NATIONAL INSTITUTES OF HEALTH
DEPARTMENT OF HEALTH AND HUMAN SERVICES
National Institute of Nursing Research (NINR)
FY 2010 Budget Table of Contents FY 2010 Budget
Organization chart
Appropriation language
Amounts available for obligation
Budget mechanism table
Budget authority by activity
Major changes in budget request
Summary of changes
Budget graphs
Justification narrative
Budget authority by object
Salaries and expenses
Authorizing legislation
Appropriations history
Detail of full-time equivalent employment (FTE)
Detail of positions
New positions requested

NATIONAL INSTITUTES OF HEALTH
National Institute of Nursing Research
For carrying out section 301 and title IV of the Public Health Services Act with respect to nursing research, [$141,879,000], $143,749,000 (Department of Health and Human Services Appropriation Act, 2009)
National Institutes of Health
National Institute of Nursing Research
Amounts Available for Obligation 1/
| Source of Funding | FY 2008 Actual | FY 2009 Estimate | FY 2010 PB |
|---|---|---|---|
| Appropriation | $139,920,000 | $141,879,000 | $143,749,000 |
| Rescission | -2,444,000 | 0 | 0 |
| Supplemental | 731,000 | 0 | 0 |
| Subtotal, adjusted appropriation | 138,207,000 | 141,879,000 | 143,749,000 |
| Real transfer under Director's one-percent transfer authority (GEI) | -216,000 | 0 | 0 |
| Comparative transfer under Director's one-percent transfer authority (GEI) | 216,000 | 0 | 0 |
| Subtotal, adjusted budget authority | 138,207,000 | 141,879,000 | 143,749,000 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | -1,000 | 0 | |
| Subtotal, adjusted budget authority | 138,206,000 | 141,879,000 | 143,749,000 |
| Unobligated balance lapsing | 0 | 0 | 0 |
| Total obligations | 138,206,000 | 141,879,000 | 143,749,000 |
| 1/ Excludes the following amounts for reimbursable activities carried out by this account: FY 2008 -$450,000 FY 2009 -$450,000 FY 2010 -$450,000 | |||
NATIONAL INSTITUTES OF HEALTH
National Institute of Nursing Research
(Dollars in Thousands) Budget Mechanism -Total
| MECHANISM | FY 2008 Actual | FY 2009 Estimate | FY 2010 PB | Change | ||||
|---|---|---|---|---|---|---|---|---|
| Research Grants: | No. | Amount | No. | Amount | No. | Amount | No. | Amount |
| Research Projects: | ||||||||
| Noncompeting | 188 | $71,924 | 173 | $71,746 | 174 | $72,558 | 1 | $812 |
| Administrative supplements | (6) | 442 | (6) | 442 | (6) | 442 | (0) | 0 |
| Competing: | ||||||||
| Renewal | 8 | 3,568 | 9 | 2,905 | 9 | 3,021 | 0 | 116 |
| New | 69 | 21,060 | 73 | 24,096 | 74 | 24,843 | 1 | 747 |
| Supplements | 0 | 0 | 1 | 330 | 1 | 336 | 0 | 6 |
| Subtotal, competing | 77 | 24,628 | 83 | 27,331 | 84 | 28,200 | 1 | 869 |
| Subtotal, RPGs | 265 | 96,994 | 256 | 99,519 | 258 | 101,200 | 2 | 1,681 |
| SBIR/STTR | 17 | 3,267 | 17 | 3,345 | 17 | 3,389 | 0 | 44 |
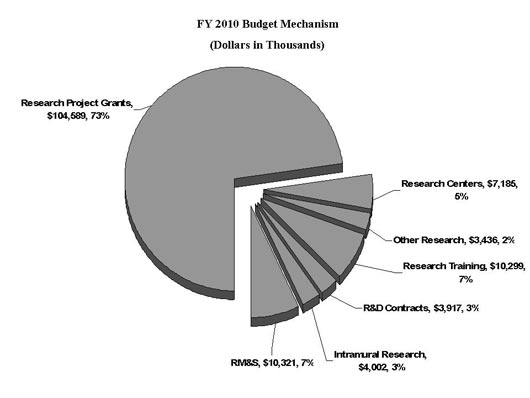
| Subtotal, RPGs | 282 | 100,261 | 273 | 102,864 | 275 | 104,589 | 2 | 1,725 |
| Research Centers: | ||||||||
| Specialized/comprehensive | 21 | 7,851 | 19 | 7,185 | 19 | 7,185 | 0 | 0 |
| Clinical research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biotechnology | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comparative medicine | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Research Centers in Minority Institutions | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subtotal, Centers | 21 | 7,851 | 19 | 7,185 | 19 | 7,185 | 0 | 0 |
| Other Research: | ||||||||
| Research careers | 32 | 3,145 | 33 | 3,236 | 33 | 3,236 | 0 | 0 |
| Cancer education | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cooperative clinical research | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Biomedical research support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Minority biomedical research support | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 0 | 397 | 0 | 300 | 0 | 200 | 0 | -100 |
| Subtotal, Other Research | 32 | 3,542 | 33 | 3,536 | 33 | 3,436 | 0 | -100 |
| Total Research Grants | 335 | 111,654 | 325 | 113,585 | 327 | 115,210 | 2 | 1,625 |
| Research Training: | FTTPs | FTTPs | FTTPs | |||||
| Individual awards | 77 | 2,446 | 94 | 3,025 | 93 | 3,025 | (1) | 0 |
| Institutional awards | 153 | 6,587 | 168 | 7,324 | 166 | 7,274 | (2) | -50 |
| Total, Training | 230 | 9,033 | 262 | 10,349 | 259 | 10,299 | (3) | -50 |
| Research & development contracts | 0 | 3,751 | 0 | 3,859 | 0 | 3,917 | 0 | 58 |
| (SBIR/STTR) | (0) | (9) | (0) | (9) | (0) | (9) | (0) | (0) |
| FTEs | FTEs | FTEs | FTEs | |||||
| Intramural research | 9 | 3,854 | 10 | 3,943 | 10 | 4,002 | 0 | 59 |
| Research management and support | 34 | 9,915 | 40 | 10,143 | 41 | 10,321 | 1 | 178 |
| Construction | 0 | 0 | 0 | 0 | ||||
| Buildings and Facilities | 0 | 0 | 0 | 0 | ||||
