All Funded Institutions
Alabama
UAB Center for Clinical and Translational Science
Birmingham, Alabama
Principal Investigator
Robert Kimberly, M.D., University of Alabama at Birmingham

Victor Darley-Usmar, Ph.D., and postdoctoral fellow Elena Ulasova perform research to understand how diabetes and dietary factors affect mitochondrial function in the heart. A better understanding of these processes will help in designing new therapies for treatment of diabetic patients.
The vision of the UAB Center for Clinical and Translational Science (CCTS) is to transform the university's environment by building productive and efficient interdisciplinary research teams through educational ingenuity, regulatory reorganization, resource coordination and methodological innovation. The mission is to develop a transformative infrastructure that spans the spectrum from preclinical research to bench-to-bedside translation to community implementation. This center builds upon a long-standing collaborative network that involves Historically Black Colleges and Universities and underprivileged communities in its region.
Using the community health advisor model, UAB CCTS investigators have a strong record of NIH- and CDC-funded community-based participatory research involving the Alabama “Black Belt,” one of the nation's most underserved areas. Further, in collaboration with Alabama's Historically Black Colleges and Universities, they have built an extensive network for training the next generation of health disparities researchers. CCTS will provide the crucible to bring these activities to the next level. Through its innovative “One Great Community” component, CCTS will support three community incubators (Urban Lay, Health Professionals, and University), as well as a Research Incubator to insure the bidirectional flow of information between the lay and the research communities that will generate new knowledge at the intersection between science and community needs.
Arkansas
UAMS Center for Clinical and Translational Research
Little Rock, Arkansas
Principal Investigator
Curtis L. Lowery, M.D., University of Arkansas for Medical Sciences

Curtis Lowery, M.D., chairman of the UAMS Department of Obstetrics and Gynecology, consults with a couple about their baby's ultrasound via a unique telemedicine program that is the first of its kind in the nation. Known as ANGELS (Antenatal & Neonatal Guidelines, Education and Learning System), the award-winning network links obstetric physicians at 40 sites around the state with UAMS maternal fetal medicine specialists. (UAMS Photo/Johnpaul Jones)
The University of Arkansas for Medical Sciences (UAMS) formed the Arkansas Center for Clinical and Translational Research (CCTR) to synergize clinical and translational research programs and revamp institutional research endeavors. CCTR unites UAMS' colleges and graduate school to establish an integrative center that transforms the pace, effectiveness and quality of translational research, resulting in better health for all Arkansans.
The goals are to educate tomorrow's physicians and scientists in collaborative translational science; develop partnerships with communities to assure that research benefits them and addresses their needs; champion innovation and collaboration in research and discovery to bring new technologies to Arkansans; and provide administrative structure that promotes productive interactions among basic science, clinical, health services and health policy researchers.
CCTR will foster the formation of a cross-disciplinary, multifaceted interface among the laboratory bench, patient bedside and broader community through an interactive network of scientists. The center will build on Arkansas' statewide telemedicine program to support community-based research efforts. UAMS is developing a new Division of Informatics to bring added value to Arkansas and represent a model program for research that extends to rural communities.
CCTR will facilitate the training of future clinical and translational investigators across health sciences and forge collaborations among researchers, community clinicians, clinical research networks, professional societies, industry and policymakers.
The ultimate goal of the Arkansas CCTR is to translate successful health care research projects directly to patient care delivery and assure that all Arkansans have access to the same state-of-the-art care — no matter where they live.
California
Scripps Translational Science Institute
La Jolla, California
Principal Investigator
Eric J. Topol, M.D., Scripps Research Institute

Kelly Frazer, Ph.D., director of genomics research at Scripps Translational Science Institute in San Diego, is working on an integrated Omics Program that studies the intersection of genomics, proteomics and glycomics and their influence on disease. She is researching how a piece of chromosome 9 (called 9p21) contributes to heart disease.
The Scripps Translational Science Institute (STSI) will emphasize three dimensions of translation: traditional bench-to-bedside, bedside-to-bench and back to bedside, and bedside to the community and practice of medicine. The vision of this program provides the appropriate amalgam of integration and innovation, and it capitalizes on particular strengths at The Scripps Research Institute, Scripps Health and partnering institutions as well as strengths of faculty members that have led to the development of the goals of STSI.
Building upon and establishing specific collaborations with leading translational science and clinical investigators will accelerate discovery that has impact across multiple research disciplines. Collaborations with a large subset of the 550 life science companies in San Diego provide an extraordinary advantage for accessing innovative technology and catalyzing benefits to patients. STSI exploits the excitement of today's scientific advances to speed tomorrow's preventions, improve health and train future leaders of academic medicine.
Spectrum: The Stanford Center for Clinical and Translational Education and Research
Palo Alto, California
Principal Investigator
Harry B. Greenberg, M.D., Stanford University

A team reviews tape of a simulated birth at the Center for Pediatric Education at Lucile Packard Children's Hospital. The facility is the world's first dedicated pediatric and obstetric medical simulation center, allowing health-care professionals to hone their skills in a simulated medical environment. The hospital is a participating institution in the Stanford Center for Clinical and Translational Education and Research. (Lucile Packard Children's Hospital Photo)
Spectrum: The Stanford Center for Clinical and Translational Education and Research will pursue a multidisciplinary approach to transform and integrate critical components of clinical and translational research related to human health across Stanford University's academic and clinical enterprise. The goals of the center are to effectively convert basic discoveries into practical methods that will improve human health and to prepare the next generation of research leaders to ensure that the translation of discoveries into benefits for human health continues into the future. This mission will be accomplished through a series of coordinated and synergistic transformative changes in their educational and mentoring programs, institutional governance structure, research support infrastructure, and the professoriate, which are all intended to promote clinical and translational research at Stanford and in the community.
The vision for the Stanford center is to transform the goals of the institution to incorporate the needs and priorities of the local community while continuing to promote research. To realize this vision, Stanford is creating an Office of Community Research to create and establish bidirectional information flow between the community and investigators, making the community a true partner in setting the research agenda and priorities. The Office of Community Research will serve as a single point of contact for community groups and Stanford investigators, with the goals that include enhancing understanding of local community needs and priorities and improving dissemination of key research results to the local community to promote health and improved clinical practice.
UCLA Clinical and Translational Science Institute
Los Angeles, California
Principal Investigator
Steven M. Dubinett, M.D., University of California Los Angeles

Pediatric research is an essential aspect of the UCLA Clinical and Translational Science Institute. Melinda Braskett is a pediatric and adult allergist who conducts research in the Food and Allergy Care Center. (UCLA Photo)
The UCLA Clinical and Translational Science Institute (CTSI) is an academic-clinical-community partnership designed to accelerate scientific discoveries and clinical breakthroughs for improved health. The cultural and economic diversity of Los Angeles County poses challenges for health and disease research. CTSI is creating transdisciplinary teams focused on these challenges and community health needs.
The CTSI mission is to create a borderless clinical and translational research institute that brings UCLA innovations and resources to bear on the health needs of Los Angeles. To accomplish its mission, CTSI has established five goals: 1) create an academic home for clinical and translational science that integrates the many strengths of UCLA and its partners; 2) build transdisciplinary research teams to translate discoveries for improved health; 3) transform educational and career development programs to promote the next generation of clinician investigators and translational scientists; 4) expand strong bidirectional academic-community partnerships to ensure that new scientific discovery is relevant to community needs; and 5) serve as a national resource for collaborative research through regional, statewide and national CTSA consortia.
CTSI will reach underrepresented populations and develop translational strategies for health improvements nationwide.
UC Davis Clinical and Translational Science Center
Sacramento, California
Principal Investigator
Lars Berglund, M.D., Ph.D., University of California, Davis

A cadre of leading scientists and physicians will be overseeing the new UC Davis Clinical and Translational Science Center. A few program leaders include: Ann Bonham, Ph.D., executive associate dean for research and education; Lars Berglund, M.D., Ph.D., assistant dean of clinical research and director of the new center; and Fitz-Roy Curry, associate dean for research.
The University of California, Davis (UC Davis) is proposing to create a Clinical and Translational Science Center (CTSC) that will transform its medical research enterprise into a highly effective “open” academic home for clinical and translational research by building on three key assets: 1) a long-standing commitment as a land-grant university to serve the geographically dispersed and ethnically diverse populations of inland and northern California with a health care system enabled by one of the broadest and most extensive telecommunications programs in the world; 2) the collaborative culture of UC Davis, which has one of the most extensive and interdisciplinary life science environments in the country; and 3) an established CTSC pilot facility — the UC Davis Clinical Research Investigator Services Program (CRISP) — that serves as the physical home for clinical and translational research, and for faculty training and career development. CRISP is a fundamentally important CTSC testing ground where many perceived CTSC barriers have been explored and solutions have been tested. Through CRISP, UC Davis has completed the planning phase for CTSC.
In the structure of CTSC, considerable attention is paid to create an organization that is: 1) responsive and familiar to investigators; 2) flexible; 3) well linked to university leadership, participating academic units and the community; and 4) focused on the goal of reducing barriers and facilitating the translation of research gains into medical practice. Key features are an education program focused on team science, extensive collaborations across UC Davis colleges and centers, introduction of catalyst functions, such as collaborative research facilitators and translational postdoctoral fellowships, dissemination through teletechnology, flexible use of resources for patient-oriented research, and a community engagement program emphasizing trust and respect.
CTSC is under the leadership of two co-principal investigators, Drs. Berglund M.D., Ph.D., and Joseph M.D., Ph.D., supported by a team of directors and co-directors that oversees each of the nine CTSC programs, and a comprehensive committee structure, designed to firmly anchor CTSC with institutional leadership, faculty, trainees and the community. CTSC will be implemented through a carefully designed collaborative plan, and the activity will be guided through continuous evaluations and corrections.
University of California, Irvine Institute for Clinical and Translational Science
Irvine, California
Principal Investigator
Dan M. Cooper, M.D., University of California, Irvine

ICTS is dedicated to innovation in clinical science. In this photograph, ICTS Research Nurse Connie Parido is drawing blood in a regional middle school as part of an NIH-funded research effort to reduce obesity in children at high-risk for developing type 2 diabetes. (Paul R. Kennedy Photo)
The University of California, Irvine (UCI) Institute for Clinical and Translational Science (ICTS) is designed to identify, test and implement innovative ways to break down barriers that impede biomedical discovery. The overarching vision of ICTS is to: 1) Nurture novel collaborations by building multidisciplinary research teams, such as chemists and clinicians exploring breath biomarkers in the human “ventilome.” ICTS experts in team science will work with investigators to identify and remove obstacles to successful collaboration. 2) Create new research tools by assessing new technologies for clinical investigators including iophotonics and microdevices, ubiquitous computing for field research, approaches for qualitative and comparative-effectiveness research, and metrics of human performance that link genomic information with dynamic disease phenotypes. 3) Share information by bringing together clinicians, hospital information technology staff and UCI scholars in the Center for Biomedical Informatics. Infrastructure for clinical data interoperability is embedded in the data warehouse. 4) Engage our community by championing new approaches such as PEER (Participant Experience Enhancement in Research), a program that views research volunteers as partners in the process of discovery. Research outreach and dissemination activities are targeted to a variety of timely health care issues, such as mitigating elder abuse and preventing sudden death in pediatric athletes. 5) Training clinical and translational researchers by sponsoring Crossing Boundaries, a set of degree and certificate programs along with mentorship interaction that tackles key issues in translational science.
Finally, UCI is working with regional academic centers to actualize the CTSA vision of collaborative translational science throughout Southern California.
UC San Diego Clinical Translational Research Institute
La Jolla, California
Principal Investigator
Gary Steven Firestein, M.D., University of California, San Diego

Victor Nizet, M.D., chief of the division of pediatric pharmacy and drug discovery at the UCSD School of Medicine department of pediatrics and Skaggs School of Pharmacy and Pharmaceutical Sciences, analyzes a gel related to a novel target for treatment of MRSA and anthrax infections with post-doctoral fellow Shauna McGillivray. (UCSD Photo)
The goals of the University of California, San Diego (UCSD) Clinical and Translational Research Institute (CTRI) are to: 1) provide an academic home for the discipline of clinical and translational science; 2) establish an integrated educational pipeline to train and support clinical and translational scientists; 3) develop a robust clinical research infrastructure that replaces silos with integrated research; 4) enhance bioinformatics capabilities that leverage unique UCSD resources; 5) develop novel technologies to improve research, such as biomarker and imaging; 6) form a Translational Research Alliance with research institutes and industry; and 7) form a Community Alliance with community physicians and the general public to translate scientific discoveries into best practices, increase research into health care disparities and involve the general public in biomedical science.
CTRI will transform education in clinical and translational science by coordinating disparate programs, providing breadth of education from high school through predoctoral students, and providing training to postdoctoral fellows and faculty. The institute also will transform the conduct of clinical research by providing guidance and support from initial planning through data analysis and sharing. The new structure will foster development of novel technologies to facilitate clinical research and provide support for the services and resources necessary to conduct clinical investigation and improve health. CTRI will place a special emphasis on several areas of strength, such as imaging, biomarkers, community outreach and the translation of basic science discoveries to clinical science.
UCSF Clinical and Translational Science Institute
San Francisco, California
Principal Investigator
S. Clay Johnston, M.D., Ph.D., University of California, San Francisco

