August 2012
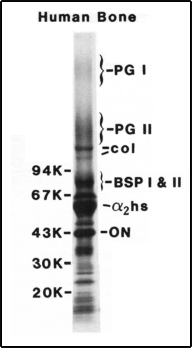 Original gel showing
Original gel showing
PGI and PGII
John Termine finally got the word in 1983. He had been selected to serve as chief of the brand-new Bone Research Branch in the National Institute of Dental Research’s intramural program. Not only would his branch function as the lone laboratory and hub for bone research on the NIH campus in Bethesda, Maryland, Termine proposed to pull out all of the existing scientific stops to take the field further down a road less traveled. Small proteins.
Termine proposed to extract all of the small proteins trapped, like insects fossilized in amber, when the bone matrix mineralizes and hardens during development. For the traditional physical chemists who had dominated bone research since the halcyon days of the light microscope, his was a fool’s errand. The microscope didn’t lie. Bone matrix was an inorganic mesh of rod-like mineral crystals threaded in and around the lattice-like protein collagen frozen in a calcified state. The other proteins, comprising only about 10 percent of the organic content, lingered there in various states of degradation. How could they be relevant?
But for Termine, discovering the assumed dozen or so distinct types of non-collagenous proteins in bone was like digging for biochemical gold. The reason hinged on a fundamental question. Why does the predominant collagen mineralize in bone but not in connective tissues such as skin and tendon? Termine noticed nothing special about the collagen in bone. That left the non-collagenous proteins and the assumption that, like yeast to dough, they must be essential to give rise to the mineralization process.
“At the time, it was the next phase,” Termine recalled recently, referring to his decision to catalogue the proteins, working back to the then-unmapped genome to locate the encoding genes and fully characterize their functions.
Termine, a physical chemist by training, recruited help from other biological disciplines to get his project up and running. “What I was looking for at the time was somebody with experience in connective tissue protein chemistry,” he said of assembling staff to start the lab. “That was Larry Fisher. I wanted a cell biologist who had experience with connective tissue and could easily adapt to bone. That was Pam Robey. Then we needed a molecular biologist who was experienced in connective tissue. That was Marian Young. All were highly regarded, top-choice people.”
 Dr. Larry Fisher
Dr. Larry Fisher
Fisher, the protein chemist formerly at Case Western Reserve in Cleveland, headed the branch’s matrix biochemistry group. His initial task was to take developing subperiosteal bone from various species, partially dissolve the mineral, and tease out the various proteins hidden within for further characterization.
One of the presumed hidden proteins was bone proteoglycan. Proteoglycans, which are found in most tissues throughout the body, consist of a core protein that is linked to one or more distinctive carbohydrate chains called a glycosaminoglycan. Think of a dragonfly (the core) with a single wing or a varied number of paired sets of wings (the chains).
In the early 1980s, researchers referred to bone proteoglycan in the singular. But Fisher and colleagues soon pulled out one and then a fragment of another proteoglycan from the bone matrix. They were “structurally, chemically, and immunologically distinct,” the researchers reported, later earning the generic designations small proteoglycan (PG) I and II to differentiate them.
But in 1989, Fisher and Marian Young deduced the amino-acid sequences of each, allowing for a more descriptive renaming. For the single-chained PG II, the name decorin already had begun to make the rounds. The idea being, PG-II seemed to decorate the surface of the collagen fibrils, helping to determine their diameter.
That left LG I. “Larry said maybe we should call it biglycanin,” recalled Young, the hybrid name referring to its two distinctive carbohydrate chains. “I said, ‘I can’t even pronounce that word.’ So Larry said, ‘How about if we shorten it to biglycan?’”
The following year, the plot thickened. While characterizing the proteins, Fisher, Young, and colleagues discovered decorin indeed seemed to be a full-time matrix component. Biglycan wasn’t. It appeared to localize on the surface of cells or within the fluid-filled gaps between them.
What biglycan was doing there, not hooked in with decorin in the matrix, they hadn’t a clue.
