Rock Talk Archives for February 2013
Another Approach to Transparency
As you know, in August 2011 we issued a final rule in the Federal Register revising the regulations on financial conflicts of interest (FCOI) of extramural investigators, and these regulations were implemented in August 2012. One of the major changes in the new regulations was the requirement that institutions make publicly accessible certain information concerning identified financial conflicts of interest held by senior/key personnel. This requirement was included to help the public monitor the integrity of the research funded by the Department of Health and Human Services (HHS) and to underscore our commitment to fostering transparency and accountability.
This public accessibility requirement in the FCOI regulations is in line with a number of recent public disclosure initiatives. One such example is the “Sunshine Rule”, which was included in the Affordable Care Act passed in March 2010. Today, a final rule implementing the Sunshine Rule provisions was posted in the Federal Register by the Centers for Medicare & Medicaid Services (CMS).
The rule, called the “National Physician Payment Transparency Program: Open Payments”, finalizes the provisions that require manufacturers of drugs, devices, biologicals, and medical supplies covered by Medicare, Medicaid, or the Children’s Health Insurance Program (CHIP) to report payments or other transfers of value they make to physicians and teaching hospitals to CMS. CMS will post that data to a public website. The final rule also requires manufacturers and group purchasing organizations (GPOs) to disclose to CMS physician ownership or investment interests. Applicable manufacturers and applicable group purchasing organizations must begin to collect the required data on August 1, 2013 and report to CMS by March 31, 2014. The final rule contains a lot more information on reporting these payments or other transfers of value and their subsequent publication on the website.
There are some major differences between these two rules. For example, the FCOI regulations cover financial interests of investigators receiving or applying for funding from the Public Health Service including the NIH, while the new CMS rule covers payments from the entities listed above to physicians and teaching hospitals. As I’ve shown before, around 30% of NIH-funded principal investigators have an MD or MD/PhD degree. Therefore, some individuals may be covered by both rules.
It is extremely important that we all strive to maintain the public trust in biomedical research and its applications. The National Physician Payment Transparency Program is part of this evolving landscape.
One Step Closer: OMB Asks for Comments on Proposed Rule on Federal Grant Policy Reforms
You may recall that back in February 2012 I blogged about OMB’s request for comment on proposed reforms to federal grant policies contained in Office of Management and Budget (OMB) circulars such as A-21, A-133, and A-122. OMB proposed these reforms to streamline Federal policies relating to grants. The essential idea behind this reform is to reduce the “red tape” and unnecessary or overly burdensome requirements so that grantees can better focus their efforts achieving their research objectives.
The prior Advanced Notice of Proposed Guidance (ANPG) received more than 350 public comments, all of which OMB has considered carefully in drafting the proposed rule issued on February 1, 2013 in the Federal Register (note that there are two primary documents: the preamble and the full text of the proposed rule). I am pleased to note that the public’s feedback has influenced the draft guidance greatly and OMB has opened the latest version of the proposed rule for public comment once again.
Providing comments and feedback on the proposed rule is an important opportunity for the grants community because their comments can be used to further refine the reforms before the final guidance is issued. I described the scope of the proposed reform in my previous blog post, and I suggest that you read the Federal Register notice in full, but I want to highlight some key areas on which OMB seeks specific feedback, as these topics are highly relevant to the NIH research administration community:
- Proposed audit language for time and effort reporting requirements. OMB is asking whether the language proposed adequately provides enough flexibility for institutions to meet these standards in the way most appropriate to their particular organizations.
- Revisions to reimbursement for utility costs to institutions of higher education.
- One time extension of indirect (“F&A”) costs for all types of institutions, i.e. the option of extending negotiated rates for up to 4 years subject to approval of the indirect cost cognizant agency.
There are many more issues raised in the proposed rule; again, I encourage you to read the notice in its entirety. Please note the 26% limitation on reimbursement of administrative costs remains for institutions of higher education and would not apply to other types of institutions such as non-profit organizations.
Also, please note that OMB offers a number of resources and supporting documents comparing current requirements and proposed changes to help you navigate the proposed changes. Some of you may also be interested in viewing the upcoming webcast on Feb. 8 at 11 am EST, hosted by OMB and the Council on Financial Assistance Reform.
