Staying Alive: Cancer Cells Expressing Mutant KRas Depend on ERH for Survival
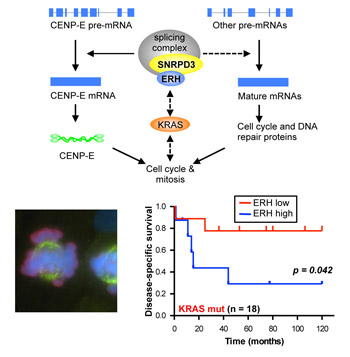
Splicing vulnerability. Scientists in Luo’s laboratory discovered that ERH is a new mRNA splicing factor that facilitates the expression of a subset of cell cycle genes and is required for the survival of KRas mutant CRC cells (top). In the absence of ERH, KRas mutant cells fail to align chromosomes during mitosis and suffer from increased genomic instability (bottom left). Clinically, CRC patients with tumors bearing KRas mutations showed better survival if their tumors have low levels of ERH (bottom right), suggesting the mRNA splicing machinery could be exploited for therapeutic benefit.
The small G-protein KRas acts like a molecular switch, turning on and off pro-growth signaling pathways within cells when appropriate. In a large number of cancers, KRas is permanently turned on by a variety of mutations and drives the constant growth of these tumor cells. KRas itself has proved to be a poor drug target so researchers in the laboratory of Ji Luo, Ph.D., in CCR’s Medical Oncology Branch decided to look for other pathways that are essential for the growth of cells expressing mutant KRas. These pathways could present new drug targets, and blocking their activities might selectively affect cells that express mutant KRas.
Using a small interfering RNA screening method to decrease the expression of genes across the genome, one at a time, the researchers identified a number of candidates that appeared to be essential for KRas mutant cell survival. They selected one gene, ERH, for further study because the function of this gene is poorly understood, although it is evolutionarily well conserved.
The research team first verified that ERH protein was required for the viability of cells expressing a KRas mutation. Decreasing the expression of ERH in two different colorectal cancer (CRC) cell lines caused more KRas mutant cells to die than cells expressing normal (i.e. wild type) KRas. Loss of ERH also prevented the growth of KRas mutant cells in soft agar, suggesting that ERH is required for the cells’ tumor-forming ability. The dependence of tumor cells on ERH was specific for KRas mutant cells since cells that express mutant PI3K, another oncogene, were insensitive to ERH depletion.
Because ERH is evolutionarily conserved with a unique sequence, it likely plays an important role in cell function. The investigators noticed that decreased ERH did not alter the level of KRas or decrease its signaling through the MAP kinase pathway. Instead, reduced expression of ERH caused KRas mutant cells to stop progressing through the cell cycle just before mitosis. By using a fluorescent marker for chromosomes, the researchers saw that the chromosomes of KRas mutant cells lacking ERH failed to properly align at the midline in mitosis, which leads to increased error in chromosome segregation and genomic instability.
The scientists noticed that the chromosome defects they observed with loss of ERH were similar to those that occur in cells lacking the expression of CENP-E, a motor protein that moves chromosomes from the cellular spindle poles to the midline. Depletion of ERH reduced the amount of CENP-E protein as well as its messenger RNA (mRNA) but did not affect the expression of other proteins that help regulate chromosome segregation.
To investigate how ERH regulates CENP-E expression, the researchers first looked to see whether ERH binds to the promoter region of the CENP-E gene. The lack of association between ERH and the CENP-E promoter suggested that ERH does not stimulate the transcription of CENP-E. The investigators wondered whether ERH needs to interact with additional proteins to control the level of CENP-E and used mass spectrometry to find potential ERH-associated proteins. One protein identified in the screen was SNRPD3, which plays a role in pre-mRNA splicing. Using real-time quantitative polymerase chain reaction (PCR), the research team found that cells lacking ERH failed to properly splice the CENP-E mRNA, which led to its rapid degradation and prevented CENP-E protein expression.
Using gene expression microarrays, the investigators found that 87 additional genes, many involved in cell cycle regulation and DNA replication or repair, were also regulated by ERH. The gene signature generated by loss of ERH was negatively correlated with two established KRas mutant signatures in CRC cell lines and tumor samples. These results suggest that KRas mutant cells depend on ERH expression because of the complex gene network it controls, and the level of ERH in KRas mutant tumors may reflect their aggressiveness.
To explore this hypothesis, the researchers compared patient survival with ERH mRNA levels in two independent groups of CRC patients. They found no significant difference in ERH expression in tumors from patients with or without KRas mutation. There was no difference in survival times for patients with tumors expressing normal KRas regardless of ERH level. However, for patients with KRas mutant tumors those with low levels of ERH expression lived significantly longer than those with high ERH.
Taken together, these studies show that KRas mutant cells are uniquely dependent upon ERH. This pathway appears to have important clinical implications for patients with CRC, and inhibition of the cell splicing machinery may be a viable option for treating patients with KRas mutant tumors. Multiple CCR labs, as well as NHLBI scientists and NIH extramural investigators contributed to this study.
Summary Posted: 12/2012
Reference
Reference: Evolutionarily conserved protein ERH controls CENP-E mRNA splicing and is required for the survival of KRAS mutant cancer cells. Weng M-T, Lee J-H, Wei S-C, Li Q, Shahamatdar S, Hsu D, Schetter AJ, Swatkoski S, Mannan P, Garfield S, Gucek M, Kim MKH, Annunziata CM, Creighton CJ, Emanuele MJ, Harris CC, Sheu J-C, Giaccone G, and Luo J. PNAS. December 10, 2012. PubMed Link
Note: All questions should be directed to TellCCR
- A Role for Small Antibody Fragments to Bind and Neutralize HIV
- Sunitinib Proves Beneficial in Metastatic Kidney Cancer
- CD22: A Promising Target for Acute Lymphoblastic Leukemia Treatment
- Newly-Identified Fragile Sites Push Stressed Cells toward Cancer
- Intermittent IL-7 Signaling Essential for T cell Homeostasis
- Staying Alive: Cancer Cells Expressing Mutant KRas Depend on ERH for Survival
- RNAi Functions in Adaptive Reprogramming of the Genome
- Putting on the Brakes: Blocking the Growth of Metastases
- Finding a Chink in the Armor by Investigating the Structure of HIV
- What a Shock: No Apoptosis without Heat Shock Protein 90α
- New Web-Based Tools Make Systems Pharmacology More Accessible Using Data from the NCI-60
- CTCF, a Novel Regulator of Alternative Splicing
- Smurf2 Regulates DNA Repair and Packaging to Prevent Tumors
- Brachyury Protein: A Potential Target in Lung Cancer Therapy

