E, Stromme SB, Hornslien K,
Dahl AA, Fossa SD. Effectiveness of physical activity on cardiorespiratory
fitness and health-related quality of life in young and middle-aged cancer
patients shortly after chemotherapy. J.Clin.Oncol. 2005 Apr
1;23(10):2378-88.
- Pinto BM, Frierson GM, Rabin C, Trunzo JJ,
Marcus BH. Home-based physical activity intervention for breast cancer
patients. J.Clin.Oncol. 2005 May 20;23(15):3577-87.
- Mustian KM, Katula JA, Zhao H. A pilot study to
assess the influence of tai chi chuan on functional capacity among breast
cancer survivors. J.Support.Oncol. 2006 Mar;4(3):139-45.
- Hutnick NA, Williams NI, Kraemer WJ,
Orsega-Smith E, Dixon RH, Bleznak AD, Mastro AM. Exercise and lymphocyte
activation following chemotherapy for breast cancer. Med.Sci.Sports Exerc. 2005
Nov;37(11):1827-35.
- Herrero F, San Juan AF, Fleck SJ, Balmer J,
Perez M, Canete S, Earnest CP, Foster C, Lucia A. Combined aerobic and
resistance training in breast cancer survivors: A randomized, controlled pilot
trial. Int.J.Sports Med. 2006 Jul;27(7):573-80.
- Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman
R, Roalfe A. Randomized trial of exercise therapy in women treated for breast
cancer. J.Clin.Oncol. 2007 May 1;25(13):1713-21.
- Culos-Reed SN, Carlson LE, Daroux LM,
Hately-Aldous S. A pilot study of yoga for breast cancer survivors: physical
and psychological benefits. Psychooncology. 2006 Oct;15(10):891-7.
- Bennett JA, Lyons KS, Winters-Stone K, Nail LM,
Scherer J. Motivational interviewing to increase physical activity in long-term
cancer survivors: a randomized controlled trial. Nurs.Res. 2007
Jan;56(1):18-27.
- Basen-Engquist K, Taylor CL, Rosenblum C, Smith
MA, Shinn EH, Greisinger A, Gregg X, Massey P, Valero V, Rivera E. Randomized
pilot test of a lifestyle physical activity intervention for breast cancer
survivors. Patient.Educ.Couns. 2006 Dec;64(1-3):225-34.
- Demark-Wahnefried W, Peterson BL, Winer EP,
Marks L, Aziz N, Marcom PK, Blackwell K, Rimer BK. Changes in weight, body
composition, and factors influencing energy balance among premenopausal breast
cancer patients receiving adjuvant chemotherapy. J.Clin.Oncol. 2001 May
1;19(9):2381-9.
- Irwin ML, Crumley D, McTiernan A, Bernstein L,
Baumgartner R, Gilliland FD, Kriska A, Ballard-Barbash R. Physical activity
levels before and after a diagnosis of breast carcinoma: the Health, Eating,
Activity, and Lifestyle (HEAL) study. Cancer 2003 Apr 1;97(7):1746-57.
- Sprod LK, Drum SN, Bentz AT, Carter SD,
Schneider CM. The effects of walking poles on shoulder function in breast
cancer survivors. Integr.Cancer Ther. 2005 Dec;4(4):287-93.
- Ahmed RL, Thomas W, Yee D, Schmitz KH.
Randomized controlled trial of weight training and lymphedema in breast cancer
survivors. J.Clin.Oncol. 2006 Jun 20;24(18):2765-72.
- Courneya KS, Friedenreich CM, Sela RA, Quinney
HA, Rhodes RE, Handman M. The group psychotherapy and home-based physical
exercise (group-hope) trial in cancer survivors: physical fitness and quality
of life outcomes. Psychooncology. 2003 Jun;12(4):357-74.
- Cho OH, Yoo YS, Kim NC. Efficacy of
comprehensive group rehabilitation for women with early breast cancer in South
Korea. Nurs.Health Sci. 2006 Sep;8(3):140-6.
- Sandel SL, Judge JO, Landry N, Faria L,
Ouellette R, Majczak M. Dance and movement program improves quality-of-life
measures in breast cancer survivors. Cancer Nurs. 2005 Jul;28(4):301-9.