Both Federal agencies and the public are invited to submit comments at www.regulations.gov; the last day comments will be accepted is May 2, 2013. My office has been providing comments throughout the development of this guidance, and will do so again now. I encourage you to share your thoughts with OMB too.
Does Your Academic Training Destine Your Choice of Research Subject?
As I’ve been discussing the biomedical research workforce with people in the extramural community over the past few months, I’ve heard people say things like “PhDs don’t do much research with human subjects”. I decided to look into data that could support or refute statements such as this, and started with checking data on the use of animal and human subjects in research.
As you can see in the graphs below, we looked at awards by degree over the past 10 years and grouped them into four categories based on the research described in the grant applications: 1) human subjects research only (defined as having been reviewed by an Institutional Review Board), 2) animal subjects research only (defined as having been reviewed and approved by an Institutional Animal Care and Use Committee), 3) no human or animal subjects, or 4) both human and animal subjects.
To put these data in context, you’ll remember that about 70% of NIH-funded PIs have PhDs, with most of the remaining PIs having either an MD or an MD/PhD. Since the number of PIs with other degrees (DDS or DVM only, for instance) is very small, we’ve provided those data separately – see the footnote below.
First, we took a look at human and animal subject use by each degree category: PhDs, MD/PhDs, and MD. As shown in figure 1, among PIs with a PhD degree, about 45% of PhDs engage in research involving only animal subjects, about 22% engage in human subjects research, a little less than 30% do research with no human or animal subjects, and only 5% conduct research with both. These proportions haven’t changed much over time. 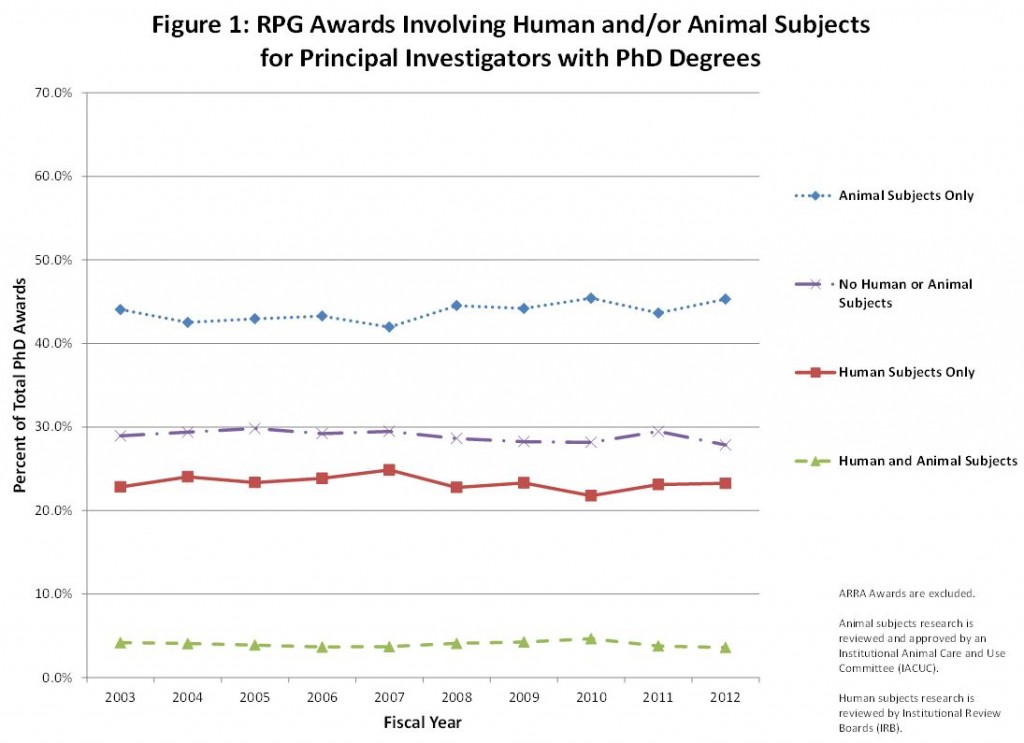
The breakdown for PIs with MD/PhD degrees is slightly different (figure 2). A higher proportion – over 50% – engage in research with animal subjects only, nearly 22% conduct research involving human subjects only, while around 10% do research with no human or animal subjects and another 10% with both.