| Total, NINR | 43 | 138,207 | 50 | 141,879 | 51 | 143,749 | 1 | 1,870 |
| Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research | ||||||||
National Institutes of Health
National Institute of Nursing Research
BA by Program
(Dollars in thousands)
FY 2006 Actual | FY 2007 Actual | FY 2008 Estimate | FY 2008 Comparable | FY 2009 Estimate | FY 2010 PB | Change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extramural Research Detail | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount | FTEs | Amount |
| Self-Management, Symptom Management, and Caregiving | 47,137 | 43,548 | 42,847 | 42,921 | 44,079 | 44,642 | 563 | |||||||
| Health Promotion and Disease Prevention | 35,640 | 38,155 | 40,073 | 40,143 | 41,225 | 41,752 | 527 | |||||||
| Research Capacity Development | 23,622 | 20,348 | 19,432 | 19,466 | 19,990 | 20,246 | 258 | |||||||
| Technology Integration | 9,987 | 10,939 | 10,368 | 10,386 | 10,666 | 10,802 | 136 | |||||||
| End-of-Life | 9,361 | 11,490 | 11,502 | 11,522 | 11,833 | 11,984 | 151 | |||||||
| Subtotal, Extramural | 125,747 | 124,480 | 124,222 | 124,438 | 127,793 | 129,426 | 1,633 | |||||||
| Intramural research | 9 | 2,411 | 10 | 3,348 | 9 | 3,854 | 9 | 3,854 | 10 | 3,943 | 10 | 4,002 | 0 | 59 |
| Res. management & support | 34 | 9,089 | 30 | 3,360 | 34 | 9,914 | 34 | 9,915 | 40 | 10,143 | 41 | 10,321 | 1 | 178 |
| TOTAL | 43 | 137,247 | 40 | 131,188 | 43 | 137,990 | 43 | 138,207 | 50 | 141,879 | 51 | 143,749 | 1 | 1,870 |
| Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research | ||||||||||||||
Major Changes in the Fiscal Year 2010 Budget Request
Major changes by budget mechanism and/or budget activity detail are briefly described below. Note that there may be overlap between budget mechanism and activity detail and these highlights will not sum to the total change for the FY 2010 budget request for NINR, which is $1.870 million more than the FY 2009 estimate, for a total of $143.749 million.
Research Project Grants (+$1.725 million, total $104.589 million): NINR plans to support a total of 275 Research Project Grant (RPG) awards in FY 2010. Noncompeting RPGs will increase by 1 award and $812 thousand. Competing RPGs are expected to increase by 1 award and $869 thousand. The NIH Budget policy for RPGs in FY 2010 is to provide a 2% increase in noncompeting awards and a 2% increase in the average cost for competing RPGs.
NATIONAL INSTITUTES OF HEALTH
National Institute of Nursing Research
Summary of Changes
| FY 2009 estimate | 141,879,000 | ||||
| FY 2010 estimated budget authority | 143,749,000 | ||||
| Net change | 1,870,000 | ||||
| 2009 Current Estimate Base | Change from Base | ||||
| CHANGES | FTEs | Budget Authority | FTEs | Budget Authority | |
A. Built-in: 1. Intramural research: | |||||
| a. Annualization of January 2009 pay increase | $1,495,000 | $150,000 | |||
| b. January FY 2010 pay increase | 1,495,000 | 22,000 | |||
| c. Zero less days of pay | 1,495,000 | 0 | |||
| d. Payment for centrally furnished services | 621,000 | 12,000 | |||
| e. Increased cost of laboratory supplies, materials, and other expenses | 1,827,000 | 30,000 | |||
| Subtotal | 214,000 | ||||
2. Research management and support: | |||||
| a. Annualization of January 2009 pay increase | $5,200,000 | ($72,000) | |||
| b. January FY 2010 pay increase | 5,200,000 | 78,000 | |||
| c. Zero less days of pay | 5,200,000 | 0 | |||
| d. Payment for centrally furnished services | 880,000 | 18,000 | |||
| e. Increased cost of laboratory supplies, materials, and other expenses | 4,063,000 | 66,000 | |||
| Subtotal | 90,000 | ||||
| Subtotal, Built-in | 304,000 | ||||
| CHANGES | No. | Amount | No. | Amount | |
B. Program: 1. Research project grants: | |||||
| a. Noncompeting | 173 | $72,188,000 | 1 | $812,000 | |
| b. Competing | 83 | 27,331,000 | 1 | 869,000 | |
| c. SBIR/STTR | 17 | 3,345,000 | 0 | 44,000 | |
| Total | 273 | 102,864,000 | 2 | 1,725,000 | |
2. Research centers | 19 | 7,185,000 | 0 | 0 | |
3. Other research | 33 | 3,536,000 | 0 | (100,000) | |
4. Research training | 262 | 10,349,000 | (3) | (50,000) | |
5. Research and development contracts | 0 | 3,859,000 | 0 | 58,000 | |
| Subtotal, extramural | 1,633,000 | ||||
| FTEs | FTEs | ||||
| 6. Intramural research | 10 | 3,943,000 | 0 | (155,000) | |
| 7. Research management and support | 40 | 10,143,000 | 1 | 88,000 | |
| Subtotal, program | 141,879,000 | 1,566,000 | |||
| Total changes | 50 | 1 | 1,870,000 | ||
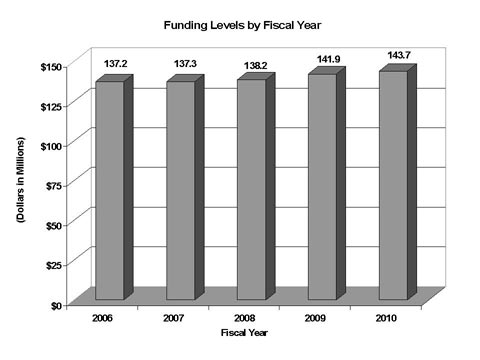
Fiscal Year 2010 Budget Graphs
History of Budget Authority and FTEs:
Distribution by Mechanism:
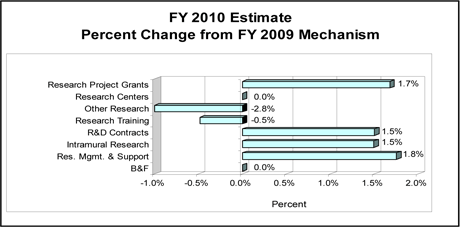
Change by Selected Mechanism:
Justification
National Institute of Nursing Research
| Authorizing Legislation: | Section 301 and title IV of the Public Health Service Act, as amended. | ||||