Joseph DeRisi, Ph.D., in his lab at the University of California, San Francisco. (UCSF Photo/Felix Aburto)
Despite explosive gains in our understanding of the basic mechanisms of human disease, meaningful translation of this knowledge to the treatment and prevention of disease has moved slowly. To accelerate the pace at which discoveries in basic science can serve the health of our patients and community, the University of California, San Francisco (UCSF) intends to establish a Clinical and Translational Science Institute (CTSI). Its mission will be to create a comprehensive, integrated academic home that promotes research and education in clinical and translational science at UCSF, at affiliated institutions, and in participating communities.
Its goals are: 1) to support, enhance and integrate existing training programs, increasing the number of trainees from diverse disciplines and improving the quality of their training in clinical and translational research methods; 2) to support, improve and integrate existing infrastructure to enhance the design and implementation of clinical and translational studies, fostering collaborations to achieve a diverse spectrum of high-quality, original research; 3) to enhance career development of clinical and translational researchers by providing mentoring and opportunities to catalyze original research, and by changing the academic culture to appropriately reward multidisciplinary collaborative work; and 4) to create a virtual home providing contemporary communications to simplify collaboration, to provide an optimal informatics matrix for conducting innovative research, and to nurture the growth of clinical and translational science.
To reach these goals, UCSF is transforming its clinical and translational research organization to establish 13 interrelated programs that will provide the training, services and opportunities needed. These programs are led by senior scientists drawn from diverse disciplines in each of UCSF's four health science schools — dentistry, medicine, nursing and pharmacy — and its graduate division. The plans reflect input from more than 200 interested, energetic and committed participants from throughout the community, including most of UCSF's academic leaders. These individuals worked collaboratively to ensure inclusion, transparency and flexibility in the design and planned implementation of CTSI.
UCSF believes that this infusion of new energy and resources will create and sustain a rich environment for innovative research and drive the realization of UCSF's full potential to educate and to support the work of clinical and translational scientists. If so, biomedicine will be advanced, and the health of our patients and the community will benefit.
Southern California Clinical and Translational Science Institute
Los Angeles, California
Principal Investigator
Thomas A. Buchanan, M.D., University of Southern California
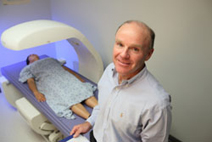
Thomas A. Buchanan, M.D., administers a bone density scan. Dr. Buchanan is associate dean for clinical research at the Keck School of Medicine of the University of Southern California (USC), and principal investigator and director of the Los Angeles Basin Clinical and Translational Science Institute. (Phil Channing Photo)
The vision of the Southern California Basin Clinical and Translational Science Institute (CTSI) is to improve the health of the diverse and underserved population of urban Los Angeles and gain knowledge to improve health in urban settings and large cities across the globe. There are four main goals for the CTSI: 1) to build on many independent strengths to create an integrated academic home that places a high priority on clinical and translational science, 2) to create new translational research teams and conduct projects that leverage the unique populations and partnerships to address the best scientific opportunities and most important health priorities of the local communities, 3) to transform education and training to create a core group of people with a major career focus on clinical and translational research, and 4) to implement and disseminate the findings to improve health in Los Angeles communities and advance translational research through regional and national networks and collaborations.
To achieve these goals, CTSI is creating: 1) outstanding resources for active development of new research projects and teams; 2) a robust infrastructure to promote and support basic, clinical and community research; 3) cutting-edge methodologies for data acquisition, integration, management and analysis; 4) integrated training of basic, clinical and community researchers using distance education and a focus on research for the diverse communities; 5) a novel approach to sharing its findings using professional communications expertise; and 6) a professional evaluation group to track and evaluate the progress and impact.
Colorado
Colorado Clinical and Translational Sciences Institute
Aurora, Colorado
Principal Investigator
Ronald J. Sokol, M.D., University of Colorado Denver
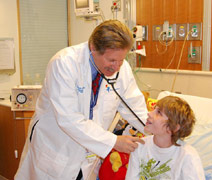
Ronald J. Sokol, M.D., principal investigator for the University of Colorado Denver's CTSI, examines 14-year-old Matt Gordon, who is participating in research studies that will translate discoveries into new treatments for cystic fibrosis.
The University of Colorado Denver and its affiliates are using this award to speed biomedical discoveries from laboratories to the lives of citizens. The university and its partners created an unprecedented statewide network of research, health care and community facilities. Working together, the new Colorado Clinical and Translational Sciences Institute (CCTSI) turns biomedical findings into improved patient and community health. CCTSI coordinates the efforts of scientists, health care providers and advocates from two research universities, six health care professional schools, five hospitals, a health care network and more than a dozen community health programs.
The institute's five goals are to: 1) convert laboratory discoveries into clinical use, 2) bring clinical advances into communities, 3) apply new technologies to deliver personalized medicine, 4) train future researchers, and 5) advance child and maternal health. A dynamic Partnership of Academicians and Communities for Translation (PACT) facilitates exchanges between communities and academic programs that let scientists share discoveries with communities, but at the same time let communities tell scientists what medical and public health needs should be addressed. CCTSI commits personnel and informatics resources to six diverse and well-developed community partnerships as its initial focus for launching this shared translational research agenda. Following initial testing in these communities, CCTSI will adapt successful models to the remaining PACT communities. Eventually, PACT should have an impact on the health care of all of Colorado's more than 4 million residents and the 1,300 physician practices and 300 hospitals that serve them.
Connecticut
Yale Center for Clinical Investigation
New Haven, Connecticut
Principal Investigator
Robert S. Sherwin, M.D., Yale University
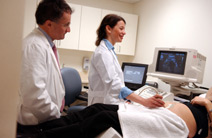
Charles J. Lockwood, M.D., chair of Obstetrics, Gynecology & Reproductive Sciences at Yale School of Medicine, consults with a patient on an ultrasound with Sifa Turan, former postdoctoral fellow and sonographer at Yale Ob/Gyn. (Yale University Photo)
The Yale Center for Clinical Investigation (YCCI) was created to provide a home for training the next generation of clinical investigators. Key programmatic goals are: 1) Attract highly talented students and junior faculty across medicine, nursing, public health, biological sciences and biomedical engineering; train them in the use of state-of-the-art research tools; give them the skills to work within complex research teams; and support their professional development. 2) Foster the translation of disease-related discoveries from the laboratory into the community by stimulating the creation of interdisciplinary teams; making available state-of-the-art core facilities and expanded biostatistical and bioinformatics resources; establishing organizational and regulatory infrastructure for clinical studies; and forging a dynamic new partnership that will integrate community leaders, physicians and health centers. Participating institutions include the schools of medicine, nursing and public health; the Department of Biomedical Engineering; and graduate programs in biological and biomedical sciences.
The investigative medicine program (IMP) is central to YCCI's education and training efforts. It is a unique doctoral program that offers Ph.D. degrees in health sciences research to highly qualified M.D. fellows embarking on careers in translational or clinical research. IMP will be expanded with CTSA support to include nursing, public health biological sciences, and M.D.-Ph.D. students. YCCI has also created a Society of YCCI Faculty Mentors who will participate actively in the training and nurturing of the students and junior faculty members identified as YCCI Clinical Scholars. Pilot and feasibility grants will be awarded for: 1) junior faculty, 2) interdisciplinary translational teams, 3) new technologies, and 4) community-based projects. YCCI will cluster research cores around common themes, including imaging, specimen analysis, physiology, cognition and behavior, drug development, and cell therapy. A new office of research services will provide facilities for one-stop shopping for regulatory, biostatistical, bioinformatics, recruitment and other support services. YCCI will have an office to coordinate the university's efforts to address health issues facing our community. The university's decision to immediately provide substantial support to establish the YCCI reflects its strong commitment to an innovative redesign of our clinical and translational research activities.
District of Columbia
Clinical and Translational Science Institute at Children's National
Washington, District of Columbia
Principal Investigator
Lisa M. Guay-Woodford, M.D., Children's National Medical Center
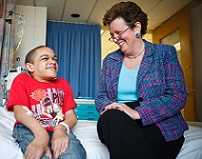
Lisa Guay-Woodford, M.D., principal investigator at the Clinical and Translational Science Institute at Children’s National, talks with patient Michael Lewis during his visit to the Clinical Research Center. Lewis has mucopolysaccharidosis IVA (Morquio A syndrome).(Stephen Bobb Photo/Paula Darte)
The Clinical and Translational Science Institute at Children's National (CTSI-CN) offers a unique set of resources in translating discovery to improved health. CTSI-CN provides highly integrated, cost-effective, investigator-focused resources designed to overcome research barriers, promote collaborative research and provide research training in the area of children's health. With an emphasis on health disparities and childhood antecedents to adult diseases, this center builds upon its pediatric research strengths in areas such as rare diseases, asthma and neurodevelopmental disabilities to collaborate with a national network of 1,200 community health centers.
The specific objectives of CTSI-CN are to: 1) provide state-of-the-art, flexible resources required by clinical and translational researchers, 2) promote multidisciplinary clinical and translational research (CTR), 3) strengthen CTR education and training for diverse trainees at all stages of career development, 4) promote demographic diversity and address health disparities, 5) incorporate effective and sustained collaboration with community partners, 6) ensure research efficiency, and 7) establish bidirectional collaborations with the CTSA network. CTSI-CN lends its unique perspective on pediatric health issues and urban health disparities to the already distinguished members of the CTSA network.
Some key features of CTSI-CN are to expand CTR education and training, making it available from high school through mid-career, encourage community-based research at diverse locations, and overcome gaps in innovative research methodologies to support research.
Georgetown-Howard Universities Center for Clinical and Translational Science
Washington, District of Columbia
Principal Investigators
Joseph G. Verbalis, M.D., Georgetown University
Thomas A. Mellman, M.D., Howard University

Thomas Mellman of Howard University and Joseph Verbalis of Georgetown University (right) share leadership of the Georgetown-Howard Universities Center for Clinical and Translational Science. (Georgetown University Photo)
The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) is a collaborative research center that includes two major universities and three affiliated hospital and research systems.
The specific aims of GHUCCTS are to accelerate improvements in human health by stimulating innovative, multidisciplinary and cross-institutional research among GHUCCTS investigators; to support the careers of clinical and translational investigators through a variety of educational programs paired with focused mentorship; and to enhance local and national clinical and translational research in underserved populations, including minorities, the elderly and those with disabilities.
To accomplish these aims, the GHUCCTS team integrates existing research and training programs with an innovative infrastructure to enhance practice-, laboratory- and community-based clinical and translational research. GHUCCTS includes a coordinated multi-institutional biomedical informatics infrastructure, an expanded clinical research operation with new community-based clinical research units, a new community engagement resource to support and enhance community-based research, and expanded resources in regulatory knowledge and ethics. GHUCCTS also supports collaborative research projects, using the supercomputing and translational tools of the Oak Ridge National Laboratory to explore and develop novel translational methodologies.
Resource integration across the GHUCCTS institutions is accompanied by a joint research education, training and career development program, including new master's degree and scholars' programs in clinical and translational science. GHUCCTS multidisciplinary and cross-institutional research enables the Washington, D.C., community to benefit from the generation and application of new discoveries in clinical and translational science.
Florida
UF Clinical and Translational Science Institute
Gainesville, Florida
Principal Investigator
David Robert Nelson, M.D., University of Florida
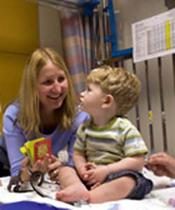
Dr. Jennifer Miller visits with a young patient who was involved in a clinical study at the University of Florida. (University of Florida Photo/Sarah Kiewel)
To speed the translation of basic science discoveries to early investigations in humans and the translation of clinical research into better medical practice and health care delivery, the University of Florida (UF) has invested in new research and training resources and restructured its traditional reporting, research and training operations to create the Clinical and Translational Science Institute (CTSI). The institute will provide the intellectual home for clinical and translational research and training, integrating and synergizing the scientific and educational activities of 12 colleges, two academic and clinical campuses, two regional health care systems and Florida's 67 counties.
CTSI aims to: 1) create an environment through which individuals from diverse disciplines can interact; resources, services and technologies can be identified and accessed; and local and regional barriers to collaborative research can be overcome; 2) train a workforce of clinical and basic science investigators, clinical trialists, laboratory technicians, study coordinators and other personnel required to establish and support multi- and interdisciplinary clinical and translational research teams; 3) enhance the quality and availability of cutting-edge technologies and novel research programs to accelerate discovery, development and application of diagnostic and therapeutic modalities; and 4) create opportunities for clinical scientists and Floridians to collaborate in advancing education and research into the causes, prevention, diagnosis, treatment and cure of human disease.
CTSI will enable UF to transform how it conducts multi- and interdisciplinary clinical and translational research and training and how it engages citizens across Florida in community-based participatory research, education, health care and health care delivery.
Miami Clinical and Translational Science Institute
Miami, Florida
Principal Investigator
José Szapocznik, Ph.D. University of Miami