- Mansel RE, Fallowfield L, Kissin M, Goyal A,
Newcombe RG, Dixon JM, Yiangou C, Horgan K, Bundred N, Monypenny I, et al.
Randomized multicenter trial of sentinel node biopsy versus standard axillary
treatment in operable breast cancer: the ALMANAC Trial. J.Natl.Cancer Inst.
2006 May 3;98(9):599-609.
- Paskett ED, Naughton MJ, McCoy TP, Case LD,
Abbott JM. The epidemiology of arm and hand swelling in premenopausal breast
cancer survivors. Cancer Epidemiol.Biomarkers Prev. 2007 Apr;16(4):775-82.
- Petrek JA, Naughton MJ, Case LD, Paskett ED,
Naftalis EZ, Singletary SE, Sukumvanich P. Incidence, time course, and
determinants of menstrual bleeding after breast cancer treatment: a prospective
study. J.Clin.Oncol. 2006 Mar 1;24(7):1045-51.
- van Akkooi AC, Bouwhuis MG, van Geel AN,
Hoedemaker R, Verhoef C, Grunhagen DJ, Schmitz PI, Eggermont AM, de Wilt JH.
Morbidity and prognosis after therapeutic lymph node dissections for malignant
melanoma. Eur.J.Surg.Oncol. 2007 Feb;33(1):102-8.
- Wrightson WR, Wong SL, Edwards MJ, Chao C,
Reintgen DS, Ross MI, Noyes RD, Viar V, Cerrito PB, McMasters KM. Complications
associated with sentinel lymph node biopsy for melanoma. Ann.Surg.Oncol. 2003
Jul;10(6):676-80.
- Karakousis CP, Driscoll DL, Rose B, Walsh DL.
Groin dissection in malignant melanoma. Ann.Surg.Oncol. 1994
Jul;1(4):271-7.
- Okeke AA, Bates DO, Gillatt DA. Lymphoedema in
urological cancer. Eur.Urol. 2004 Jan;45(1):18-25.
- Werngren-Elgstrom M, Lidman D. Lymphoedema of
the lower extremities after surgery and radiotherapy for cancer of the cervix.
Scand.J.Plast.Reconstr.Surg.Hand Surg. 1994 Dec;28(4):289-93.
- Haberthur F, Almendral AC, Ritter B. Therapy of
vulvar carcinoma. Eur.J.Gynaecol.Oncol. 1993;14(3):218-27.
- Karakousis CP, Heiser MA, Moore RH. Lymphedema
after groin dissection. Am.J.Surg. 1983 Feb;145(2):205-8.
- Ingvar C, Erichsen C, Jonsson PE. Morbidity
following prophylactic and therapeutic lymph node dissection for melanoma--a
comparison. Tumori 1984 Dec 31;70(6):529-33.
- Urist MM, Maddox WA, Kennedy JE, Balch CM.
Patient risk factors and surgical morbidity after regional lymphadenectomy in
204 melanoma patients. Cancer 1983 Jun 1;51(11):2152-6.
- de Vries M, Vonkeman WG, van Ginkel RJ,
Hoekstra HJ. Morbidity after inguinal sentinel lymph node biopsy and completion
lymph node dissection in patients with cutaneous melanoma. Eur.J.Surg.Oncol.
2006 Sep;32(7):785-9.
- McKenzie DC, Kalda AL. Effect of upper
extremity exercise on secondary lymphedema in breast cancer patients: a pilot
study. J.Clin.Oncol. 2003 Feb 1;21(3):463-6.
- Courneya KS, Mackey JR, Bell GJ, Jones LW,
Field CJ, Fairey AS. Randomized controlled trial of exercise training in
postmenopausal breast cancer survivors: cardiopulmonary and quality of life
outcomes. J.Clin.Oncol. 2003 May 1;21(9):1660-8.
- Demark-Wahnefried W, Clipp EC, Morey MC, Pieper
CF, Sloane R, Snyder DC, Cohen HJ. Lifestyle intervention development study to
improve physical function in older adults with cancer: outcomes from Project
LEAD. J.Clin.Oncol. 2006 Jul 20;24(21):3465-73.