****************
 Dr. Marian Young and
Dr. Marian Young and
Dr. Agnes Berendsen
Three decades later Marian Young, still running her own lab at the National Institute of Dental and Craniofacial Research (NIDCR) had published more than 30 journal articles on biglycan. She and her colleagues around the world still had yet to solve the basic question of what biglycan did around the cell surface. But Young now had a hunch – and a new post doctoral student in her lab to test it.
The post doc was Agnes Berendsen. She had spent the past four years in Amsterdam immersing herself in cell biology and a project to learn the ins and outs of engineering a replacement periodontal ligament. Four first-author papers later, Berendsen wanted to hone her skills in molecular biology, too. Her lab chief in The Netherlands contacted Young, a research acquaintance, about Berendsen doing a fellowship in her lab. The paperwork was completed, and Berendsen soon found herself sitting across the table from Young and processing an area of science as unfamiliar to her as navigating Washington’s color-coded subway system.
Young laid out her philosophy of taking the scientific road less traveled. She said while many researchers focus inside bone-forming cells called osteoblasts to connect “the balls and chains,” i.e., the diagrammatic dots and lines that depict the behavior-changing inputs and outputs of a signaling pathway, she was interested in the activity on the surface of the bone matrix. That’s where most signals originated and diffused, like fish through coral, to influence the behavior of the osteoblasts and their bone-resorbing cousins the osteoclasts.
That brought Young to biglycan. The Young lab and others had found that the proteoglycan seems to help the formation of new bone during fracture healing. Her lab had data from cell culture studies that biglycan modulates the signals of established growth factors in bone (TGF-β and two BMPs), providing a possible rationale for the proteoglycan’s positive effects on fracture healing. But this work was just a start. A more-detailed mechanistic understanding was essential to develop a rational approach to exploit the lead.
Enter Young’s hunch. “I’m of the opinion that proteins aren’t limited to carrying out a single function,” said Young. “They probably do a number of things. We knew that the canonical Wnt pathway was extremely important in bone. We also knew biglycan was around the cell surface in abundance. So we just guessed that there might be a connection.”
Berendsen recognized the term canonical Wnt pathway. It was the common evolutionary thread for all metazoans, from humans all the way down to the ocean sponges, to have the ability to pattern their three-dimensional body plans during embryogenesis. The pathway was biologically profound, painfully complicated, and still poorly characterized.
She also knew that the thread had been extended well beyond development. Through the 1990s and into the 2000s, the canonical Wnt pathway kept turning up in the scientific literature as helping to maintain various already-developed body systems, from the brain to the lungs.
In 2001, bone joined the list. A protein called LRP5 had been found to play a role in determining bone mass in people. Subsequent work showed LRP5, one of the many protein receptors displayed like satellite dishes on the cell surface, was wired into the canonical Wnt pathway. If LRP5 functioned normally, the pathway operated efficiently. If the protein faltered, so did the pathway. The similar finding was published a few years later involving the related LRP6 protein.
The finding had many thinking that such a major pathway must employ not only LRP5 and LRP6, but additional coreceptors and chemical factors nearby to further fine-tune its signal as needed in bone.
Young asked Berendsen to investigate, as step one in building a mechanistic understanding, whether biglycan indeed plugs into the canonical Wnt pathway on the cell surface. If correct – still a big if wrapped in a guess - future fellows in the Young lab would tackle the next incremental steps of delineating how the process works to help heal fractured bone.
“I realized from the outset that the project would be a huge challenge,” said Berendsen. “But I was up to it. I love challenges.”
Young added two additional challenges that would loom large over the project. One, Berendsen would need to start from scratch, throwing out a small body of preliminary data that Young’s lab already had generated in support of the idea. The hope being, Berendsen could build a stronger case this way for a higher impact publication.
Two, Young asked Berendsen to complete the project in three years. “My philosophy is the optimal time for a fellowship in my lab is three years,” said Young. “After that, it becomes much tougher to leave. Agnes had a visa for five years, so I said to her, ‘Spend three years here and complete your last two years in another lab, where you can continue to build your lab skills.’”
Berendsen agreed.
And the clock started to tick.
******************
As the first days passed, Young could see that the project was in good hands. Berendsen was as meticulous in planning the sequence of experiments required to build the case. There would be no investigative wrong turns.