Olveen Carrasquillo, M.D., M.P.H., director of community engagement and cultural diversity in the Miami CTSI, is pictured with a study participant in a National Heart, Lung and Blood Institute study examining the role of community health workers in improving the health status of Latino diabetics. (John Zillioux Photo)
In a community with approximately 80 percent racial/ethnic minorities, members of the Miami Clinical and Translational Science Institute (CTSI) work together to drive scientific discovery and its translation into evidence-based practices that improve the health of South Florida. The Miami CTSI is co-funded by the NIH National Institute on Minority Health and Health Disparities and headed by a minority investigator dedicated to research with racial/ethnic minorities. The Miami CTSI is dedicated to making significant contributions in minority health and health disparities and in training underrepresented minorities in clinical and translational research. Involving the larger Miami community in a transformational process, the Miami CTSI is building a visionary, far-reaching research enterprise that encompasses internationally renowned clinical services for 1.6 million patients unmatched in cultural diversity; world-class research in areas of highly-visible public concern (i.e., child health, obesity, diabetes, drug abuse, HIV, spinal cord injury, cancer and eye disease); globally-recognized ethics programs that house the Collaborative Institutional Training Initiative human subjects protection training program; pioneering medical training initiatives throughout the hemisphere; and a nationally-recognized 35-year record of exemplary community engagement and research. Fundamental to accomplishing the Miami CTSI's mission is the orchestration of research programs, services, resources and training in novel ways that foster excellence in discovery, innovation and translation; elevate research ethics; promote collaboration among diverse disciplines; and build full research partnerships with diverse communities.
Georgia
Atlanta Clinical and Translational Science Institute
Atlanta, Georgia
Principal Investigator
David S. Stephens, M.D., Emory University

Nana Gletsu Miller, Ph.D., is an assistant professor in the Department of Surgery at Emory University School of Medicine. Her research program studies patients undergoing weight loss and the effects of excess adipose tissue on obesity-related diseases. She is performing an ELISA assay to measure concentrations of adipose tissue cytokines in plasma samples obtained from these patients. (Emory University Photo)
The Atlanta Clinical and Translational Science Institute (Atlanta-CTSI) is led by Emory University, along with partners Morehouse School of Medicine, Georgia Institute of Technology and Children's Healthcare of Atlanta. These institutions already are partners in health care, education and cutting-edge, interdisciplinary research that will be propelled by the Atlanta-CTSI.
The established partnerships and diverse faculty enable the Atlanta-CTSI to combine strong clinical, translational, training and basic discovery programs at Emory with the health disparities, training and community outreach focus of Morehouse School of Medicine, together with the engineering and bioinformatics achievements of Georgia Tech and the excellence in pediatrics of Children's Healthcare of Atlanta. Collaborations with the private nonprofit Georgia Bio organization and the Georgia Research Alliance, the state-sponsored academic-industry partnership, create additional synergies that foster and accelerate development and application of new and emerging technologies. Finally the Atlanta-CTSI creates dynamic community, public health, informatics and population studies programs through partnerships and collaborations with Kaiser Permanente of Georgia, the Centers for Disease Control and Prevention, and the Atlanta Veterans Affairs Medical Center.
Illinois
Northwestern University Clinical and Translational Sciences Institute
Evanston and Chicago, Illinois
Principal Investigator
Philip Greenland, M.D., Northwestern University
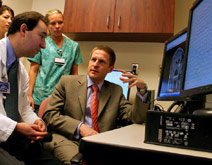
Dr. Stephan Schuele (right) leads the Northwestern University Comprehensive Epilepsy Center based at Northwestern Memorial Hospital. The center's team of neurologists, neurosurgeons, radiologists, neuropsychologists, electrophysiologists and nuclear medicine specialists apply the latest advances in molecular biology, imaging and pharmacology to diagnose and treat epilepsy.
The Northwestern University Clinical and Translational Sciences Institute (NUCATS) is dedicated to facilitating, supporting and promoting research that results in better medical treatments and improved health care. NUCATS is the physical home and central hub for translational research across the Northwestern University enterprise. The institute is composed of five centers, and participation spans several geographic locations, including the involvement of all four NU-affiliated hospitals and six NU schools located on two campuses: the Feinberg School of Medicine, the Kellogg School of Management, the McCormick School of Engineering and Applied Science, the School of Communication, the School of Education and Social Policy, and the Weinberg College of Arts and Sciences. Basic scientists, medical practitioners, community-based medical practitioners and community-based organizations are working together with leaders in the fields of communications, education, business and public health to eliminate barriers to innovation.
University of Chicago Institute for Translational Medicine
Chicago, Illinois
Principal Investigator
Julian Solway, M.D., University of Chicago
The ultimate goals of the University of Chicago (UC) CTSA program are: 1) to train scientists and health care providers at the university, partner institutions and community to determine the molecular underpinnings of disease and disease predisposition in any individual patient; 2) to develop, test, implement and make readily available to community residents personalized therapies directed toward those individual underpinnings; and 3) to do this in a way that is rigorous, valid, efficient, ethical, and respectful of our community's needs and values. In a robust alliance with Argonne National Laboratory, the Illinois Institute of Technology and two large health care organizations, the UC CTSA will undertake three bold new steps that will transform clinical and translational research: 1) creation of an Institute for Translational Medicine, a new university-wide structure to collect, integrate and disseminate the intellectual, organizational and resource infrastructure needed to promote and support multidisciplinary translational research collaborations; 2) synergistic research interaction with a new Urban Health Initiative which, through partnership with community stakeholders, aims to improve community health care access and quality, to build health literacy and trust throughout the community, to enhance a translational research program informed by and responsive to the needs of the community, and so to reduce health disparities; and 3) establishment of a new academic Committee on Clinical and Translational Science and of multiple novel training programs to encourage and develop careers in clinical and translational research, intended for high school students through university faculty and across the entire translational research spectrum. CTSA investigators will employ a systems medicine approach to leverage their particular expertise in social science, genetic medicine and integrative therapeutics.
UIC Center for Clinical and Translational Science
Chicago, Illinois
Principal Investigator
Larry Tobacman, M.D., University of Illinois at Chicago
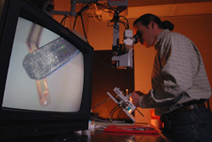
John Hetling, associate professor in bioengineering, is working on a CCTS-funded study to produce the first human data from the multi-electrode electro-retinogram, which maps retinal health. (Roberta Dupuis-Devlin Photo)
The University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) will fundamentally alter clinical and translational investigation at UIC and our partnering institutions. Harnessing our diverse backgrounds, interests and expertise, CCTS will catalyze collaborative thinking and innovation. The center will organize, finance and house the infrastructure, expertise and resources for clinical and translational investigators within a single academic home, crossing administrative boundaries to harness and enhance existing UIC resources.
CCTS' goals: 1) Create and develop an academic home for clinical translational research at UIC that will provide a flexible, adaptable infrastructure to stimulate collaborative thinking, generative discourse and collective action, facilitating clinical and translational investigation. This will include establishing a robust pilot grant program, a Clinical and Translational Science Academy, a Web-based and geographic single point-of-access for investigators, and a matchmaking service to identify novel collaborations. 2) Establish the research service infrastructure (six cores) to provide research support services. 3) Provide multifaceted educational experiences for pre- and postdoctoral trainees, junior faculty and established faculty who want to extend their thinking beyond current disciplinary boundaries.
The administrative reorganization represented by CCTS will lead to rationalization and integration of significant and mature UIC resources for clinical translational research. CCTS will add to these resources to produce not only something different, but something better for clinical translational researchers and trainees at UIC and at partner institutions.
Indiana
Indiana Clinical and Translational Science Institute
Indianapolis, Indiana
Principal Investigator
Anantha Shekhar, M.D., Ph.D., Indiana University School of Medicine

Mu Wang, Ph.D., (right) director of the Protein Analysis and Research Center, and Attaya Suvannasankha, M.D., assistant professor of medicine, are employing proteomics tools to find biomarkers for multiple myeloma at Indiana University School of Medicine.
Indiana Clinical and Translational Science Institute (CTSI) will increase translational biomedical research and improve the health of the people of Indiana and beyond. The Indiana CTSI has developed enhanced educational programs to train translational researchers, a newly designed community engagement activity to produce effective and bidirectional community partnerships, a streamlined process for all available research infrastructure to accelerate translational projects, and a partnership with commercial and philanthropic organizations in Indiana.
The critical link connecting all of these efforts is the university's medical informatics program that enables all parties to interact in an easy, responsive and prompt manner. Thus, the Indiana CTSI brings together the research resources of the entire state. It will provide the national network of CTSAs with a statewide laboratory to experiment with innovative methods aimed at transforming research in biomedical sciences, health economy, health care delivery and health policy. It will create pilot projects, train translational researchers, foster community engagement, build research resources and technologies, and leverage the resources of the Greater Indiana Community.
Iowa
Institute for Clinical and Translational Science at the University of Iowa
Iowa City, Iowa
Principal Investigator
Gary E. Rosenthal, M.D., University of Iowa

Michael Hildebrand studying gene therapies for hearing loss. (University of Iowa Photo)
The Institute for Clinical and Translational Science at the University of Iowa includes 39 established University centers and institutes representing all 11 UI colleges. Examples include the UI Hospitals and Clinics' General Clinical Research Center; the Holden Comprehensive Cancer Center at the UI; the Law, Health Policy, and Disability Center; the Prairielands Addiction Technology Transfer Center; the Northern Plains Native American Health Disparities Center; and the Upper Midwest Public Health Training Center. Partnerships with the University of Arizona and Iowa State University add richness and diversity to the institute's efforts.
The institute will energize strong UI research programs in areas like optical science, oral and maxillofacial implants, nanotechnology, and advanced imaging, and nurture newer initiatives like community-based research. The institute's statewide network of community practitioners, hospitals and health organizations will help identify areas for further study; improve public perception of clinical research; and make cutting-edge research, discoveries and treatments available to patients wherever they live. Looking even further ahead, the institute's master's and Ph.D. degree programs in clinical and translational science will prepare tomorrow's researchers. CTSA support will advance research at the UI in innumerable ways.
Kansas
Heartland Institute for Clinical and Translational Research
Kansas City, Kansas
Principal Investigators
Richard J. Barohn, M.D., The University of Kansas Medical Center
Lauren S. Aaronson, Ph.D., R.N., The University of Kansas Medical Center
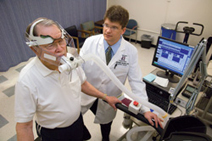
Jeffrey Burns, M.D., director of the University of Kansas Heartland Institute for Clinical and Translational Research's Participant and Clinical Interactions Resources Program, and its Clinical and Translational Science Unit, and director of the Alzheimer's and Memory Program, monitors Howard Kemper, a participant in the Brain Aging Project. The program is studying the relationship between fitness and Alzheimer's disease. Mr. Kemper, who does not have Alzheimer's disease, is participating in this study as a "healthy" control. (Donna Peck Photo)
The University of Kansas Heartland Institute for Clinical and Translational Research, known locally as the Frontiers program, is an academic home for clinical and translational research serving Kansas and the greater Kansas City region. The vision of Frontiers is to create a novel and transformative translational research enterprise from bench to bedside to community. Drawing on many years of experience reaching the frontiers of Kansas with educational, research and health care programs, the program supports scientists and actively involves the community so that discoveries and research findings are more rapidly brought to the point of care. Frontiers also promotes innovative public/private partnerships for developing new drugs and devices and integrates patient-centered health and health systems outcomes into evidence-based risk models that inform clinical care. Through these approaches, Frontiers is improving the health of all Kansans, especially those in rural and underserved communities.
The specific aims of Frontiers are to: 1) create a new academic home with innovative training programs for clinical and translational investigators; 2) provide an enhanced coordinated translational research infrastructure; and 3) actively engage the community in developing, testing and disseminating research. The proposed infrastructure and educational programs of Frontiers address the challenges facing clinical and translational investigators by enhancing and integrating existing resources for easier access, developing new innovative resources, and capitalizing on resources residing in research centers across the university and the region.
Kentucky
UK Center for Clinical and Translational Science
Lexington, Kentucky
Principal Investigator
Philip A. Kern, M.D., University of Kentucky
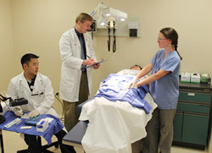
Dr. Philip Kern (center), director of the Center for Clinical and Translational Science, works with College of Health Sciences postdoctoral graduate Jonah Lee (left) and physician assistant Jessica Brown (right) at the University of Kentucky. A muscle biopsy has just been obtained from a volunteer, and the sample is being processed and frozen for subsequent histochemistry and measurement of gene expression. Muscle biopsies are routinely performed as part of studies related to obesity, diabetes and fibromyalgia. (Kristi Lopez Photo)
The goal of the University of Kentucky (UK) Center for Clinical and Translational Science (CCTS) is to transform the pace, effectiveness and quality of translational research leading to novel discoveries that impact health care. CCTS seeks to integrate research strengths of UK's scientists from disciplines focused on common diseases, such as cancer, heart disease and diabetes, and from such disciplines as pharmaceutical sciences and biomedical engineering, which are essential to development of novel drugs and medical devices. UK also has significant scientific strength in the study of risk-related behaviors, such as smoking, alcohol, drug abuse and obesity — all of which are major factors in the high rates of cancer, heart disease and diabetes in Kentucky and across Appalachia.
UK is a land grant university with 16 colleges, including engineering, agriculture and six health science colleges, located all on the Lexington campus. In addition to integrating this UK expertise, CCTS is collaborating with Marshall University, CTSAs at the Ohio State University and the University of Cincinnati, and regional academic institutions to form the Appalachian Translational Research Network. This network will engage investigators in clinical and translational science; foster collaborations, joint pilot studies and mentoring; and develop strong programs in community-based participatory research.
CCTS and its partners will prepare the next generation of scientists; break down barriers to translational research; accelerate the pace of scientific discoveries; and improve the health of citizens in Kentucky, the Appalachian region and the nation.
Maryland
Johns Hopkins Institute for Clinical and Translational Research
Baltimore, Maryland
Principal Investigator
Daniel E. Ford, M.D., M.P.H., Johns Hopkins University