| Budget Authority: | |||||
| FY 2008 | FY 2009 | FY 2009 Recovery | FY 2010 President's | FY 2010 +/- 2009 |
BA | $138,207,000 | $141,879,000 | $35,877,000 | $143,749,000 | +$1,870,000 |
FTE | 43 | 50 | 0 | 51 | +1 |
This document provides justification for the Fiscal Year (FY) 2010 activities of the National Institute of Nursing Research (NINR), including HIV/AIDS activities. Details of the FY 2010 HIV/AIDS activities are in the "Office of AIDS Research (OAR)" Section of the Overview. Details on the Common Fund are located in the Overview, Volume One. Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
In FY 2009, a total of $35,877,000 American Recovery and Reinvestment Act (ARRA) funds were transferred from the Office of the Director. These funds will be used to support scientific research opportunities that help support the goals of the ARRA. The ARRA allows NIH to execute these funds via any NIH funding mechanism. Funds are available until September 30, 2010. These funds are not included in the FY 2009 Omnibus amounts reflected in this document.
DIRECTOR'S OVERVIEW
The National Institute of Nursing Research (NINR) supports clinical and basic research to build the scientific foundation for clinical practice, prevent disease and disability, manage and eliminate symptoms caused by illness, and enhance end-of-life and palliative care. The Institute's scientific focus spans multiple disciplines and unites the biological and behavioral sciences to better understand the complex interactions between the physiological factors of health and disease and the knowledge, beliefs, and behavior of the individual. Across all NINR scientific programs, research addresses the special needs of at-risk and underserved populations with an emphasis on health disparities.
The breadth and depth of NINR's research portfolio is ideally suited to explore some of the most important challenges affecting the health of the American people. These converging issues include:
- an aging population living longer with chronic diseases that require complex management by the patient, informal caregivers, and/or clinicians;
- the growth of diverse racial and cultural populations in the U.S. and the associated issue of health disparities;
- our ability to translate emerging patient management technologies into clinical practice and home-based use, and;
- the increased demand for nurses and other clinicians, both current and projected.
Confronting these issues requires shifting to a patient management paradigm that is increasingly person-centered rather than disease-oriented, that focuses on preventing the development of chronic illness rather than treating it, and that features the person as an active participant in his or her own health.
The science supported by NINR seeks to advance this new model. For example, NINR research activities address a changing reality in which individuals of all ages are increasingly living with multiple chronic illnesses such as heart disease, diabetes, and cancer. NINR supports research to improve quality of life for these individuals by finding more effective ways to address their adverse symptoms, by improving their ability to manage their own illnesses, and by developing approaches to ease the burden on their family caregivers. NINR scientists also design and test new technologies that allow clinicians to monitor chronic illnesses in individuals without ready access to health care facilities, and that allow these individuals to better manage their own health. Finally, an aging population inevitably increases the need for improved awareness of the multiple concerns surrounding end-of-life care. As the lead NIH Institute for end-of-life research, NINR supports studies that explore key end-of-life areas such as clinician/family member communication, decision-making, and issues of pediatric end-of-life and palliative care.
Ultimately, the best way to address the problems of chronic illnesses is to prevent the development of such illnesses in the first place. Therefore, NINR-supported scientists develop research-based health promotion and disease prevention programs, with a special focus on populations that experience health disparities. For example, NINR scientists have designed culturally sensitive interventions that have successfully reduced HIV risk behaviors in minority populations, and other interventions that have reduced risk behaviors for obesity and diabetes in rural populations. Overall, NINR research in disease prevention explores the connections among lifestyle, biology, behavior, environment, geography, socio-cultural contexts, technologies, and economic factors, and applies this in-depth knowledge to reducing society's future burdens of chronic illnesses.