Berendsen also had the scientific equivalent of a village to help her frame the right questions. The village consisted of the five stories of Building 30, the red-brick home of the NIDCR intramural research program. In addition to Young as her main day-to-day mentor, Berendsen worked next to Larry Fisher’s lab. His door was always open to discuss biochemistry and design protein-protein binding assays to establish that two proteins interact. Just across the hall was Pam Robey. Her door was open to discuss transplants. Several more doors down the hall, Berendsen could find cancer researcher Silvio Gutkind, who was well versed in the canonical Wnt pathway. He was happy to help.
Berendsen’s first question was an obvious one: Does biglycan interact with a protein that activates the canonical Wnt pathway? Berendsen chose the protein Wnt3a, one of four Wnt proteins known to activate the pathway, as the key to insert to initiate the signal.
She obtained a recombinant version of biglycan from former NIDCR fellow Dave McQuillan and borrowed from Fisher Wnt3a and other needed reagents. She then targeted each protein with labeled antibodies and got an indirect and then a direct hit. Biglycan’s core protein, as opposed to its carbohydrate chains, indeed interacts with Wnt3a.
The data gave Berendsen her next lead. Because Wnt3a activates the pathway via the LRP6 receptor, maybe biglycan did the same. This brought her to the cell surface and straight down into one of the most complex signals in biology. It is mediated by six known types of receptors: Frizzled (10 different kinds), LRP5, LRP6, Derailed/RYK, ROR, and FRL1/crypto.
Think of a choir. While a solo is always nice, a duet, trio, or even quartet can help to amplify tone and range, generating a distinctive timbre of sound. The same is true of the choir of Wnt receptors. A distinctively toned duet of LRP6 and biglycan, after binding Wnt3a, might generate a distinctive signal within a cell. But how would Berendsen know it?
The intracellular protein b-catenin was the answer.
Here’s why. The Wnts and their medley of proteins receptors form the biological equivalent of an on-off switch. When no Wnts reside on the cell surface and the switch is off, b-Catenin is biochemically marked inside the cell for degradation. It sings an executioner’s song. But when Wnts are on the cell surface and the canonical pathway thus punched, the degradation stops. b-catenin accumulates in the cell and interacts with certain family of transcription factors, leading to the activation of several genes that ramp up cell cycle progression and proliferation.
Canonical Wnt Pathway
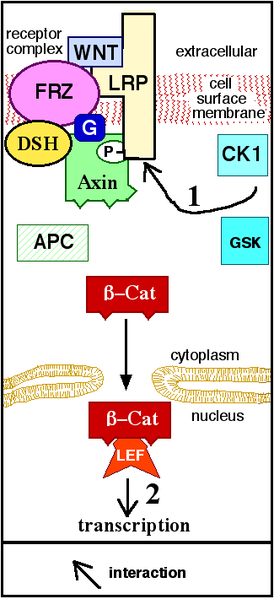
Diagram courtesy John Schmidt
After a series of cell culture experiments, Berendsen had a verdict in hand. It was a split decision. She found biglycan wasn’t the driving force that directly navigated the pathway to its destination, blocking input from Wnt3a and LRP6 once the signal left the station. This realization hung on two key pieces of evidence. One, Wnt3a induced b-catenin to localize to the nucleus regardless of whether biglycan was present. Two, biglycan alone had no direct bearing on b-catenin activity within the cell, meaning it had no obvious mechanistic influence on the course of the pathway.
Biglycan wasn’t off the hook, though. Berendsen found that minus biglycan, the expression levels of some of the pathway’s known target genes dropped by as much as 60 percent. Biglycan thus had an important hand in the signal and its outcome, just not an iron fist.
This raised an interesting possibility. Biglycan might not necessarily function to power a dedicated on-off switch that triggered the canonical Wnt pathway. It might be more of a signal modulator that, like the volume button on a cell phone, turns up or down the intensity of the newly generated signal. What’s more, the modulation seemed to take place on the cell surface or within the greater extracellular space.
Molecular biologists are interested in solving exploitable mechanistic questions that tell them precisely how, when, and why things work. Although Berendsen was getting much closer, the precise mechanism by which biglycan influenced the canonical Wnt pathway remained unclear.