Johns Hopkins University students in a laboratory. (Johns Hopkins University Photo)
The new Johns Hopkins Institute for Clinical and Translational Research synergizes many existing translational research efforts across the Johns Hopkins schools of engineering, medicine, nursing and public health. The institute will create new opportunities to partner with patient communities and for-profit organizations that are dedicated to moving new medical interventions into practice. Incorporating new partners for clinical research among community hospitals and primary care organizations also will be a priority. The institute will provide comprehensive training programs for clinical and translational research that will be targeted for the full range of learners. Clinical and translational researchers will be supported by new programs in biostatistics, innovative methodology, patient recruitment, navigating through regulatory offices, clinical research management systems, bioinformatics, data safety and monitoring programs, building community bridges, research ethics consultations, and the Accelerated Translational Incubator Program (pilot program). New translational cores in drug, device and vaccine development; proteomics; genetics; and imaging will create new translational research teams. Basic science and translational science forums will be used to create and support new research teams that span the translational pathway.
Massachusetts
BU Clinical and Translational Science Institute
Boston, Massachusetts
Principal Investigator
David M. Center, M.D., Boston University
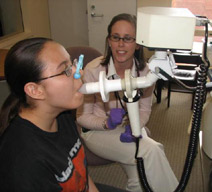
Lisa Gagalis, R.N. (right), a study coordinator at Boston University Medical Campus, performs lung function testing on research participant Ashley Mendez, who is enrolled in a clinical trial of omalizumab, an antibody-based treatment for asthma. This trial is being conducted as part of the Inner-City Asthma Consortium funded by NIAID. (Vivian Borek Photo)
The Boston University Clinical and Translational Science Institute (BU CTSI) will integrate, connect and expand research and programs across traditional academic departments and schools. The institute will act as a bridge between disciplines to facilitate interactions by incorporating multiple key programs that support the university-wide commitment to a home for translational research.
The CTSA grant will allow the institute to build on existing strengths to create an environment linking faculty members, trainees and university programs to speed the translation of innovations in medical science to improve maintenance of health and diagnosis and treatment of diseases and share these innovations with other university-based CTSAs. The BU CTSI environment also will support the bidirectional development and translation of ideas that begin in the clinic to the BU scientific community and back to identify new ways to improve health and delivery of health care services. Moreover, the institute will significantly enhance existing partnerships with Boston's community health centers, transforming the conduct of clinical and translational research by infusing it with community-based perspectives and needs.
Harvard Catalyst: The Harvard Clinical and Translational Science Center
Boston, Massachusetts
Principal Investigator
Lee Marshall Nadler, M.D., Harvard University

Akiko Yabuuchi, a Ph.D. in George Daley's lab at Children's Hospital Boston, conducts stem cell research inside a clean room. Daley is a faculty member at Harvard Medical School and part of the Harvard Stem Cell Institute. (Harvard University Photo/Justin Ide)
Harvard Catalyst: The Harvard Clinical and Translational Science Center will alter the culture of clinical and translational research at Harvard by creating structured and effective methods to connect and support individual investigators and teams of investigators. The center will create managed approaches to focusing the skills of experts in diverse disciplines to find innovative solutions to challenging questions in clinical and translational research. It will deploy both new and old resources more effectively, lowering the barriers to the initiation and conduct of clinical and translational research within and across institutions.
In parallel, the center will build a structure to encourage initiation of new clinical and translational research projects and provide mechanisms for bringing together interdisciplinary and cross-institutional teams, opening the doors of the clinical and translational research enterprise to researchers and engineers with diverse backgrounds, skills and resources. The center will educate the broader Harvard research community on the opportunities, challenges and goals of clinical and translational research.
The overarching goals focus on the individual development of clinical and translational researchers and alignment of incentives with desired outcomes. Structured processes will be created that will enhance the ability of investigators to identify information, seek expertise and access tools necessary to conceive and successfully complete clinical and translational experiments.
Tufts Clinical and Translational Science Institute
Boston, Massachusetts
Principal Investigator
Harry P. Selker, M.D., M.S.P.H., Tufts University

Hans Klingemann, M.D., is director of the Bone Marrow and Hematopoietic Cell Transplant Program at Tufts Medical Center. This program is an example of the clinical and translational research conducted by Tufts Medical Center and Tufts University researchers. (Tufts Medical Center Photo)
The Tufts Clinical and Translational Science Institute will build on long-standing traditions of multidisciplinary collaboration. The institute will leverage that history to produce ground-breaking translational research to bring therapies to patients more quickly. Among the endeavors the institute will undertake will be a program aimed at cultivating connections between researchers and community groups, transforming the research process by developing ways for these groups to learn about each other's needs, interests and perspectives. The program includes annual needs assessments with community partners to identify perceived health needs and research priorities. This partnership will facilitate the recruitment of diverse participants in research efforts to accelerate the adoption of evidence-based care into clinical practice settings. The emphasis on community involvement uniquely reflects both Tufts University and Tufts Medical Center's dedication to active citizenship and biomedical research by including multiple hospitals, community organizations, health plans, industry and others in Massachusetts and nationally.
University of Massachusetts Center for Clinical and Translational Science
Worcester, Massachusetts
Principal Investigator
John Lewis Sullivan, M.D., University of Massachusetts Medical School, Worcester

Drs. Thomas Mayer, Craig Lilly and Joanne Meisner stand in front of a robotic, liquid-handling instrument used to isolate plasma, DNA and RNA from volunteer blood samples in the Biorepository Laboratory at the University of Massachusetts. (University of Massachusetts Medical School Photo)
The University of Massachusetts Center for Clinical and Translational Science, housed within the only public medical school in Massachusetts, has transformed the conduct of clinical and translational research by providing an academic home for all university bench-to-bedside-to-population investigators. By recruiting leaders and establishing innovative research core facilities, we aim to: 1) accelerate early phase translational studies and develop new therapies, devices and interventions based upon UMass discoveries; 2) integrate unique networks of clinical research and health care delivery in Central New England and Massachusetts to build and expand clinical effectiveness research and implementation research capacity and enhance patient and community outreach and participation in clinical/population-based research; 3) work collaboratively with all campuses and schools of the UMass system in developing programs, curricula and faculty support systems that promote careers in clinical and translational research.
The university has established the UMass Advanced Therapeutics Cluster (UMATC), which includes the RNA Therapeutics Institute, the Center for Stem Cell Biology and Regenerative Medicine, and the Gene Therapy Center. UMATC joins the Massachusetts Biologic Laboratories, Meyers Primary Care Institute and Commonwealth Medicine to speed discoveries in clinical and translational research.
Michigan
Michigan Institute for Clinical and Health Research
Ann Arbor, Michigan
Principal Investigator
Thomas P. Shanley, M.D., University of Michigan at Ann Arbor
Basic and clinical researchers in the Allergy and Clinical Immunology Division at the University of Michigan Medical School form collaborative relationships with other units to speed discoveries to patients. (University of Michigan Photo)
The Michigan Institute for Clinical and Health Research creates partnerships among the relevant units of the university, NIH, external industry partners and the community. The overwhelming majority of University of Michigan (UM) schools, colleges and institutes are participating, including: the top-ranked Schools of Business, Dentistry, Medicine, Nursing, Social Work and Public Health; the Colleges of Engineering, Pharmacy, Literature, and Science and Arts; the Division of Kinesiology; the Institute of Social Research; and the Life Sciences Institute. The university-owned Health System, which includes integrated outpatient and inpatient facilities, is contributing significantly to a strong partnership with the UM CTSA site. In addition to the grant resources, the institution is contributing substantial in-kind support, cost-sharing, support of pilot and recruitment programs, and renovation costs, a more than 1:1 match of NIH dollars. The UM CTSA program includes an Education Program that reaches a wide spectrum of audiences, from undergraduates to mid-career faculty, from basic scientists to population researchers, and from staff to community members.
Minnesota
Mayo Clinic Center for Translational Science Activities
Rochester, Minnesota
Principal Investigator
Robert Rizza, M.D., Mayo Clinic
The goal of the Mayo Clinic CTSA application is to present May''s vision for the integration and expansion of its innovative clinical and translational research activities, so that a highly functional academic home for clinical and translational research is developed at the Mayo Clinic. The Center for Translational Science Activities is founded on the Mayo Clinic's long-standing excellence in and commitment to clinical and translational research, which includes the support of key infrastructure and a commitment to career development. To achieve this goal, Mayo will take a comprehensive approach to the key elements of the CTSA Request for Applications and focus on enhancing: 1) clinical research core resources that provide innovative tools to investigators; 2) career development and education programs that prepare the next generation of investigators; 3) compliance and regulatory affairs support that ensures patient safety and privacy, and customer service-oriented approaches to support investigative teams; 4) community affairs support to enhance participation, diversity and community support for clinical and translational research; 5) collaboration with industry and clinical practices to translate research discoveries into routine clinical practice; and 6) continued and expanded institutional support that includes an academic home for clinical and translational research. The Mayo Clinic also proposes a consolidated governance plan that incorporates strong data-driven evaluation of each Center for Translational Science Activities element and the program as a whole. In principle, the CTSA program is consistent with the historical, conceptual and philosophical underpinnings of the Mayo Clinic, and this application clearly articulates how the overarching and transformative goals of the CTSA program can be met at Mayo. To summarize, the Mayo Clinic Center for Translational Science Activities will bring together all the resources of the five schools within the Mayo Clinic College of Medicine, and more than 100 years of scientific and medical research expertise, to discover innovative new methods that will speed the translation of research results into therapies, tools and patient care practices that impact all members of the local and national communities. This vision is entirely consistent with the stated mission of the Mayo Clinic to provide the best care to every patient, every day, through integrated clinical practice, education and research.
University of Minnesota Clinical and Translational Science Institute
Minneapolis, Minnesota
Principal Investigator
Bruce R. Blazar, M.D., University of Minnesota Twin Cities