NINR training opportunities seek to educate research scholars to become the nursing faculty of the future. This will have a direct impact on improving the ability of academic institutions to educate new nurses. Through NINR-supported efforts such as research training grants, the NIH Graduate Partnership Program, and hands-on research training programs on the NIH campus in association with the NINR Intramural Research Program, the Institute prepares future scientists for independent research careers.
NINR's future priorities and scientific objectives are reflected in its research programs and proposed research activities for FY 2010. Specific activities will include an effort to advance palliative care research for children experiencing serious illness or bereavement. In addition, NINR will continue to support research centers to enhance research capacity and advance health promotion, quality of life, and end-of-life research.
Broadly, NINR will continue to support innovative studies in research areas highlighted in its strategic plan, including: self-management, symptom management, and caregiving; health promotion and disease prevention; research capacity development; technology integration; and end-of-life science. Results from these studies will inform future strategies as NINR begins to consider its strategic plan beyond 2010. In addition, input from stakeholders, trans-NIH planning and priority setting processes such as the NIH Roadmap, Neuroscience Blueprint, and Pain Consortium, and changing public health concerns will continue to shape the future directions of NINR research.
NINR is committed to improving clinical practice through the generation of new knowledge and leaders in science. In strategically focusing its research on enhancing quality of life, improving patient self-management, and discovering better ways to prevent disease, NINR promotes the translation of scientific discoveries into general practice and ensures that current public health needs are proactively addressed.
Overall Budget Policy: Investigator-initiated research projects, supporting new investigators, research training, and career development continue to be the Institute's highest priorities. The NINR will follow the NIH Budget policy for RPGs in FY 2010, which is to provide a 2% inflationary increase in noncompeting awards and a 2% inflationary increase in the average cost for competing RPGs. NINR carefully evaluates investigator-initiated grant applications for all research programs. Scientific reviews are conducted, and the results are presented to the National Advisory Council for Nursing Research to determine the level of recommended support for the application, if any. The level of support provided for Institute-initiated projects is also evaluated. The Institute maintains a careful balance between solicitations issued to the extramural community in areas that need stimulation and funding made available to support investigator-initiated projects. Intramural Research and Research Management and Support each receive increases of 1.5% to help cover the cost of pay and other associated costs. NINR will continue to support new investigators and to maintain an adequate number of competing RPGs.
In addition, consistent with the President's multi-year commitment to double funding for cancer research, and with a government-wide initiative to enhance support for autism research, NINR plans to increase supportfor these two areas of research in FY 2010. Increased efforts in cancer and autism research will be accomplished across NINR's research programs, and include research projects consistent with NINR's mission and strategic plan.
FY 2010 JUSTIFICATION BY ACTIVITY DETAIL
Program Descriptions and Accomplishments
Self-Management, Symptom Management, and Caregiving
Improving the quality of life of individuals, both in clinical practice as well as the home setting, is a fundamental tenet of the NINR mission. NINR studies quality of life as a continuum composed of three key elements: self-management, symptom management, and caregiving. The NINR Self-Management, Symptom Management, and Caregiving program seeks to address the challenges of short- and long-term disease and disability management by enhancing the individual's role in managing disease, relieving symptoms, and improving health outcomes for individuals and caregivers.Current research projects supported by this program explore: interventions that reduce symptom burden; interventions to improve support for caregivers of patients with chronic disease; and techniques to promote healthy behaviors that improve the ability of patients to be partners in managing their own care. Among many other recent program activities in this area, NINR is sponsoring an initiative under the auspices of the NIH Pain Consortium to explore mechanisms, measurement, and management of acute and chronic pain. Pain research sought under this initiative spans all areas of science, from research on the basic neural pathways of pain, to pain-related behavioral and social sciences research.
Budget Policy: The FY 2010 budget estimate for this program is $44.642 million, an increase of $563 thousand or 1.3 percent above the FY 2009 Estimate. NINR plans in FY 2010 to continue to address the many challenges and opportunities that exist in the areas of self-management, symptom management, and caregiving as an important part of a strategically balanced research portfolio.
Health Promotion and Disease Prevention
The NINR Health Promotion and Disease Prevention program studies the key biological, behavioral, and social factors that prevent the development of disease and achieve long-term, positive health outcomes in individuals of all ages. Research supported under this activity seeks scientific discoveries of health predictors and prevention strategies across conditions, diseases, and settings. Under this wide scope of research, efforts can range from promoting behavioral changes in individuals, to evaluating health risks in diverse communities, to assessing issues of patient safety.
In FY 2008, NINR solicited proposals for Centers in Health Promotion/Disease Prevention or Symptom Management Research. These Centers will support shared resources and highly collaborative, interdisciplinary research environments for groups of investigators pursuing scientific inquiries into innovative, effective methods for promoting health and preventing disease and disability. The first awards under this program are expected to be made in late FY 2009.
Budget Policy: The FY 2010 budget estimate for this program is $41.752 million, an increase of $527 thousand or 1.3 percent above the FY 2009 estimate. NINR plans in FY 2010 to continue to address the many challenges and opportunities that exist in the areas of health promotion and disease prevention as an important part of a strategically balanced research portfolio.
