Converting Brain Signals into Action
At the University of Pittsburgh, preliminary studies with animals have enabled a research team to explore a new device that may one day help paralyzed people move again.
Today, 8 million Americans are living with paralysis or have lost limbs. Many could benefit from technologies that would help them carry out daily activities, but high-tech prosthetics or other such devices are not always sufficient to meet these needs, particularly for those who are paralyzed. To improve the quality of life for these individuals, NCATS-supported investigators at Pitt are exploring two different computerized chips that convert brain signals into an action simply through the patient's thinking about the action.
"The problem has always been the control of the prosthetic by someone with limited ability to move," said Michael Boninger, professor and chair of the Department of Physical Medicine and Rehabilitation at the Pitt School of Medicine. Boninger and Andrew Schwartz, a professor of neurobiology at the medical school, have built a collaborative research program to develop brain-computer interface devices that interpret the brain's still-intact command abilities and convey them to high-tech prosthetics and assistive devices.
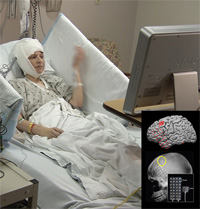
A team of NCATS-supported researchers at the University of Pittsburgh developed a micro-electrocorticography grid that may help paralyzed individuals move again. The device, which is implanted in the brain's movement-controlling motor cortex (see image inset), helps this study participant practice simple computer tasks using only her mind. A computer system interprets her brain's electrical impulses captured by the device then converts the signals into movement controls in virtual environments. (University of Pittsburgh School of Medicine Photo)
"There is a really collaborative group of researchers at Pitt," said Boninger. "It's a critical mass supported by the Clinical and Translational Science Institute." The university's CTSI is one of about 60 research institutions supported by NCATS' Clinical and Translational Science Awards (CTSA) program, which aims to move scientific innovations into clinical practice. Currently, the research team is working in parallel on two devices with unique features. One of these, the micro-electrocorticography (ECoG) electrode grid, is placed beneath the skull and on the surface of the brain's movement-controlling motor cortex. A computer system interprets the electrical impulses in the brain captured by the micro-ECoG technology and then converts the signals into movement controls in virtual environments. The group at Pitt developed the device as a smaller, less invasive and higher-resolution version of an ECoG grid that is used to monitor intractable epileptic seizures prior to surgery.
"We wanted to accelerate the translation of this into clinical work, starting with what was available and approved for clinical use," recalled team member and biomedical engineer Wei Wang, who is an assistant professor in Boninger's department and a co-principal investigator of the CTSI-funded pilot research.
"Our first phone call was to the CTSI," said Boninger. Before each phase of research into their micro-ECoG grid, the collaborators met with the regulatory experts at the CTSI for guidance on the requirements for human research. Wang added, "I think that without their help, it would have been a much tougher route to take, and may not have happened."
In addition to specialized expertise, Boninger's research team received two grants from the CTSI to facilitate the project. First, a Translational Tool Pilot Project award to co-principal investigators Wang and Elizabeth Tyler-Kabara, assistant professor of neurosurgery and bioengineering at Pitt, made it possible for the team to map out the brain signals corresponding to specific hand movements. The team then worked with patients who were undergoing epilepsy monitoring for a week and were willing to have the experimental micro-ECoG grid implanted alongside their clinical ECoG grid. Using only their minds via the brain interface device, the volunteers practiced computer-screen tasks and a video game.
Building upon this work, Wang received a training grant from the CTSI's Clinical Research Scholars Program. This grant enabled continued research funding and formal mentorship support from Boninger and Schwartz as well as educational opportunities in various aspects of clinical and translational research. Leveraging funding from NIH's National Institute for Neurological Disorders and Stroke, Schwartz and Pitt bioengineer Jennifer Collinger are working with Wang to test the micro-ECoG technology with people who have tetraplegia, or paralysis in all four limbs. In this study, volunteers will have more time to master use of the implanted brain interface device, spending 25 hours per week for nearly a month testing their control of computer cursors and assistive devices.
The group also is developing a second brain-interface technology, an intracortical microarray. This device enables the user to control movement with thoughts, but with a higher resolution and potentially greater control than the micro-ECoG because the tiny chip's 100 miniscule, spike-like electrode probes descend into the surface of the motor cortex. The probes read the signals coming from individual neurons. Because the arrays are embedded into the brain, this interface device could cause more scar tissue than the micro-ECoG. With support from the Defense Advanced Research Projects Agency, Schwartz is leading research using the array in animals paired with a dexterous prosthetic arm engineered at Johns Hopkins University.
With coaching from CTSI and other university experts, the team received approval from the U.S. Food and Drug Administration to test the intracortical array in humans. Now supported by the U.S. Department of Defense, Schwartz is continuing his animal model work, and Boninger is leading its testing in volunteers with tetraplegia.
"Our work is a great example of translational research that accomplishes things that we're not going to get from a pill," Boninger said. "Support of bioengineering research is an absolutely critical part of the CTSI program."
Posted March 2012
Social Media Links