Looking at PIs with MD degrees, we see MDs are likely to engage in research solely involving human subjects (approximately 45%), and also likely to engage in research involving only animal subjects (nearly 35%). However, they are the least likely to conduct research that involves no human or animal subjects – only about 8% of MDs conduct research that does not involve human or animal subjects (figure 3).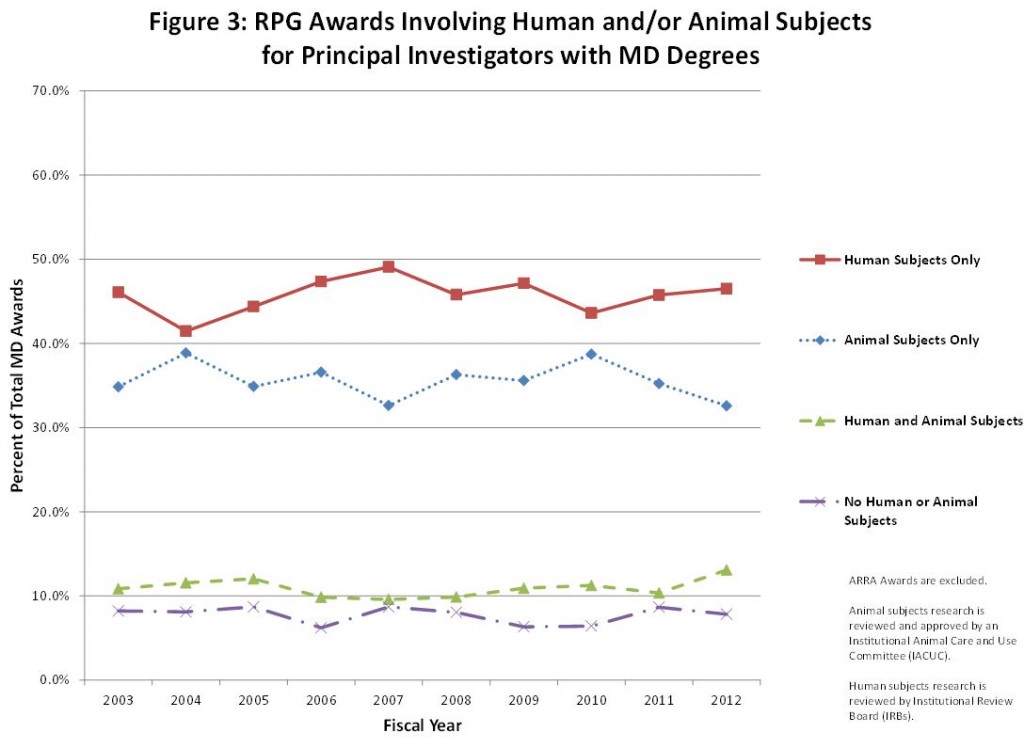
In this last graph we’ve pulled out the human subjects only percentages for each of these 3 degree categories and compared them all on one graph. As shown in figure 4, investigators with MD degrees do tend to work on research involving only human subjects. This is not too surprising, though it is interesting that such a significant and similar percentage of PIs with a PhD and MD/PhD do research exclusively with human subjects.
Taken together, these data support the idea that many PIs are conducting research that fits with their academic training, but a substantial number of PhD-trained scientists are doing research with human subjects and many clinician-scientists (either MDs or MD/PhDs) conduct research with neither human nor animal subjects. Also, changes in the extramural community over the past decade do not seem to have affected these proportions significantly. These data may prove useful to the new advisory committee to the director’s working group on clinician-scientists.
—-
Note: The data table corresponding to these figures is posted on RePORT and can be downloaded in an Excel file. The table includes data for other advanced degrees (e.g. DVM, DDS, and DMD), which were not plotted on the charts because of their small numbers.