Research Capacity Development
The NINR Research Capacity Development program builds research capacity through centers programs and fosters interdisciplinary training for the next generation of scientists in basic, translational, and clinical research by means of individual and institutional training awards. Through these activities, NINR addresses the national shortage of nurses by contributing to the development of the nursing faculty needed to teach and mentor individuals entering the field. Among these opportunities, NINR provides support for trainees from underrepresented and disadvantaged backgrounds.
In FY 2008, NINR made its first awards of program project grants under a new initiative entitled, "Nursing Science Research on Interventions in Chronic Illness." These awards, intended for investigators and institutions with proven and long-established research programs and funded through the P01 grant mechanism, support shared resources and a collaborative research effort for several large research projects. The awards allow a group of investigators, using multiple approaches funded as individual subprojects, to conduct innovative, high-impact, and interdisciplinary research on topics of strategic importance to the Institute.
Budget Policy: The FY 2010 budget estimate for this program is $20.246 million, an increase of $256 thousand or 1.3 percent above the FY 2009 estimate. The proposed level of funding will allow NINR to cover its current commitments, including NINR's Centers of Excellence in Symptom Management or Health Promotion/Disease Prevention Research. This level would also allow new individual training grants to be awarded in FY 2010, which will directly impact the ability of schools of nursing to educate new nurses. NINR plans in FY 2010 to continue its commitment to developing the next generation of innovative investigators and enhance overall research capacity in strategically important areas of research as part of an overall balanced program portfolio.
Technology Integration
The NINR Technology Integration program supports innovative, interdisciplinary studies to develop new and adapt existing technologies to improve clinical care and quality of life. For example, research conducted under this program seeks to improve quality of life by developing technologies that assist patients in monitoring and reporting indicators of health status, such as breathing status, blood pressure, and blood glucose levels.
Current activities under this program include: developing devices to facilitate clinical decision support for care providers in hospitals and clinics, creating software modules that assist patients with making decisions on advanced care planning, and developing a speech translation device for improving patient-provider communications. Investigators are also designing a system that will allow chemotherapy patients to report their symptoms over the internet or phone to nurses in a clinic. This type of technology allows for better management of patient symptoms at home, and eliminates unnecessary trips to the clinic, which is especially useful for patients in rural locations without ready access to health care services.
Budget Policy: The FY 2010 budget estimate for this program is $10.802 million, an increase of $136 thousand or 1.3 percent above the FY 2009 estimate. In FY 2010, NINR plans to continue supporting investigators who are innovative in their use and development of novel technologies that address current and future clinical care and patient management needs, and who will work to incorporate these technologies into standard practice. This level of funding will allow NINR to cover current commitments and fund additional awards in this emerging area of research as part of a strategically balanced program portfolio.
End-of-Life
End-of-life science seeks to understand dying with respect to the needs of dying persons and formal and informal caregivers. It includes research on issues such as: alleviation of symptoms; psychological care; near-death preferences; advance directives; and family decision-making. Likewise, end-of-life research addresses the cultural, spiritual, age- and disease-specific factors that make each person's experience at the end of life unique. The NINR End-of-Life research program applies biological, behavioral, and social science strategies to advance the understanding of the dynamic interactions of these various factors, and to develop interventions that optimize patient and caregiver quality of life across care settings and cultural contexts.
NINR currently supports Centers in Self-Management or End-of-Life Research. These Centers serve as a nexus for the emergence of end-of-life research as an interdisciplinary science, training investigators from multiple backgrounds and enhancing collaboration to increase the quality and quantity of innovative, interventional research projects in end-of-life and palliative care science.
Portrait of a Program: End-of-Life Research
FY 2009 Level: $11.833 million
FY 2010 Level: $11.984 million
Change: $ 0.151 million
The life expectancy of the American people has reached a historic high, but along with increased life expectancy comes an increase in the number of people living with, and dying from, chronic debilitating diseases. Prolonged courses of decline at the end of life, palliative treatment options, and life-sustaining technologies have raised many important research questions within the last decade. In addition, the needs of dying children and their families are coming into greater focus.1 Although fewer pediatric patients die, death in childhood stands out as a particular tragedy and a unique end-of-life experience for all involved. In 1997, the Director of NIH designated NINR as the lead NIH Institute for end-of-life research, providing an important opportunity for NINR-supported science to set strategic priorities in this crucial area of science. Consistent with this role, NINR has sponsored numerous research initiatives related to end-of-life science, and currently supports Centers of Excellence in End-of-Life or Self-Management Research. NINR recently co-sponsored an initiative to develop and test interventions to enhance end-of-life and palliative care that providers can implement across multiple settings, illnesses, and cultural contexts. In FY 2010, NINR will support a new effort under its end-of-life program to conduct pediatric palliative care research. This research will seek to improve quality of life for children who are facing a life-threatening illness, and to intervene in helping children cope while a parent or sibling faces a terminal illness.
1 Institute of Medicine (2002). When children die: Improving palliative and end-of-life care for children and their families. Washington, DC: The National AcademiesPress.
Budget Policy: The FY 2010 budget estimate for this program is $11.984 million, an increase of $151 thousand or 1.2 percent above the FY 2009 estimate. Given the enormous potential and great need for improving the quality of life of dying patients and their caregivers, NINR plans to expand end-of-life research efforts in FY 2010 to build upon continuing accomplishments in this program area. This level of funding will allow NINR to support existing commitments, including the Institute's Centers of Excellence in End-of-Life Research, and to fund additional awards in this critical area of research, as part of an overall balanced program portfolio. Also in FY 2010, NINR will support a new effort in end-of-life research that will seek to advance palliative care research for children experiencing serious illness or bereavement.