- Matthews CE, Wilcox S, Hanby CL, Der AC, Heiney
SP, Gebretsadik T, Shintani A. Evaluation of a 12-week home-based walking
intervention for breast cancer survivors. Support.Care Cancer 2007
Feb;15(2):203-11.
- Ohira T, Schmitz KH, Ahmed RL, Yee D. Effects
of weight training on quality of life in recent breast cancer survivors: the
Weight Training for Breast Cancer Survivors (WTBS) study. Cancer 2006 May
1;106(9):2076-83.
- Ryan JL, Carroll JK, Ryan EP, Mustian KM,
Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist.
2007;12 Suppl 1:22-34.
- Pinto BM, Clark MM, Maruyama NC, Feder SI.
Psychological and fitness changes associated with exercise participation among
women with breast cancer. Psychooncology. 2003 Mar;12(2):118-26.
- McTiernan A. Mechanisms linking physical
activity with cancer. Nat.Rev.Cancer 2008 Jan 31.
- Bernstein L. Epidemiology of endocrine-related
risk factors for breast cancer. J.Mammary.Gland.Biol.Neoplasia. 2002
Jan;7(1):3-15.
- Roddam AW, Allen NE, Appleby P, Key TJ.
Endogenous sex hormones and prostate cancer: a collaborative analysis of 18
prospective studies. J.Natl.Cancer Inst. 2008 Feb 6;100(3):170-83.
- Eliassen AH, Missmer SA, Tworoger SS,
Spiegelman D, Barbieri RL, Dowsett M, Hankinson SE. Endogenous steroid hormone
concentrations and risk of breast cancer among premenopausal women.
J.Natl.Cancer Inst. 2006 Oct 4;98(19):1406-15.
- Kaaks R, Berrino F, Key T, Rinaldi S, Dossus L,
Biessy C, Secreto G, Amiano P, Bingham S, Boeing H, et al. Serum sex steroids
in premenopausal women and breast cancer risk within the European Prospective
Investigation into Cancer and Nutrition (EPIC). J.Natl.Cancer Inst. 2005 May
18;97(10):755-65.
- Winer EP, Hudis C, Burstein HJ, Wolff AC,
Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, et al.
American Society of Clinical Oncology technology assessment on the use of
aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone
receptor-positive breast cancer: status report 2004. J.Clin.Oncol. 2005 Jan
20;23(3):619-29.
- Lukanova A, Lundin E, Micheli A, Arslan A,
Ferrari P, Rinaldi S, Krogh V, Lenner P, Shore RE, Biessy C, et al. Circulating
levels of sex steroid hormones and risk of endometrial cancer in postmenopausal
women. Int.J.Cancer 2004 Jan 20;108(3):425-32.
- Tammela T. Endocrine treatment of prostate
cancer. J.Steroid Biochem.Mol.Biol. 2004 Nov;92(4):287-95.
- Thompson IM, Goodman PJ, Tangen CM, Lucia MS,
Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, et al. The
influence of finasteride on the development of prostate cancer. N.Engl.J.Med.
2003 Jul 17;349(3):215-24.
- McTiernan A, Ulrich C, Slate S, Potter J.
Physical activity and cancer etiology: associations and mechanisms. Cancer
Causes Control 1998 Oct;9(5):487-509.
- Loucks AB. Energy availability, not body
fatness, regulates reproductive function in women. Exerc.Sport Sci.Rev. 2003
Jul;31(3):144-8.
- Loucks AB, Redman LM. The effect of stress on
menstrual function. Trends Endocrinol.Metab 2004 Dec;15(10):466-71.
- Williams NI, Helmreich DL, Parfitt DB,
Caston-Balderrama A, Cameron JL. Evidence for a causal role of low energy
availability in the induction of menstrual cycle disturbances during strenuous
exercise training. J.Clin.Endocrinol.Metab 2001 Nov;86(11):5184-93.
- Bonen A. Recreational exercise does not impair
menstrual cycles: a prospective study. Int.J.Sports Med. 1992
Feb;13(2):110-20.
