News
BRCA1 Variants Co-Conspire
with MicroRNA-155
Recently discovered microRNAs (miRNAs) play an important biological role by switching “on” and “off” at different times during cell growth, death, development, and differentiation. They regulate gene expression by blocking messenger RNA’s instructions for protein production.
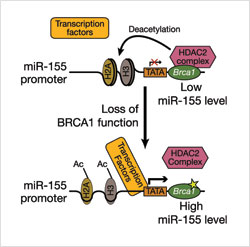 If BRCA1 is absent, or if a variant form—such as the R1699Q—is present in the cell, the interaction of the BRCA1-HDAC2 complex is disrupted. This increases the level of acetylated H2A and H3, which, in turn, activates the miR-155 promoter. This upregulation of miR-155 contributes to tumor development. (Image: S. Sharan, CCR)
If BRCA1 is absent, or if a variant form—such as the R1699Q—is present in the cell, the interaction of the BRCA1-HDAC2 complex is disrupted. This increases the level of acetylated H2A and H3, which, in turn, activates the miR-155 promoter. This upregulation of miR-155 contributes to tumor development. (Image: S. Sharan, CCR)Shyam K. Sharan, Ph.D., working with Suhwan Chang, Ph.D., a research fellow, in CCR’s Mouse Cancer Genetics Program, recently reported in Nature Medicine how one of these miRNAs can also be harmful when the “on” and “off” switching occurs at the wrong time in the wrong place. This was discovered by researching the functional consequences of a BRCA1 variant called R1699Q that does not alter its DNA repair function. Instead, the Sharan team found that this variant co-conspires with microRNA-155 over-expression to help cancer thrive.
Sharan and colleagues uncovered a new function for BRCA1, a gene most commonly associated with hereditary breast and ovarian cancer when it is mutated. Working on mouse cells, they discovered that normal BRCA1 suppresses the expression of another gene that codes for a microRNA called miR-155, which is known to be cancer- causing. These findings suggest that BRCA1 functions as a tumor suppressor not only by playing a role in DNA repair, as known previously, but also by silencing oncogenic miR-155.
Using a mouse embryonic stem- cell-based assay, Sharan and colleagues also investigated precisely how normal BRCA1 silences miR-155 in cells. They discovered that BRCA1, through its interaction with another protein called histone deacetylase2 (HDAC2), modifies proteins called histones that wrap around DNA and help maintain its structure. As a result of these modifications, DNA is prevented from expressing miR-155. When BRCA1 is absent or a mutant BRCA1 that cannot bind to HDAC2 is present, these deacetylation modifications of DNA cannot occur, and consequently miR-155 is over-expressed.
When the researchers inactivated miR-155 in tumor cells in mice, tumor growth slowed down. If the BRCA1-associated tumors in humans are confirmed to also be dependent upon miR-155, it may be possible to treat hereditary BRCA1-mutated breast and ovarian cancers by challenging them with agents that can inactivate miR-155. In fact, expression levels of miR-155 may be a useful biomarker for BRCA1-deficient human tumors.
To learn more about Dr. Sharan’s research, please visit his CCR Web site at http://ccr.cancer.gov/staff/staff.asp?Name=sharan.