Intramural Research Program
NINR's Intramural Research Program (IRP) supports research to understand the underlying biological mechanisms of a range of symptoms, their effect on patients, and how patients respond to interventions. Recent scientific efforts have included evaluating the efficacy of novel interventions for managing symptoms associated with cancer treatment, and exploring the molecular and genetic mechanisms that influence an individual's response to analgesic treatment for acute pain. The IRP also supports research training opportunities through programs such as the NINR Career Transition Award, the NINR Summer Genetics Institute, and by participating in the NIH Graduate Partnerships Program.
Portrait of a Program: Understanding the Biological Mechanisms of Pain
FY 2009 Level: $3.943 million
FY 2010 Level: $4.002 million
Change: $ 0.059 million
Millions of Americans suffer from pain. A national survey revealed that twenty-six percent of adults experienced pain that lasted throughout the day in the month prior, and of those adults, forty-two percent experienced the pain a year or more.2 Some people develop pain as a consequence of a serious illness, such as those who experience pain related to chemotherapy treatments for cancer, while for others, chronic pain itself is a disease. Understanding the biologic and genetic mechanisms of pain is vital for developing effective treatments for managing pain and improving quality of life. Researchers in the NINR laboratories in Bethesda, Maryland are using new genetic and proteomic tools to better determine an individual's response to pain and treatments for pain. These researchers are integrating comparative studies of variations in genetics, protein biomarkers, and personal characteristics to understand individual variations in response to analgesic treatments for chronic pain conditions. Identifying these variations will allow for targeted use of gene chips to better characterize molecular-genetic mechanisms of pain and analgesia, suggest new applications, and test the efficacy and adverse reactions of newly developed or currently used drugs. Studies such as these that attempt to understand the interrelationship among an individual's personal and genetic makeup and their response to pain move us ever closer to realizing a future of personalized medicine. In FY 2010, NINR will continue to support this vital program of research and build upon the promising advances that have emanated from these scientists in recent years.
2 National Center for Health Statistics, Health, United States, 2006, With Chart book on Trends in the Health of Americans, Hyattsville, MD: 2006; p 68-70.
In 2008, the IRP sponsored the ninth annual NINR Summer Genetics Institute (SGI). The SGI provides students with a foundation in molecular genetics for use in research and clinical practice to develop and expand research capacity among graduate students and faculty in nursing. This program is approved for graduate credit in nursing at the doctoral level. Through this program, NINR prepares nurses to address important scientific questions, such as those arising from the Human Genome Project and other emerging genetic discoveries.
Budget Policy: The FY 2010 budget estimate for this program is $4.002 million, an increase of $59 thousand or 1.5 percent above the FY 2009 estimate. In FY 2010, this program will continue to support innovative research to address the scientific challenges of understanding and managing adverse symptoms such as acute and chronic pain. This program will also continue to support important training and career development opportunities for innovative investigators.
Research Management and Support
NINR Research Management and Support (RMS) activities provide administrative, budgetary, logistical, and scientific support in the review, award, and monitoring of research grants, training awards and research and development contracts. The functions of RMS also encompass strategic planning, coordination, and evaluation of the Institute's programs and liaison with other Federal agencies, Congress, and the public.
Budget Policy: The FY 2010 budget estimate for RMS is $10.321 million, an increase of $178 thousand or 1.8 percent above the FY 2009 estimate. In FY 2010, NINR plans to continue addressing the challenges and opportunities that exist in strategically managing a research portfolio that addresses areas of science critical to public health.
NATIONAL INSTITUTES OF HEALTH
National Institute of Nursing Research
Budget Authority by Object
| FY 2009 Estimate | FY 2010 PB | Increase or Decrease | Percent Change | |