The clock ticked.
*********************
Berendsen continued hammering away into year two. “Whenever I felt a little stuck, I sat down with Larry [Fisher] for biochemical advice,” she said. “I also sat down several times with Silvio [Gutkind] to get more input on the Wnt signaling pathway in general. Instead of only using bone cells, which weren’t producing enough protein to allow certain experiment, we figured out that I could use a Wnt reporter cell line to further elucidate the mechanism by which biglycan affects canonical Wnt signaling. By meeting with the right people at the right time, I was able to keep the project moving forward.”
She also moved the project in a fascinating direction. A previous report showed that a familial mutation in the LRP6 gene impaired the canonical Wnt pathway and caused an inherited form of osteoporosis and hypercholesterolemia. What if they could show in culture that biglycan rescues the pathway in cells bearing the mutation? Berendsen performed the experiment. It worked.
But bone was the Young lab’s medium. More than solving the mechanism, Berendsen needed to show more directly that biglycan indeed modulated the canonical Wnt pathway in bone-generating cells to form new tissue.
Berendsen turned her attention to bone marrow stromal cells (BMSCs), which have the potential to differentiate into bone-forming precursor cells. In additional cell culture work, she found that normal BMSCs deposited more calcium and were more responsive to Wnt3a than biglycan-deficient BMSCs.
So far so good. But Berendsen needed to extend the findings in vivo, or in the body, to confirm that the data held under more biological conditions. That meant transplanting the normal and biglycan-deficient BMSCs into mice. Berendsen had never performed such a study, called an ex vivo transplant assay. Across the hall, Pam Robey was an expert.
About two months later, Berendsen had her answer. The biglycan-deficient cells produced poorly formed bone with abnormally low mineral density. In addition, a Wnt-upregulated protein marker was expressed at low levels, an indication the lack of biglycan affected the canonical Wnt pathway.
Berendsen and Young decided to prepare a manuscript and summarize their data for publication. Meanwhile, Berendsen also continued to help organize a Gordon research seminar on teeth and bone. The session, held in Switzerland, was slated for June 2011.
By midsummer, Berendsen had good news. Per Young’s advice to limit her NIDCR fellowship to three years, she had arranged a second fellowship at Harvard University. Berendsen started in September.
While Berendsen began working out the logistics of moving to Boston, her computer inbox brought crushing news. Her manuscript had been rejected. The reviewers wanted more data to address two general issues: (1) the experiments established that biglycan interacts with Wnt3A and LRP6 individually, but it was still unclear whether all three interact simultaneously, and (2) more data were needed to show that the biglycan-Wnt bone axis is indeed connected in vivo.
What now? Young and Berendsen worked through the various possible scenarios and timeframes. They finally decided to move forward and address the reviewers’ comments. They clicked on the bright red appeal button on the computer screen, and Berendsen began mapping out her next experiments.
The clock now thumped.
“I just went for it,” she said. “I kept thinking, ‘I have to make this work.’”
Berendsen labored day and night for weeks to reach the finish line. She finally got her data and formally resubmitted the paper. The next day, she moved to Boston.
Last October, the Proceedings of the National Academy of Sciences published “Modulation of canonical Wnt signaling by the extracellular matrix component biglycan.” By then, Berendsen was ensconced in Boston, where she works on BMSCs and their role in osteoporosis.
“The three years went by very fast, and I really wanted to focus on getting a good paper out of the project,” she recalled. “I feel like I did.”
Would she recommend an NIDCR fellowship?
“Absolutely. At NIH, everything is there scientifically, and that carries over into training. As for grant writing, now that I’ve left NIH, I feel like I’m forced to do it. But I can do it. I went to a grant-writing seminar at NIH, and my writing skills certainly were in play and under development while I was in Bethesda.”
For Young, the biglycan story that began with Larry Fisher early in their careers continues. “The progress that we’ve made builds on the hard work of not only Agnes but obviously many other outstanding fellows and post doctoral students who have passed through my lab and others in Building 30.”
“Many questions remain to be answered about biglycan,” Young continued. “But its potential in fracture healing and also to treat osteoporosis and other bone-thinning conditions is now in a much tighter focus.”