- Rogol AD, Weltman A, Weltman JY, Seip RL, Snead
DB, Levine S, Haskvitz EM, Thompson DL, Schurrer R, Dowling E, et al.
Durability of the reproductive axis in eumenorrheic women during 1 yr of
endurance training. J.Appl.Physiol 1992 Apr;72(4):1571-80.
- Bullen BA, Skrinar GS, Beitins IZ, Carr DB,
Reppert SM, Dotson CO, Fencl MD, Gervino EV, McArthur JW. Endurance training
effects on plasma hormonal responsiveness and sex hormone excretion.
J.Appl.Physiol 1984 Jun;56(6):1453-63.
- Bullen BA, Skrinar GS, Beitins IZ, von MG,
Turnbull BA, McArthur JW. Induction of menstrual disorders by strenuous
exercise in untrained women. N.Engl.J.Med. 1985 May 23;312(21):1349-53.
- Keizer HA, Kuipers H, de HJ, Janssen GM,
Beckers E, Habets L, van KG, Geurten P. Effect of a 3-month endurance training
program on metabolic and multiple hormonal responses to exercise. Int.J.Sports
Med. 1987 Dec;8 Suppl 3:154-60.
- Tworoger SS, Missmer SA, Eliassen AH, Barbieri
RL, Dowsett M, Hankinson SE. Physical activity and inactivity in relation to
sex hormone, prolactin, and insulin-like growth factor concentrations in
premenopausal women - exercise and premenopausal hormones. Cancer Causes
Control 2007 Sep;18(7):743-52.
- Chan MF, Dowsett M, Folkerd E, Bingham S,
Wareham N, Luben R, Welch A, Khaw KT. Usual physical activity and endogenous
sex hormones in postmenopausal women: the European prospective investigation
into cancer-norfolk population study. Cancer Epidemiol.Biomarkers Prev. 2007
May;16(5):900-5.
- McTiernan A, Wu L, Chen C, Chlebowski R,
Mossavar-Rahmani Y, Modugno F, Perri MG, Stanczyk FZ, Van HL, Wang CY. Relation
of BMI and physical activity to sex hormones in postmenopausal women.
Obesity.(Silver.Spring) 2006 Sep;14(9):1662-77.
- Verkasalo PK, Thomas HV, Appleby PN, Davey GK,
Key TJ. Circulating levels of sex hormones and their relation to risk factors
for breast cancer: a cross-sectional study in 1092 pre- and postmenopausal
women (United Kingdom). Cancer Causes Control 2001 Jan;12(1):47-59.
- McTiernan A, Tworoger SS, Ulrich CM, Yasui Y,
Irwin ML, Rajan KB, Sorensen B, Rudolph RE, Bowen D, Stanczyk FZ, et al. Effect
of exercise on serum estrogens in postmenopausal women: a 12-month randomized
clinical trial. Cancer Res. 2004 Apr 15;64(8):2923-8.
- McTiernan A, Tworoger SS, Rajan KB, Yasui Y,
Sorenson B, Ulrich CM, Chubak J, Stanczyk FZ, Bowen D, Irwin ML, et al. Effect
of exercise on serum androgens in postmenopausal women: a 12-month randomized
clinical trial. Cancer Epidemiol.Biomarkers Prev. 2004 Jul;13(7):1099-105.
- De Souza MJ, Miller BE. The effect of endurance
training on reproductive function in male runners. A 'volume threshold'
hypothesis. Sports Med. 1997 Jun;23(6):357-74.
- Hawkins VN, Foster-Schubert K, Chubak J,
Sorensen B, Ulrich CM, Stancyzk FZ, Plymate S, Stanford J, White E, Potter JD,
et al. Effect of Exercise on Serum Sex Hormones in Men: A 12-Month Randomized
Clinical Trial. Med.Sci.Sports Exerc. 2008 Feb;40(2):223-33.
- Kaaks R, Lukanova A. Energy balance and cancer:
the role of insulin and insulin-like growth factor-I. Proc.Nutr.Soc. 2001
Feb;60(1):91-106.
- Wolf I, Sadetzki S, Catane R, Karasik A,
Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol. 2005
Feb;6(2):103-11.