|---|---|---|---|---|
| Total compensable workyears: | ||||
| Full-time employment | 50 | 51 | 1 | 2.0 |
| Full-time equivalent of overtime and holiday hours | 0 | 0 | 0 | 0.0 |
| Average ES salary | $0 | $0 | $0 | 0.0 |
| Average GM/GS grade | 12.2 | 12.2 | 0.0 | 0.0 |
Average GM/GS salary | $89,184 | $90,789 | $1,605 | 1.8 |
Average salary, grade established by act of July 1, 1944 (42 U.S.C. 207) | $106,666 | $109,972 | $3,306 | 3.1 |
| Average salary of ungraded positions | 69,252 | 77,147 | 7,895 | 11.4 |
| OBJECT CLASSES | FY 2009 Estimate | FY 2010 PB | Increase or Decrease | Percent Change |
| Personal Compensation: | ||||
| 11.1 Full-time permanent | $3,623,000 | $3,737,000 | $114,000 | 3.1 |
11.3 Other than full-time permanent | 1,026,000 | 1,090,000 | 64,000 | 6.2 |
11.5 Other personnel compensation | 146,000 | 151,000 | 5,000 | 3.4 |
| 11.7 Military personnel | 246,000 | 274,000 | 28,000 | 11.4 |
11.8 Special personnel services payments | 189,000 | 211,000 | 22,000 | 11.6 |
| Total, Personnel Compensation | 5,230,000 | 5,463,000 | 233,000 | 4.5 |
| 12.0 Personnel benefits | 1,348,000 | 1,410,000 | 62,000 | 4.6 |
| 12.2 Military personnel benefits | 117,000 | 130,000 | 13,000 | 11.1 |
| 13.0 Benefits for former personnel | 0 | 0 | 0 | 0.0 |
| Subtotal, Pay Costs | 6,695,000 | 7,003,000 | 308,000 | 4.6 |
| 21.0 Travel and transportation of persons | 171,000 | 169,000 | (2,000) | -1.2 |
| 22.0 Transportation of things | 3,000 | 3,000 | 0 | 0.0 |
23.1 Rental payments to GSA | 0 | 0 | 0 | 0.0 |
23.2 Rental payments to others | 89,000 | 83,000 | (6,000) | -6.7 |
23.3 Communications, utilities and miscellaneous charges | 61,000 | 61,000 | 0 | 0.0 |
24.0 Printing and reproduction | 21,000 | 21,000 | 0 | 0.0 |
25.1 Consulting services | 49,000 | 49,000 | 0 | 0.0 |
25.2 Other services | 1,147,000 | 1,124,000 | (23,000) | -2.0 |
25.3 Purchase of goods and services from government accounts | 8,594,000 | 8,596,000 | 2,000 | 0.0 |
| 25.4 Operation and maintenance of facilities | 305,000 | 289,000 | (16,000) | -5.2 |
25.5 Research and development contracts | 211,000 | 266,000 | 55,000 | 26.1 |
25.6 Medical care | 0 | 0 | 0 | 0.0 |
25.7 Operation and maintenance of equipment | 39,000 | 38,000 | (1,000) | -2.6 |
| 25.8 Subsistence and support of persons | 0 | 0 | 0 | 0.0 |
| 25.0 Subtotal, Other Contractual Services | 10,345,000 | 10,362,000 | 17,000 | 0.2 |
| 26.0 Supplies and materials | 267,000 | 250,000 | (17,000) | -6.4 |
31.0 Equipment | 293,000 | 288,000 | (5,000) | -1.7 |
32.0 Land and structures | 0 | 0 | 0 | 0.0 |
33.0 Investments and loans | 0 | 0 | 0 | 0.0 |
41.0 Grants, subsidies and contributions | 123,934,000 | 125,509,000 | 1,575,000 | 1.3 |
| 42.0 Insurance claims and indemnities | 0 | 0 | 0 | 0.0 |
| 43.0 Interest and dividends | 0 | 0 | 0 | 0.0 |
| 44.0 Refunds | 0 | 0 | 0 | 0.0 |
| Subtotal, Non-Pay Costs | 135,184,000 | 136,746,000 | 1,562,000 | 1.2 |
| Total Budget Authority by Object | 141,879,000 | 143,749,000 | 1,870,000 | 1.3 |
| Includes FTEs which are reimbursed from the NIH Roadmap for Medical Researc | ||||
NATIONAL INSTITUTES OF HEALTH
National Institute of Nursing Research
Salaries and Expenses
| OBJECT CLASSES | FY 2009 Estimate | FY 2010 PB | Increase or Decrease | Percent Change |
|---|---|---|---|---|
| Personal Compensation: | ||||
| Full-time permanent (11.1) | $3,623,000 | $3,737,000 | $114,000 | 3.1 |
| Other than full-time permanent (11.3) | 1,026,000 | 1,090,000 | 64,000 | 6.2 |
| Other personnel compensation (11.5) | 146,000 | 151,000 | 5,000 | 3.4 |
| Military personnel (11.7) | 246,000 | 274,000 | 28,000 | 11.4 |
| Special personnel services payments (11.8) | 189,000 | 211,000 | 22,000 | 11.6 |
| Total Personnel Compensation (11.9) | 5,230,000 | 5,463,000 | 233,000 | 4.5 |
| Civilian personnel benefits (12.1) | 1,348,000 | 1,410,000 | 62,000 | 4.6 |
| Military personnel benefits (12.2) | 117,000 | 130,000 | 13,000 | 11.1 |
| Benefits to former personnel (13.0) | 0 | 0 | 0 | 0.0 |
| Subtotal, Pay Costs | 6,695,000 | 7,003,000 | 308,000 | 4.6 |
| Travel (21.0) | 171,000 | 169,000 | (2,000) | -1.2 |
| Transportation of things (22.0) | 3,000 | 3,000 | 0 | 0.0 |
| Rental payments to others (23.2) | 89,000 | 83,000 | (6,000) | -6.7 |
| Communications, utilities and miscellaneous charges (23.3) | 61,000 | 61,000 | 0 | 0.0 |
| Printing and reproduction (24.0) | 21,000 | 21,000 | 0 | 0.0 |
| Other Contractual Services: | ||||
| Advisory and assistance services (25.1) | 49,000 | 49,000 | 0 | 0.0 |
| Other services (25.2) | 1,147,000 | 1,124,000 | (23,000) | -2.0 |
| Purchases from government accounts (25.3) | 4,983,000 | 4,982,000 | (1,000) | 0.0 |