Aaron Friedman, M.D., vice president for health sciences and dean of the University of Minnesota Medical School, left, discusses a new method to replicate regulatory T cells for bone marrow transplant patients with Bruce Blazar, M.D., who leads the Clinical and Translational Science Institute at the university. Blazer is Regent's professor of pediatrics in the Division of Blood and Marrow Transplantation, and a pioneer in the discovery and use of T cells to boost success for transplant patients.
The University of Minnesota (UMN) Clinical and Translational Science Institute (CTSI) creates an academic home to promote clinical and translational research. CTSI is developing an ongoing alignment with major statewide health care organizations and insurers, electronic networks, special and rural community populations, the state department of health, and the Mayo Clinic. As a result, CTSI is in a position to have a strong impact on workforce training, health care outcomes and policy in Minnesota.
CTSI aims to improve health and well-being by accelerating discoveries into practice, from the scientist's laboratory to the patient's bedside. UMN provides a full spectrum of research expertise and technologies to foster this acceleration.
The CTSI goals are to: 1) create an academic home and an adaptive, sustainable infrastructure to support clinical translational science research at UMN; 2) foster meaningful relationships and transparent interactions between UMN and our communities to improve health statewide; and 3) train and reward interdisciplinary clinical translational science teams at UMN and in our communities.
Increasingly integrated functions in university cores will support clinical translational science research trainees and junior faculty with learner-tailored curricula. Others will accelerate bench-to-bedside translation and commercial applications. The university's Biomedical Health Informatics initiative will provide networked clinical data and biospecimen resources while training future informatics scholars.
The CTSI vision is for an environment that transforms relationships in the health care community, forging a true partnership to facilitate discovery, translation and knowledge dissemination that identify and address changing community needs to have an ongoing positive impact on people's health.
Missouri
Washington University in St. Louis Institute of Clinical and Translational Sciences
St. Louis, Missouri
Principal Investigator
Bradley Evanoff, M.D., M.P.H., Washington University of St. Louis

Samuel Klein, M.D., professor of medicine, and Jennifer McCrea, research coordinator, offer health and nutrition tips to 10-year-old Van Carter at the Adams Park Elementary School Wellness Fair. Dr. Klein will be the director of the CTSA Clinical Interactions Resources Core at the Washington University Institute of Clinical and Translational Sciences. (Washington University Photo)
The CTSA at Washington University (WU) in St. Louis will be implemented by creating a new Institute of Clinical and Translational Sciences designed to conceptually and operationally reinvent and reinvigorate clinical and translational research and research training. The institute incorporates existing programs (K12, K30, and T32) and WU's new BioMed 21 strategic initiative in multidisciplinary, collaborative research in genome sciences, biological imaging and clinical investigation. It also involves an unprecedented level of partnership with other academic, health care, community and scientific institutions in the St. Louis area. Partners include BJC HealthCare; Saint Louis University School of Public Health, Graduate School and Doisy College of Health Sciences; University of Missouri St. Louis College of Nursing; Southern Illinois University Edwardsville School of Nursing; St. Louis College of Pharmacy; key organizations promoting community health; and biomedical and pharmaceutical companies in the St. Louis area. The Institute of Clinical and Translational Sciences will oversee 15 key programs, each designated to facilitate the safe and ethical conduct of research in humans.
New Mexico
The University of New Mexico Clinical and Translational Science Center
Albuquerque, New Mexico
Principal Investigator
Richard S. Larson, M.D., Ph.D., University of New Mexico
A nurse practitioner meets with a patient and his mother at the University of New Mexico Health Sciences Center. (University of New Mexico Photo)
The vision of the Clinical and Translational Science Center (CTSC) at the University of the New Mexico (UNM) Health Sciences Center is to continue expanding and refining a transformative, novel academic home for essential clinical and translational health sciences discovery in New Mexico and the Mountain West region.
The UNM CTSC will integrate the efforts of community leaders and clinicians; basic, clinical and translational investigators; health care and research collaborators; and industry partners to advance meaningful human health discovery, and accelerate its applications in New Mexico communities.
With its numerous, diverse partners in New Mexico and the Mountain West region, CTSC has the expertise, infrastructure and resources to: 1) synergize multidisciplinary clinical and translational research to catalyze the application of new knowledge and techniques on the patient-care front lines; 2) recruit, train and advance talented, highly skilled investigators and research teams strong in cultural sensitivity, health disparity and biotechnology; 3) create an incubator for innovative research, information technologies and research informatics; and 4) expand existing partnerships between UNM Health Sciences Center researchers, practicing clinicians and communities to speed the development of medical research.
New York
Einstein-Montefiore Institute for Clinical and Translational Research
Bronx, New York
Principal Investigator
Harry Shamoon, M.D., Albert Einstein College of Medicine
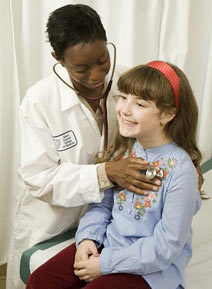
Pediatric clinical research is a vital component of the enterprise sponsored by the Einstein-Montefiore Institute for Clinical and Translational Research.
The Einstein-Montefiore Institute for Clinical and Translational Research (ICTR) is founded on successful interdependent programs comprising pediatric and adult patient research facilities, core laboratories, and expanded clinical research training programs, including a Ph.D. in clinical investigation. Jointly supported by the Albert Einstein College of Medicine and Montefiore Medical Center, ICTR encourages the development of new methods and approaches to bidirectional clinical and translational research. One focus will be on the improvement of education, training and mentoring to aid new investigators in navigating the increasingly complex research system, while taking advantage of newly designed and improved biomedical research informatics tools. ICTR actively engages the ethnically diverse communities of the Bronx, made up of 1.4 million people, collaborating with regional and affiliated institutions to expand research and training opportunities throughout the Bronx community. ICTR builds upon existing clinical research programs, such as the Hispanic Community Health Study; on interdisciplinary centers in diabetes, cancer, liver, health disparities, neuroscience, transplantation and HIV/AIDS; and on an expanding base of translational research faculty in genetics, systems biology and stem cell research, many of whom are housed in a new state-of-the-art facility. ICTR will enhance community collaboration and participation in its research, as well as expand and develop partnerships with community organizations in the Bronx. During the past two years, the Clinical and Translational Science Award development process has involved a broad range of faculty from numerous disciplines (including medicine, dentistry, nursing, epidemiology, social work and biomedical sciences) through these transformative partnerships.
Irving Institute for Clinical and Translational Research
New York, New York
Principal Investigator
Henry Ginsberg, M.D., Columbia University

The CTSA grant supports clinical and translational research at Columbia University Medical Center, such as Dr. Howard Kaufman's research on tumor immunology and cancer vaccines. (Columbia University Medical Center Photo)
Columbia University Medical Center's (CUMC) CTSA program will transform the culture of clinical and translational research so that CUMC can develop and retain an outstanding cadre of senior faculty to lead the next generation of clinical and translational investigators. With enhanced support from the CUMC-Herbert Irving Endowment, which will increase from its present level of $15 million to $25 million, CUMC will accomplish this goal by creating the Irving Institute for Clinical and Translational Research (IICTR). IICTR will be the academic and intellectual home for the next generation of clinical and translational investigators. The senior faculty will provide leadership and stature for IICTR, and serve as mentors for both the junior faculty and IICTR trainees. The junior faculty, called Irving Fellows, will work with senior faculty to develop novel approaches to advancing multi- and interdisciplinary clinical and translational research. The Irving Fellows will be role models for the way multi- and interdisciplinary research should be conducted at CUMC. The resources provided by IICTR will include outstanding support and research in biomedical informatics, biostatistical and clinical trial design, bioethics, regulatory issues, patient-oriented research, and core laboratory resources. The Irving Fellows will be supported by CUMC and CTSA, as will pilot and collaborative research grants awarded annually by IICTR. CUMC has invested in the development of new space for IICTR, including space for pediatric inpatient/outpatient research and the Center for Clinically Oriented Research Education, which will be a home for trainees and faculty. CUMC will also develop a new off-campus research center to support community-based clinical and translational research, and education. As part of the CTSA program, CUMC has created an integrated educational program that includes a new multidisciplinary, patient-oriented research master's degree, a novel K12 scholars mentored research program in muti- and interdisciplinary research, and a pioneering multidisciplinary, patient-oriented research Ph.D. program. The goal of IICTR and the CTSA program is to enable more direct utilization of research advances to benefit patients and the community.
Conduits, The Institutes for Translational Sciences at the Mount Sinai School of Medicine
New York, New York
Principal Investigator
Barbara T. Murphy, M.D., Mount Sinai School of Medicine
Hugh Sampson, M.D. (left), dean for translational biomedical sciences and the Kurt Hirschhorn Professor of Pediatrics at Mount Sinai School of Medicine, discusses the results of an experiment investigating the cellular mechanisms of peanut allergy. Joining him are student Steven Woo (center) and Wayne Shreffler, M.D., Ph.D. (right), assistant professor of pediatrics at Mount Sinai School of Medicine. (Matthew Septimus Photo)
The goal of Conduits, the Institutes for Translational Sciences at the Mount Sinai School of Medicine is to establish a research paradigm that will: 1) facilitate bench-to-bedside translation of cutting-edge research, 2) create an academic home for clinical and translational investigators, and 3) supply the governance and resources needed to allow clinical investigators to benefit from Mount Sinai's Translational Research Institutes and potential collaborators.
To accomplish this, Conduits will redesign its research infrastructure to integrate research functions across departments, enhance and promote interactions between basic scientists and clinical investigators, and streamline administrative procedures for new clinical trials and dissemination of results. It will establish a Translational Discoveries Program to provide consultation, oversight and facilities for clinical and translational research. Conduits also will engage the community and its affiliates to translate health benefits to the public. And it will develop methodologies to improve trial design and reduce participant burden.
To recruit and retain clinical and translational researchers, Conduits will train and support new investigators in a multidisciplinary, doctoral degree-granting program in clinical and translational research. It also will develop a recruitment program and enhance career development. An innovative Experimental Therapeutics & Technologies Program will identify and develop novel clinical and translational research projects and connect basic and clinical researchers, caregivers and laboratories through an integrated network of information.
Conduits will create an effective, efficient and centralized research administrative structure. It will foster and reward interdisciplinary collaborations, educate and retain new clinical and translational investigators, and enable translation of basic scientific discoveries into clinical practice. Conduits also will deliver to its diverse community new therapies and an improved standard of care.
NYU/HHC Clinical and Translational Science Institute
New York, New York
Principal Investigators
Bruce Neil Cronstein, M.D., New York University
Judith S. Hochman, M.D., New York University
Gbenga Ogedegbe, M.D., M.P.H., M.S., as seen with his patient, is an associate professor of medicine in the Division of General Internal Medicine and director of the Center for Healthful Behavior Change at the NYU School of Medicine. Dr. Ogedegbe's research is focused on the translation and dissemination of evidence-based behavioral interventions targeted at cardiovascular risk reduction in minority and underserved populations in community settings and primary care practices. (Mike Weymouth Photo)
To transform the way research is carried out at New York University (NYU) and enhance the quality and productivity of the research effort, NYU and the New York City Health and Hospitals Corporation (HHC) has established a Clinical and Translational Science Institute (CTSI). CTSI aims to increase collaboration among clinical, translational and basic scientists across NYU to better determine the relevance and applicability of scientific advances to clinical problems. It also strives to provide leadership, infrastructure and resources to support novel science and the rapid, efficient and safe application of scientific discoveries to the community. A third aim is to support education, training and development of researchers who can conduct the investigations necessary to bring scientific advances to the public. Finally, CTSI works to enhance the ties between NYU researchers and the community in order to more rapidly identify health problems, investigate their scientific basis, apply the knowledge gained, promote use of new developments and evidence-based medicine by the community, and reduce health care disparities. CTSI links NYU and the HHC in a new venture designed to bring their resources to bear on the health problems facing New York and the nation in the 21st century. Combining strengths and synergies in research, patient care and community outreach, it provides a new and innovative engine for translation of medical advances from the laboratory to the patient and from the patient to the community.
Rockefeller University Center for Clinical and Translational Science
New York, New York
Principal Investigator
Barry Coller, M.D., Rockefeller University
The Rockefeller University Hospital, originally a General Clinical Research Center awardee since 1963, has been the continuous home for clinical and translational science at Rockefeller since 1910. It has been the site of numerous landmark scientific and clinical contributions, and many of its trainees have gone on to become academic leaders. With the new resources available under a Clinical and Translational Science Award (CTSA), a core faculty of distinguished investigators, whose research spans the basic-translational spectrum and encompasses a broad range of scientific and medical disciplines, integrates and expands Rockefeller University's scientific and educational programs in the Rockefeller University Center for Clinical and Translational Science. The new center transforms clinical and translational research by encouraging new studies, enhancing and centralizing the support structures required to conduct studies with scientific rigor, and ensuring an absolute commitment to the protection of human subjects and participant safety. The key elements in the transformation are: 1) a new governance structure reflecting the NIH cooperative agreement (U54) assistance mechanism; 2) creation of a new K12 Clinical Research Scholars Program offering master's and Ph.D.-level degrees to complement the current Clinical Scholars Program; 3) infrastructure enhancements to facilitate the development and conduct of clinical protocols under the principle of good clinical practice, including biomedical informatics, biostatistics, bionutrition, research nursing, research pharmacy, participant recruitment and community engagement, and regulatory support and oversight from the clinical research (research subject advocate) support office; and 4) development of innovative and novel core methodologies related to dendritic cell therapy; vaccine development for HIV, hepatitis C and malignancies; genetics/genomics; assessing the immune response; and metabolic phenotyping. The center continues Rockefeller's tradition of focusing on the interface between scientific discovery; human pathophysiology; and novel diagnostic, preventive and therapeutic strategies. It partners with industry, when mutually beneficial, to achieve these goals. The center is an active member of the national CTSA consortium, offering the consortium novel ideas and tools for conducting and evaluating clinical and translational research. It eagerly adopts the best practices identified by the consortium and adheres to the standards set by the consortium.
University of Rochester Clinical and Translational Science Institute
Rochester, New York
Principal Investigator
Thomas Pearson, M.D., M.P.H., Ph.D., University of Rochester Medical Center