- Boule NG, Haddad E, Kenny GP, Wells GA, Sigal
RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes
mellitus: a meta-analysis of controlled clinical trials. JAMA 2001 Sep
12;286(10):1218-27.
- Frank LL, Sorensen BE, Yasui Y, Tworoger SS,
Schwartz RS, Ulrich CM, Irwin ML, Rudolph RE, Rajan KB, Stanczyk F, et al.
Effects of exercise on metabolic risk variables in overweight postmenopausal
women: a randomized clinical trial. Obes.Res. 2005 Mar;13(3):615-25.
- Ross R, Dagnone D, Jones PJ, Smith H, Paddags
A, Hudson R, Janssen I. Reduction in obesity and related comorbid conditions
after diet-induced weight loss or exercise-induced weight loss in men. A
randomized, controlled trial. Ann.Intern.Med. 2000 Jul 18;133(2):92-103.
- Ross R, Janssen I, Dawson J, Kungl AM, Kuk JL,
Wong SL, Nguyen-Duy TB, Lee S, Kilpatrick K, Hudson R. Exercise-induced
reduction in obesity and insulin resistance in women: a randomized controlled
trial. Obes.Res. 2004 May;12(5):789-98.
- Duncan GE, Perri MG, Theriaque DW, Hutson AD,
Eckel RH, Stacpoole PW. Exercise training, without weight loss, increases
insulin sensitivity and postheparin plasma lipase activity in previously
sedentary adults. Diabetes Care 2003 Mar;26(3):557-62.
- Campbell KL, McTiernan A. Exercise and
biomarkers for cancer prevention studies. J.Nutr. 2007 Jan;137(1
Suppl):161S-9S.
- Tworoger SS, Eliassen AH, Sluss P, Hankinson
SE. A prospective study of plasma prolactin concentrations and risk of
premenopausal and postmenopausal breast cancer. J.Clin.Oncol. 2007 Apr
20;25(12):1482-8.
- Tworoger SS, Sorensen B, Chubak J, Irwin M,
Stanczyk FZ, Ulrich CM, Potter J, McTiernan A. Effect of a 12-month randomized
clinical trial of exercise on serum prolactin concentrations in postmenopausal
women. Cancer Epidemiol.Biomarkers Prev. 2007 May;16(5):895-9.
- Il'yasova D, Colbert LH, Harris TB, Newman AB,
Bauer DC, Satterfield S, Kritchevsky SB. Circulating levels of inflammatory
markers and cancer risk in the health aging and body composition cohort. Cancer
Epidemiol.Biomarkers Prev. 2005 Oct;14(10):2413-8.
- Wetmore CM, Ulrich CM. Mechanisms associating
physical activity with cancer incidence: exercise and immune function. In:
McTiernan A, editor. Cancer prevention and management through exercise and
weight control. Boca Raton: CRC Press; 2006. p. 157-76.
- Esposito K, Pontillo A, Di PC, Giugliano G,
Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes
on vascular inflammatory markers in obese women: a randomized trial. JAMA 2003
Apr 9;289(14):1799-804.
- Jakobisiak M, Lasek W, Golab J. Natural
mechanisms protecting against cancer. Immunol.Lett. 2003 Dec
15;90(2-3):103-22.
- Nieman DC. Exercise immunology: practical
applications. Int.J.Sports Med. 1997 Mar;18 Suppl 1:S91-100.
- Martinez ME, Heddens D, Earnest DL, Bogert CL,
Roe D, Einspahr J, Marshall JR, Alberts DS. Physical activity, body mass index,
and prostaglandin E2 levels in rectal mucosa. J.Natl.Cancer Inst. 1999 Jun
2;91(11):950-3.
- Abrahamson PE, King IB, Ulrich CM, Rudolph RE,
Irwin ML, Yasui Y, Surawicz C, Lampe JW, Lampe PD, Morgan A, et al. No effect
of exercise on colon mucosal prostaglandin concentrations: a 12-month
randomized controlled trial. Cancer Epidemiol.Biomarkers Prev. 2007
Nov;16(11):2351-6.
top of page
Continue to G8. Mental Health
Back to G6. Functional Health
Back to Physical Activity Guidelines Advisory Committee
Report |