| Operation and maintenance of facilities (25.4) | 305,000 | 289,000 | (16,000) | -5.2 |
| Operation and maintenance of equipment (25.7) | 39,000 | 38,000 | (1,000) | -2.6 |
| Subsistence and support of persons (25.8) | 0 | 0 | 0 | 0.0 |
| Subtotal Other Contractual Services | 6,523,000 | 6,482,000 | (41,000) | -0.6 |
| Supplies and materials (26.0) | 267,000 | 250,000 | (17,000) | -6.4 |
| Subtotal, Non-Pay Costs | 7,135,000 | 7,069,000 | (66,000) | -0.9 |
| Total, Administrative Costs | 13,830,000 | 14,072,000 | 242,000 | 1.7 |
NATIONALINSTITUTES OF HEALTH
National Institute of Nursing Research
Authorizing Legislation
| PHS Act /Other Citation | U.S. Code Citation | 2009 Amount Authorized | FY 2009 Estimate | 2010 Amount Authorized | FY2010 PB | |
|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite | $141,879,000 | Indefinite | $143,749,000 |
| National Institute of Nursing Research | Section 402(a) | 42§281 | Indefinite | Indefinite | ||
| Total, Budget Authority | 141,879,000 | $143,749,000 |
NATIONAL INSTITUTES OF HEALTH
National Institute of Nursing Research
Appropriations History
| Fiscal Year | Budget Estimate to Congress | House Allowance | Senate Allowance | Appropriation 1/ |
|---|---|---|---|---|
| 2001 | 84,714,000 2/ | 102,312,000 | 106,848,000 | 104,370,000 |
| Rescission | (20,000) | |||
| 2002 | 117,686,000 2/ | 116,773,000 | 125,659,000 | 120,451,000 |
| Rescission | (23,000) | |||
| 2003 | 129,768,000 | 131,438,000 | 131,438,000 | 131,438,000 |
| Rescission | (854,000) | |||
| 2004 | 134,579,000 | 134,579,000 | 135,579,000 | 135,555,000 |
| Rescission | (831,000) | |||
| 2005 | 139,198,000 | 139,198,000 | 140,200,000 | 138,198,000 |
| Rescission | (1,126,000) | |||
| 2006 | 138,729,000 | 138,729,000 | 142,549,000 | 138,729,000 |
| Rescission | (1,387,000) | |||
| 2007 | 137,342,000 | 136,550,000 | 137,848,000 | 137,404,000 |
| 2008 | 137,800,000 | 139,527,000 | 140,456,000 | 139,920,000 |
| Rescission | (2,244,000) | |||
| Supplemental | 731,000 | |||
| 2009 | 137,609,000 | 142,336,000 | 141,439,000 | 141,879,000 |
| 2010 | 143,749,000 | |||
| 1/ Reflects enacted supplementals, rescissions, and reappropriations. 2/ Excludes funds for HIV/AIDS research activities consolidated in the NIH Office of AIDS Research. | ||||
NATIONAL INSTITUTES OF HEALTH
National Institute of Nursing Research
Details of Full-Time Equivalent Employment (FTEs)
| OFFICE/DIVISION | FY 2008 Actual | FY 2009 Estimate | FY 2010 PB |
|---|---|---|---|
| Office of the Director | 4 | 4 | 4 |
| Office of the Administrative Management | 10 | 13 | 13 |
| Division of Intramural Research | 10 | 10 | 10 |
| Office Associate Director of Scientific Programs | 19 | 23 | 24 |
| Total | 43 | 50 | 51 |
| Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research | |||
| FTEs supported by funds from Cooperative Research and Development Agreements | (0) | (0) | (0) |
| FISCAL YEAR | Average GM/GS Grade | ||
| 2006 | 11.9 | ||
| 2007 | 12.2 | ||
| 2008 | 12.0 | ||
| 2009 | 12.2 | ||
| 2010 | 12.2 | ||
NATIONAL INSTITUTES OF HEALTH
National Institute of Nursing Research
Detail of Positions
| GRADE | FY 2008 Actual | FY 2009 Estimate | FY 2010 PB |
|---|---|---|---|
| Total, ES Positions | 0 | 0 | 0 |
| Total, ES Salary | 0 | 0 | 0 |
| GM/GS-15 | 4 | 5 | 5 |
| GM/GS-14 | 8 | 10 | 10 |
| GM/GS-13 | 14 | 15 | 16 |
| GM/GS-12 | 5 | 7 | 7 |
| GM/GS-11 | 3 | 4 | 4 |
| GM/GS-10 | 1 | 1 | 1 |
| GM/GS-9 | 5 | 5 | 5 |
| GM/GS-8 | 0 | 0 | 0 |
| GM/GS-7 | 1 | 1 | 1 |
| GM/GS-6 | 1 | 1 | 1 |
| GM/GS-5 | 0 | 0 | 0 |
| GM/GS-4 | 1 | 1 | 1 |
| GM/GS-3 | 0 | 0 | 0 |
| GM/GS-2 | 0 | 0 | 0 |
| GM/GS-1 | 0 | 0 | 0 |
| Subtotal | 43 | 50 | 51 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207): | |||
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 1 | 1 | 1 |
| Senior Grade | 0 | 0 | 0 |
| Full Grade | 0 | 0 | 0 |
| Senior Assistant Grade | 2 | 2 | 2 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 3 | 3 | 3 |
| Ungraded | 19 | 19 | 19 |
| Total permanent positions | 42 | 42 | 42 |
| Total positions, end of year | 62 | 62 | 62 |
| Total full-time equivalent (FTE) employment, end of year | 43 | 50 | 51 |
| Average ES salary | 0 | 0 | 0 |
| Average GM/GS grade | 12.0 | 12.2 | 12.2 |
| Average GM/GS salary | 86,840 | 89,184 | 90,789 |
| Includes FTEs which are reimbursed from the NIH Roadmap for Medical Research. | |||
NATIONAL INSTITUTES OF HEALTH
National Institute of Nursing Research
New Positions Requested
FY 2010 | |||
|---|---|---|---|
| Grade | Number | Annual Salary | |
| Senior Research Specialist | 13 | 1 | $106,000 |
| Total Requested | 1 | ||