The University of Rochester Clinical and Translational Science Institute provides an optimal setting for medical investigators to conduct safe, controlled, inpatient and outpatient studies. Richard T. Moxley III, M.D., professor of neurology and pediatrics at the University of Rochester School of Medicine and Dentistry, visits with a patient in the Clinical Research Center. (Ken Huth Photo)
The University of Rochester Clinical and Translational Science Institute (CTSI), funded through the Clinical and Translational Science Award program, serves as the academic home for clinical and translational science at the university. The CTSI acts as a hub, integrating clinical and translational science programs and related academic departments with four affiliated URMC research centers, namely the Center for Human Experimental Therapeutics, the Clinical Research Center, the Center for Research Implementation and Translation, and the Center for Community Health.
Since its inception in 2006, the CTSI has achieved many noteworthy accomplishments: 1) Created and supports the UNYTE Translational Research Network made up of 18 biomedical research institutions in and near Upstate New York. Among other things, UNYTE implemented a model for collaborative Institutional Review Board review of multisite protocols to accelerate the approval time for research studies conducted within the network. 2) Established a Research Navigator program to facilitate, accelerate and improve collaboration in clinical and translational research within the URMC, across the UNYTE network, and throughout the CTSA Consortium. 3) Initiated and supports the Greater Rochester Practice-Based Research Network, which consists of 85 pediatric, internal medicine and family medicine practices that serve 30 percent of the adult and 80 percent of the pediatric population in the region. 4) Created an innovative new Ph.D. program to prepare individuals for academic and clinical careers related to the translation of basic biomedical research into clinical strategies to improve health.
Clinical and Translational Science Center
New York, New York
Principal Investigator
Julianne L. Imperato-McGinley, M.D., Weill Cornell Medical College

Dr. Nasser Altorki, director of the Division of Thoracic Surgery at New York-Presbyterian Hospital/Weill Cornell Medical Center, confers with a colleague about a CT scan of a patient's chest. (Weill Cornell Medical College Photo)
The Clinical and Translational Science Center (CTSC) — comprising public/private institutions on the Upper East Side of Manhattan — is a unique and diverse biomedical complex, providing investigators with state-of-the-art resources for conducting clinical and translational research.
Weill Cornell Medical College of Cornell University, the lead institution, serves as a conduit through which technological resources and educational programs are efficiently shared and managed. Neighboring institutions contribute significantly to the CTSC. Hospital for Special Surgery, a leader in investigating musculoskeletal and autoimmune diseases, is one of two medical institutions designated by NIH as a Core Center for Skeletal Integrity. Memorial Sloan-Kettering Cancer Center is a cancer center where state-of-the-art basic science research flourishes side-by-side with clinical investigation and treatment at Memorial Hospital. Cornell University Cooperative Extension, NYC, engaged in research addressing the needs of a changing New York for over 50 years, will be the linchpin for community outreach. Hunter College Gene Center's Research Center for Minority Institutions recruits and nurtures minority talent and has established an effective electronic network with minority scientists nationwide. Hunter College School of Nursing, training nurses from a diverse urban population, will participate in community outreach and education in underserved areas.
North Carolina
Duke Translational Medicine Institute
Durham, North Carolina
Principal Investigator
Robert Califf, M.D., Duke University

Scientists at Duke University Medical Center (Martin Tornai, Ph.D. shown here) have created a new breast scanner that will dramatically improve their ability to visualize small tumors while also reducing radiation exposure to one-tenth that of normal mammograms. Moreover, the new device does not compress the breast, as do traditional mammograms. (Duke University Medical Center Photo)
Duke University established the Duke Translational Medicine Institute (DTMI) to: 1) Create an institute that transforms how fundamental discoveries are translated into improved medical care by supporting creative translational research teams. The institute provides leadership and resources for original translational and clinical research, and it develops and performs studies regarding novel methods and approaches to translational and clinical science. 2) Create an environment in which trainees at all levels, including medical and nursing school students, physical therapists, pharmacologists, house staff, fellows, graduate students, junior faculty and career transition faculty can be trained in translational and clinical research. The training is built on the principle that a rich clinical and translational research environment provides Duke University trainees with models and opportunities for success. 3) Integrate translational and clinical science by fostering collaboration among Duke University's departments, institutes, centers and schools, using human resources supported by modern bioinformatics and a clinical research unit designed to integrate intensive measurements of biological processes. 4) Develop a community model for understanding how to translate the findings of research from bench to bedside, to populations using advanced informatics and health services delivery methods. The Duke University DTMI is founded on three entities, or pillars, including Duke University Translational Research Institute (DTRI), Duke University Clinical Research Institute (DCRI) and Duke University Center for Community Research (DCCR). These three entities bring together and expand existing programs and are designed to emphasize the continuities along the spectrum of research that begins in a basic science laboratory and concludes with novel therapies that change outcomes for individual patients. These three pillars — DCRI, DTRI and DCCR — are administratively joined into DTMI, which links with other key programs, including Duke University Comprehensive Cancer Center, Duke University School of Nursing, and Duke University Institute for Genome Sciences and Policy, thereby creating a comprehensive home for clinical and translational researchers. The creation of DTMI is relevant to public health as it creates an environment that fosters speedier delivery of new interventions and health care practices to the community.
The North Carolina Translational and Clinical Sciences (TraCS) Institute
Chapel Hill, North Carolina
Principal Investigator
Marschall Runge, M.D., Ph.D., University of North Carolina at Chapel Hill

UNC students at all levels are inspired to participate in clinical and translational research. Here, two students display the results of their research project that involved an improved way to detect kidney disease.
The University of North Carolina at Chapel Hill solicited input from over 300 faculty, administrators and other stakeholders, drawn not just from the university but across the state, to establish the Translational and Clinical Sciences (TraCS) Institute. The TraCS Institute is transforming the way research is performed in the state by partnering with communities to more rapidly and efficiently translate scientific discoveries into improvements in the health of citizens. The TraCS Institute has three simple goals: 1) prepare and empower faculty, health care providers and citizens to participate in all aspects of the process involved in translating good ideas into health advances; 2) provide the advice and resources necessary to design and execute the best research projects; and 3) ensure that the best discoveries and ideas evolving from these projects are rapidly used to solve important health problems in the state. The Translational Research Advisory Board, consisting of senior faculty from across the UNC System, partners with communities to identify and prioritize important health issues and calls for project proposals that address these priorities. The TraCS study section, which includes community members, prioritizes and helps improve project proposals contributed by over 40 units across campus and the state. A special TraCS program ensures that best ideas that result from these projects are implemented throughout the state to improve the health of citizens.
Ohio
Clinical and Translational Science Collaborative
Cleveland, Ohio
Principal Investigator
Pamela B. Davis, M.D., Ph.D., Case Western Reserve University

Case Western Reserve University faculty and students have the opportunity to work collaboratively in research and academia with nearby institutions that are partners in the CTSC, including the Cleveland Clinic, MetroHealth Medical Center and University Hospitals. (Case Western Reserve University Photo)
The Clinical and Translational Science Collaborative, based at Case Western Reserve University, includes three hospital affiliates of the School of Medicine, the Cleveland Clinic, University Hospitals and MetroHealth Medical Center. Together, these hospitals cover 90 percent of the medical care delivered in the seven-county area surrounding Cleveland, Ohio, offering a wealth of clinical research opportunities. In addition, excellent programs based in the Frances Payne Bolton School of Nursing and the School of Dental Medicine, as well as those in the School of Medicine and its hospital affiliates reach into the community at many sites, some of which have become study sites in the CTSC. Together with the strong biomedical cores, the reach of these programs affords great opportunity for collaborative clinical research.
The Ohio State University Center for Clinical and Translational Science
Columbus, Ohio
Principal Investigator
Rebecca D. Jackson, M.D., Ph.D., Ohio State University

Caroline Whitacre, Ph.D., one of the world's most acknowledged authorities on autoimmune disease, is among hundreds of scientists at The Ohio State University Medical Center who translate innovative research findings into personalized patient care. (The Ohio State University Medical Center Photo)
The Ohio State University (OSU) has established the Center for Clinical and Translational Science (CCTS) to improve the quality of care for all patients in the community by creating a transformative clinical and translational science discipline that is at the core of the OSU academic culture. It supports a robust and integrated partnership between the OSU and Nationwide Children's Hospital and links these sophisticated health care systems as a laboratory for biological, clinical and behavioral research. By also working through affiliated hospital networks, a primary care network and extension offices in all 88 counties in Ohio, CCTS accomplishes clinical and translational research through innovative collaboration with the community. As part of its community engagement plans, CCTS has selected the Appalachian region of Ohio as an area of emphasis — an area with some of the state's highest poverty rates. Expanding its community-based research programs to include unique partnerships with the Appalachia Community Cancer Network and Partners for Kids, a Nationwide Children's Hospital organization, provides clinical research opportunities to this rural community.
University of Cincinnati
Cincinnati, Ohio
Principal Investigators
James E. Heubi, M.D., University of Cincinnati
Joel Tsevat, M.D., University of Cincinnati
Frank McCormack, M.D., director of University of Cincinnati's (UC) pulmonary division, is an expert on the rare lung disorder lymphangioleiomyomatosis (LAM). Collaborative research at UC and Cincinnati Children's Hospital Medical Center already has led to major discoveries about the progression and treatment of LAM. This is just one example of successful translational research. (University of Cincinnati Photo/Dan Davenport)
The Center for Clinical and Translational Science and Training (CCTST) is transforming the research environment among the University of Cincinnati and its affiliated partners in the community and industry. The CCTST will coordinate and plan the overall direction of the university's research infrastructure and training opportunities; serve investigators' needs from project concept to completion; optimize skills and foster career development of both new and experienced investigators; and ensure that community input informs research processes, and that the university's discoveries are translated to the community.
Through Research Central, researchers will have easy access to centralized study design, biostatistical, bioinformatics, regulatory and community engagement support. The new Pilot & Collaborative Studies core will expand the pilot funding program at Cincinnati Children's Hospital Medical Center to the entire university. Greatly expanded educational offerings, including a new Certificate in Clinical and Translational Research, will be developed, building on the success of the Dean's Scholars in Clinical Research, as well as a master's degree in Clinical and Translational Research program.
Through the community engagement program, CCTST will further bidirectional research linkages with the local community, breaking down bureaucratic barriers by creating Institutional Review Boards that can coordinate community-based research. Expanding services, such as nursing/coordinator support and sample processing provided by the existing General Clinical Research Center and the Cincinnati Department of Veterans Affairs Medical Center, will promote patient-oriented research for populations in the community.
New translational technologies, including proteomics, drug discovery, imaging, nanomedicine, gene transfer and stem cell biology, and translational and molecular disease modeling, will be made more accessible to researchers.
Oregon
Oregon Clinical and Translational Research Institute
Portland, Oregon
Principal Investigator
Eric Orwoll, M.D., Oregon Health and Science University
Biomedical research institutions in Oregon are prepared for a major expansion in clinical and translational investigation. The university has formed the Oregon Clinical and Translational Research Institute (OCTRI). OCTRI has fundamentally changed biomedical research by creating a vibrant academic home for clinical and translational investigation. It will leverage existing strengths and remove barriers to the pace and growth of research. At the heart of OCTRI is a robust partnership between Oregon Health & Science University (OHSU) and Kaiser Permanente's Center for Health Research (KPCHR) that brings together a strong biomedical research university and an innovative practice-based research center associated with a large patient population. The collaboration provides unique opportunities for expansion across the spectrum of human investigation and sets the stage for major advances in human health. Transformation of clinical and translational research in Oregon is enhanced by robust institutional support for the OCTRI, manifest by significant administrative change as well as the commitment of substantial financial and space resources; academic faculties at OHSU and KPCHR that fully support the OCTRI initiative and the development of a strong, multidisciplinary OCTRI leadership team; merging of resources to form a coordinated infrastructure for clinical and translational research; and strong ties to the community and the involvement of the region in the human research agenda. OHSU has identified three major goals for OCTRI, and proposed far-reaching, explicit, and feasible approaches to achieve them. The university will: 1) create an academic home specifically devoted to the discipline of clinical and translational research; 2) nurture a new cadre of highly-trained, interdisciplinary investigators through a strong, diverse educational curriculum; and 3) create a “research commons” — a coordinated infrastructure of core research tools that greatly expands research opportunities and provides a unified, effective means for their access. There are particular opportunities to accelerate progress in pediatric and child health, community based research, and human genetics.
Pennsylvania
Penn State Clinical and Translational Science Institute
Hershey, Pennsylvania
Principal Investigator
Lawrence I. Sinoway, M.D., Penn State Milton S. Hershey Medical Center
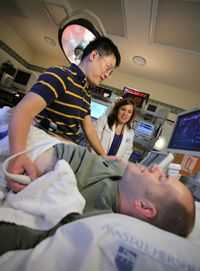
Jessica Mast, R.N., assists during an ultrasound performed by Zhaohui Gao, Ph.D., on study volunteer Josh Miller for one of many cardiac research studies at Penn State Hershey. (Penn State College of Medicine Photo/Darrell Peterson)
The overarching goal of the Penn State Clinical and Translational Science Institute (CTSI) is to revitalize the health science research and education enterprise at Penn State to better enable it to deliver on the promise of improved health. Throughout the university, the CTSI has broad support across colleges, campuses and partner organizations.
With strengths in relevant biological, social and physical sciences and an organizational structure that encourages collaboration across units, CTSI plans to: 1) bolster community alliances to strengthen trust, enhance awareness of disparities and ascertain needs; 2) cultivate new problem-driven interdisciplinary collaborations that go well beyond the traditional boundaries of biomedicine to address these needs, including partnering with industry; 3) share resources and promote their most proficient use; 4) capitalize on novel tools in information technology to collect, share and mine data, and disseminate new knowledge; and 5) educate a new generation of investigators, health professionals and community leaders who are fluent across disciplines, aware of ethical principles, sensitive to the community's needs, and able to apply their skills in partnership with others.
Some of the key features of the Penn State CTSI are to expand biomedical informatics and develop new software solutions to study genetics, epigenetics and systems biology.
Jointly with its community partners, and through the CTSA consortium, the Penn State CTSI will serve as a collaborative engine that will drive research initiatives geared to enhance wellness and better predict, prevent and treat disease in the people it serves.
University of Pennsylvania Institute for Translational Medicine and Therapeutics
Philadelphia, Pennsylvania
Principal Investigator
Garret FitzGerald, M.D., University of Pennsylvania

Biomedical research technician pipetting sample for translational therapeutic study at the University of Pennsylvania School of Medicine. (University of Pennsylvania School of Medicine Photo/Dan Naylor)
The Clinical and Translational Research Award (CTSA) has been greeted enthusiastically by the University of Pennsylvania (Penn) and its partner institutions. The Penn-led proposal was funded in the first round of CTSAs, and Penn has just completed its renewal. The overarching themes of the Penn proposal are: 1) fostering the development of translational therapeutics and 2) bridging the artificial divide between pediatric and adult physiology and disease. A strategic plan had identified clinical and translational research as a priority, leading to the formation of the Institute for Translational Medicine and Therapeutics (ITMAT) in January 2005. ITMAT anticipated many aspects of the CTSA — amongst them, inclusion of dedicated “dry” and “wet” bench space for translational research and a robust educational program, configured on a Master in Translational Research (MTR). This CTSA application prompted intra- and interinstitutional consideration of how to build on this achievement. This has forged a transformational alliance between Penn, the Children's Hospital of Philadelphia (CHOP), the Wistar Institute (WI) and the University of the Sciences in Philadelphia (USP). Faculty from 9 of the 12 schools at Penn and from the partner institutions are represented in leadership roles in the response to this CTSA. ITMAT, designated as the academic home for clinical and translational research, has been broadened to serve a transinstitutional role. Its structure has been transformed to foster interdisciplinary science from discovery of new molecules through to the study of drug action in large populations. This has been accomplished by developing interdisciplinary centers, related cores, innovative interdisciplinary programs of research and strategies to engage and inform communities and their physicians. A particular emphasis has been placed on training and innovative programs, which cover the entire career span, engaging undergraduate students through to mature clinicians. The proposal includes the flexible use of the MTR and new tracks in the Master in Clinical Epidemiology (MSCE) with the M.D., Ph.D., V.M.D., M.S.N., D.M.D., and M.B.A. degrees; dedicated slots for medical school entrants pursuing M.D.-MTR/MSCE degrees; and the flexible use of diverse faculty tracks at Penn and CHOP to broaden physician engagement in research. These initiatives will be pursued in partnership with industry, the State of Pennsylvania, the Food and Drug Administration (FDA), and a national network of institutions holding CTSAs. Specifically, Penn and the FDA signed a memorandum of understanding to pursue jointly educational and programmatic initiatives in the area of translational therapeutics under the guidance of ITMAT. In summary, this initiative has fostered: 1) an integrated strategy to develop clinical and translational research by Penn, CHOP, the WI and USP — more than 900 investigators from these institutions are now members of ITMAT; and 2) the transformation and expansion of ITMAT. This has permitted the development of interdisciplinary structures designed to foster and facilitate research and education in this emerging discipline.
University of Pittsburgh Clinical and Translational Science Institute
Pittsburgh, Pennsylvania
Principal Investigator
Steven Reis, M.D., University of Pittsburgh

Michael Tsang, Ph.D., assistant professor of developmental biology, uses zebrafish in a new high school outreach program through the University of Pittsburgh's Clinical and Translational Science Institute. Tsang's research focuses on feedback regulation of fibroblast growth factor signaling in zebrafish. (Joshua Franzos Photo)
As one of the nation's leading academic research centers, the University of Pittsburgh has embraced the opportunity and obligation to take the inherent risks associated with reengineering a successful research enterprise and to undertake a transformative initiative, resulting in the development and advancement of clinical and translational science as a distinct discipline in western Pennsylvania. The university demonstrated its commitment to transforming its culture, environment and structure to achieve this goal by forming the Clinical and Translational Science Institute (CTSI). The CTSI serves as the integrative academic home for clinical and translational scientists across the university's six health sciences schools, Carnegie Mellon University, the University of Pittsburgh Medical Center (UPMC) and the region.
CTSI focuses primarily on developing, nurturing and supporting a cadre of clinical and translational scientists by building on the university's existing clinical research training programs to establish a comprehensive program with activities ranging from early research exposure for high school students to advanced doctoral programs. Through integration and innovation, CTSI excels in the development of new biomedical knowledge and the translation of that knowledge from the basic and preclinical research settings to individuals, communities and health practice. The CTSI Center for Clinical and Translational Informatics, which is developing translational research informatics tools for the National Cancer Institute's Cancer Biomedical Informatics Grid initiative, infuses informatics tools into the entire lifecycle of clinical research studies and has developed an online collaborative research community. Innovative, interdisciplinary research initiatives have been developed through the 10 CTSI resource cores, and translated to health practice via a novel CTSI community partnership program and through centralization of UPMC's extensive clinical networks. The resulting transformations in the institution, scientists, research and health practice has improved health locally, regionally and nationally.
South Carolina
South Carolina Clinical and Translational Research Institute
Charleston, South Carolina
Principal Investigator
Kathleen Theresa Brady, M.D., Ph.D., Medical University of South Carolina
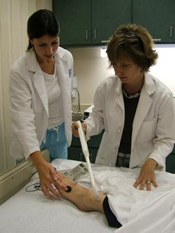
Teresa Kelechi, Ph.D., R.N. is a board-certified gerontological clinical nurse specialist and a certified wound care nurse. Dr. Kelechi's translational research involves the use of infrared thermometry to detect the potential for stasis ulcer development in individuals with chronic venous disorders, and she is developing a "smart sock" clinical intervention to prevent and treat venous leg ulcers. (Medical University of South Carolina Photo)
The goal of the South Carolina Clinical and Translational Research Institute (SCTR) is to create a sustainable home at the Medical University of South Carolina to advance clinical and translational research as a distinct discipline and facilitate collaboration across other disciplines. The overall approach focuses on implementing advances in biomedical science to create opportunities for discovery; removing barriers to link knowledge, experience and expertise across disciplinary boundaries; providing training and mentoring experiences for clinical and translational researchers with diverse training and backgrounds; and fostering community engagement with a rapidly growing underserved population to improve health outcomes and research participation.
Joining the national CTSA consortium will accelerate progress by facilitating: 1) development and interoperability of biomedical informatics systems; 2) active exchange of best processes and practices in evidence-based medicine and community engagement; 3) advancement of clinical and translational science as a discipline and career path; and 4) shared knowledge, experience and collective influence in setting regional and national research agendas and health policy designed to generate the transformative results envisioned by the NIH Roadmap.
SCTR will bring together scientists, clinicians and the lay community to address diseases that commonly impact the citizens of South Carolina. SCTR will coordinate resources and expertise statewide in efficient, innovative approaches to research. Through SCTR, a new generation of researchers will be trained to work across multiple disciplines in collaboration with community members so that scientific discovery is relevant to the public.
Tennessee
Vanderbilt Institute for Clinical and Translational Research
Nashville, Tennessee
Principal Investigator
Gordon R. Bernard, M.D., Vanderbilt University

Sandy Yoder, a senior research specialist, works in Vanderbilt University Medical Center's Pediatric Infectious Diseases Lab. She uses liquid nitrogen to keep vials at 320 degrees below zero. Yoder is putting away influenza samples obtained in vaccine trials at Vanderbilt. (Vanderbilt University Photo)
The Vanderbilt Institute for Clinical and Translational Research (VICTR) was created to focus on both bench-to-bedside, and bedside-to-practice translation. Vanderbilt intends to remove impediments and release investigators from administrative burdens, produce inspired personnel trained in the bidirectional process of translational research, foster innovation by stimulating contributions from collaborators, and enrich the translational research environment with extensive state-of-the-art informatics tools and expert biostatistics support.
The institute funds also are being used to support a Community Engagement and Research program, which leverages the very strong ties of Vanderbilt to the community. Important to the overall success of the program is a focused partnership with Meharry Medical College. Also involved within Vanderbilt is the Institute for Medicine and Public Health as well as the schools of medicine, nursing, law, business and engineering; the Peabody College of Education and Human Development; the College of Arts and Sciences; and the Kennedy Center for Research on Human Development.
Texas
Center for Clinical and Translational Sciences
Houston, Texas
Principal Investigators
David Dugald McPherson, M.D., University of Texas Health Science Center at Houston
Robert C. Bast, Jr., M.D., University of Texas Health Science Center at Houston
Daniel D. Karp, M.D., University of Texas Health Science Center at Houston
Roberta B. Ness, M.D., M.P.H., University of Texas Health Science Center at Houston

Dianna Milewicz, M.D., Ph.D., professor and director of medical genetics at The University of Texas Medical School at Houston, directs research in the Genetics Core Lab. The lab will help facilitate more rapid research results at UT-Houston's Center for Clinical and Translations Sciences. (UT Health Science Center at Houston Photo/Ester Fant)
The University of Texas Health Science Center at Houston (UTHSC-H) has established a Center for Clinical and Translational Sciences (CCTS) at the Texas Medical Center (TMC) in Houston. Participating faculty and trainees in CCTS include those from the UTHSC-H component degree-granting schools, including its Medical School, School of Public Health, Graduate School of Biomedical Sciences, School of Health Information Sciences, School of Nursing, Dental Branch, and Brown Foundation Institute of Molecular Medicine, as well as collaborating faculty/facilities of The University of Texas M.D. Anderson Cancer Center (MDACC), which also is located in the TMC. The academic home for CCTS is housed in 11,422 square feet of recently renovated space at the UT Medical School, which is physically joined to Memorial Hermann-Texas Medical Center and serves as its partner and primary teaching hospital. The CCTS home administers all aspects of CCTS and provides space and resources for faculty and trainees, along with expertise in study design, biostatistics, regulatory issues, ethics, bioinformatics, funding of pilot and feasibility studies, provision of resources, protected time for clinical and translational faculty and trainees, and interactions/collaborations with the various communities and industry. For participant and clinical interactions resources, CCTS has subsumed the UTHSC-H General Clinical Research Center (GCRC) at Memorial Hermann, the satellite UTHSC-H GCRC at Brownsville, Texas, and, in part, the MDACC Clinical and Translational Research Center, to enhance research productivity and efficiency. In its educational component, CCTS has subsumed, in part, the current Center for Clinical Research and Evidence-Based Medicine, which has developed and currently provides formal classes, mentoring, and a Master of Clinical Research degree at UTHSC-H, and an active K30 award at MDACC. Also proposed in the original application was a novel T32 application offering combinations of master's and doctoral degrees in community health sciences, biomedical sciences and/or biomedical informatics — primarily for pre-doctoral students — and a K12 application for post-doctoral trainees and junior faculty. CCTS also has subsumed core translational laboratories, including a genotyping/sequencing core; a biomarkers core offering DNA microarray, RT-PCR and proteomics services; an immune monitoring core; an MRI core; and a biobanking core. A CCTS think tank composed of highly accomplished translational and clinical investigators, basic scientists and educators, and community representatives has come together as an engine for innovation to bring forward and recommend the application of novel and emerging scientific information, methods and technologies to research into human health and diseases across specialties, disciplines and communities.
Institute for Integration of Medicine and Science
San Antonio, Texas
Principal Investigator
Robert A. Clark, M.D., University of Texas Health Science Center at San Antonio

A medical student at The University of Texas Health Science Center at San Antonio celebrates new life with a mother and baby while on obstetrics rotation at the Regional Academic Health Center, a campus of the Health Science Center in the Lower Rio Grande Valley of Texas. (UT Health Science Center at San Antonio Photo)
The University of Texas Health Science Center at San Antonio has established the Institute for Integration of Medicine and Science as the home for the Clinical and Translational Science Award. The institute's mission is to spur integration of clinical and translational research, education, training and career development across all schools and among partner organizations in South Texas. The institute will bring existing and newly developing resources and intellectual capital to bear on clinical and translational research for the improvement of human health. Meaningful two-way community participation has promoted buy-in from all stakeholders and will remain a key principle.
Institute partners have brought together major talent and a broad array of resources to create synergies that add value to all participating organizations, residents of the region and the CTSA network. Distinctive features of the institute include thriving partnerships with key public and private organizations, major investments in research resources and infrastructure, one of the world's largest primate research colonies, the largest cadre of military health care and biomedical research operations in the United States, and a 46,000-square-mile service area populated by predominantly Hispanic residents. This area includes some of the country's poorest people and has high rates of health disparities, providing an opportunity, challenge and obligation for this institute to make a significant impact on human health.
The primary vision is to work closely with all partners to translate the results of the academic- and community-based research for the direct benefit of the regional population.
Institute for Translational Sciences at UTMB
Galveston, Texas
Principal Investigator
Allan R. Brasier, M.D., University of Texas Medical Branch
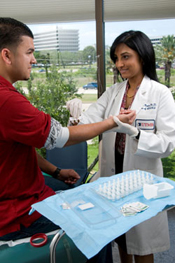
UTMB physician Meera Gupta's translational research interests include transplant immunology, particularly the mechanisms of graft tolerance and rejection. She also has clinical interests in solid organ transplantation and cystic fibrosis. UTMB's Institute for Translational Sciences is composed of eight multidisciplinary teams formed around a diversity of topics. (UTMB Photo/John Glowczwski)
The University of Texas Medical Branch (UTMB) at Galveston, through its Clinical and Translational Science Award (CTSA), seeks to facilitate translational research as a rigorous discipline, develop translational research training programs at all levels in the graduate continuum, effectively conduct and bridge step one translational research to steps two and three, and interface productively with the national CTSA consortium. In particular, this CTSA helps UTMB to build teams of researchers with diverse skills who can work effectively toward positive health outcomes. These teams also serve as exemplary learning environments for the next generation of translational investigators. Training activities include a Clinical and Translational Scholars program, an Academy of Mentors, a seminar series and team training workshops.
The CTSA is administered by UTMB's Institute for Translational Sciences (ITS), which facilitates many aspects of translational research and support at the university, enhancing researchers' ability to quickly and effectively translate basic science discoveries into improvements in human health. ITS' operations are guided by three overlying principles: 1) proactively identifying new team-oriented research opportunities; 2) prioritizing trainee involvement in a team-based culture; and 3) integrating systems biological approaches into translational research. Through the CTSA, ITS has organized 12 key resources — combinations of university core laboratories and intellectual resources, integrated by a single point of investigator/trainee contact. Key resources are designed to respond to the needs of investigators and trainees and to break down communication, technology and regulatory barriers, thereby transforming how UTMB conducts patient-oriented research.
UT Southwestern Clinical and Translational Alliance for Research
Dallas, Texas
Principal Investigator
Robert D. Toto, M.D., University of Texas Southwestern Medical Center at Dallas

Dr. Robert Toto, associate dean for clinical and translational research at UT Southwestern Medical Center, is principal investigator for UT-STAR. (UT Southwestern Medical Center Photo)
The University of Texas (UT) Southwestern Medical Center has established the UT Southwestern Clinical and Translational Alliance for Research (UT-STAR) supported by the NIH-sponsored Clinical and Translational Science Award. The University of Texas Southwestern Medical Center has established the UT Southwestern Clinical and Translational Alliance for Research (UT-STAR) supported by the NIH-sponsored Clinical and Translational Science Award. UT-STAR provides the crucial infrastructure necessary for medical scientists to discover and apply new diagnostics and therapeutics for the detection, diagnosis, treatment and prevention of disease. We focus on four major areas: 1) catalyzing research that leverages our strengths in biological sciences, 2) educating clinical investigators to perform cutting-edge clinical and translational research, 3) developing and implementing new research methods and technologies, and 4) engaging the community in improving the health of the population that we serve.
The Department of Clinical Sciences serves as the academic home for UT-STAR and is supported by faculty from UT Southwestern’s schools of allopathic and osteopathic medicine as well as from our partnering institutions’ schools of dentistry, nursing, pharmacy, public health, bioengineering and computer science. Overall, UT-STAR includes more than 200 established clinical and translational investigators, who act as faculty and mentors.
Existing infrastructure includes our Clinical and Translational Research Center, which has incorporated the Dallas Heart Study infrastructure and database, creating new blood and tissue biobanks; education programs for Clinical Research Scholars and M.Sc. degree candidates that include an innovative T1/Phase I trials training track and expanded instruction in comparative-effectiveness research (CER) and community engagement research; a Spanish-language validation service; T1 research funding pilot awards to develop novel translational diagnostics and therapeutics; an Advanced Imaging Research Center for T1 research; a large and medically diverse patient base; and the use of more than 85,000 square feet of space.
With the help of the CTSA and a strong institutional commitment, UT-STAR reduces many barriers to performing clinical and translational research through education, collaboration, research resource expansion, integration, and improved health care delivery to our community and region.
Utah
University of Utah Center for Clinical and Translational Science
Salt Lake City, Utah
Principal Investigator
Don McClain, M.D., Ph.D., University of Utah
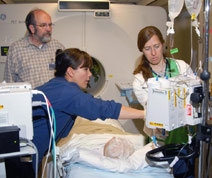
Lauren Jones, R.N., assistant nurse manager (front left), Shelley Stanton, R.N., and Donald A. McClain, M.D., Ph.D., of the University of Utah Center for Clinical and Translational Science, prepare a patient for a PET scan as part of a clinical trial.
The University of Utah Center for Clinical and Translational Science will build on the university's strengths in genetics and bioinformatics to translate promising bench science into practices that improve human health. The center serves as an academic home for clinical and translational research, developing innovative health services for the community and health researchers, providing seed funds to initiate clinical and translational research projects, and training a new generation of clinical and translational investigators.
The center and its partners are increasing the visibility, volume and quality of participatory research by connecting investigators at the university with other health care institutions, clinical practitioners, public health personnel, patients and research participants. As the sole academic health sciences center serving Utah's rapidly growing racial, ethnic and culturally diverse population, the center will support the empowerment and representation of underserved populations as stakeholders in translational research. The center also links research activities across systems that together provide health care coverage to 80 percent of Utah's population as well as patients in surrounding states.
Virginia
VCU Center for Clinical and Translational Research
Richmond, Virginia
Principal Investigator
John N. Clore, M.D., Virginia Commonwealth University
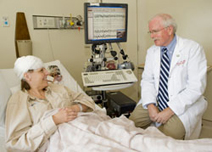
John N. Clore, professor of medicine and associate vice president for clinical research, speaks with patient Colleen A. Thoma, as she undergoes a test to monitor brain wave activity. (Virginia Commonwealth University Photo/Tom Kojcsich)
Effective delivery of research that moves from the bench to the bedside to the community requires a transformation of research practice at every level. To accomplish this, Virginia Commonwealth University (VCU) has established the Center for Clinical and Translational Research (CCTR), a comprehensive matrix center that supports VCU's efforts to strengthen ties with affiliates and community partners to better share resources and respond to community health needs. CCTR facilitates new partnerships and initiatives, extending the university's research base of federal, private and industry sponsors. Combining VCU's existing resources with those of the CTSA award, CCTR supports novel research methods in three areas of strength: substance abuse, women's health and rehabilitation science; pilot funds support innovation and community engagement research in these areas. Through CCTR, researchers benefit from centralized management, web-based data sharing, training and access to a rich array of resources, including biostatistics, ethics, research study and regulatory support. In addition, students can pursue a transdisciplinary education through the center's M.S. and Ph.D. degree programs in clinical and translational science.
Washington
Institute of Translational Health Sciences
Seattle, Washington
Principal Investigator
Mary L. Disis, M.D., University of Washington

Dr. Mary "Nora" Disis, right, and researcher Vivian Goodell are conducting research to develop a cancer vaccine that would work like immunizations against infectious diseases. (Seattle Post-Intelligencer Photo/Paul Joseph Brown)
The Institute of Translational Health Sciences represents a consortium of six University of Washington (UW) health science professional schools with multiple partners that cover 12 performance sites, involve 67 scientific key personnel and connect researchers to over 150 centers. In addition, the Institute of Translational Health Sciences integrates major research and clinical institutions across a five-state region: Washington, Wyoming, Alaska, Montana and Idaho (WWAMI) via ongoing clinical and research collaboration pathways that are part of the WWAMI program led by the UW School of Medicine.
A unique feature of this CTSA site is its community engagement plan, which considers diversity across race, ethnicity, culture, rural and urban locations, geography, health status, and health service delivery, with a targeted program for Alaska Natives and American Indians. The Institute of Translational Health Sciences supports an integrated ethics program, linking adult and pediatric medical centers and the community. An additional unique feature is the site's advanced capability for therapeutic product development and clinical testing that will enhance future health care throughout the region. The Institute of Translational Health Sciences will foster new health sciences interactions across the sites through a variety of technology, education and research support cores. The institute guides, supports and facilitates translational research efforts that focus on expanded information collection, sharing and analysis, innovative scientific technologies, and critical support services aimed at accelerating health sciences research.
Wisconsin
Clinical and Translational Science Institute
Milwaukee, Wisconsin
Principal Investigator
Reza Shaker, M.D., Medical College of Wisconsin
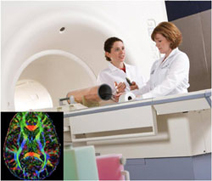
Kathleen Schmainda, Ph.D., professor of radiology and biophysics, utilizes MRI methods capable of measuring the micromolecular movement of tissue water (i.e., diffusion) to demonstrate the potential to track tumor progression and, possibly, invasion. She is seen mentoring Melissa Wagner Schuman, graduate student in the Medical Scientist Training Program. Inset -- Diffusion Tensor Imaging, MRI of white matter tracts. (Mark Dersen Photo)
The goal of the Clinical and Translational Science Institute (CTSI) is to create a borderless, collaborative environment for biomedical researchers, health care providers, educators, citizens and industry to work together synergistically and translate discoveries into better health for our citizens. CTSI has constructed a unique academic community partnership, building on the long collaborative history among the major academic and health care institutions in Wisconsin.
To accomplish this transformation, CTSI is: 1) developing a distinct academic home for the discipline of clinical and translational science, which transcends intra- and interinstitutional barriers and empowers experienced leadership to have the authority and resources to promote, facilitate, coordinate and foster the continuum of translational research from bench to bedside, to clinical practices, and to our communities; 2) increasing the number of investigators participating in clinical research through innovative programs; and 3) engaging clinical practices and the community in research that enhances public health. To train clinicians and basic scientists in the emerging discipline of clinical and translational science, CTSI has launched a Ph.D. program in basic and translational research and a Master of Science degree in clinical and translational science and is developing a Ph.D. program in Clinical and Translational Health Sciences for health professionals.
UW Institute for Clinical and Translational Research
Madison, Wisconsin
Principal Investigator
Marc K. Drezner, M.D., University of Wisconsin - Madison
The University of Wisconsin - Madison, through its Institute for Clinical and Translational Research (ICTR), addresses how to translate biomedical discoveries into practices that improve health. ICTR has taken a new approach to research by producing interdisciplinary research scientists who can address health problems along a continuum — from basic laboratory investigations through clinical trials in patients and into population health studies in communities. The institute provides researchers with an array of tools and creates feedback systems to ensure that research is relevant and addresses the health care needs of populations in Wisconsin.
The institute serves as the hub of a network that fans across the university and extends around the state. People based at five schools on campus and several hospitals in Madison are involved as are experts at academic campuses on all points of the Badger State compass. New and existing statewide partnerships have been enhanced. Physicians and public health workers in towns and communities throughout the state are essential players.
Social Media Links