
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
Below is a protocol for calibration of creatinine concentration reported for whole blood to creatinine concentration in simultaneously collected venous serum or plasma.
The NKDEP Laboratory Working Group has recommended that creatinine concentrations measured in whole blood should be adjusted and reported to providers as equivalent creatinine concentrations measured in simultaneously collected venous serum or plasma, where the serum or plasma measurements are traceable1 to a high-level isotope dilution mass spectrometry (IDMS) Reference Method2. The purpose of this protocol is to provide manufacturers of whole blood creatinine measurement devices practical guidance regarding the calibration of their devices to an IDMS-traceable serum or plasma method. The protocol may also be used by laboratories reporting creatinine measured in whole blood to verify agreement of the measurement to an IDMS traceable serum or plasma method.
Sensors for measurement of creatinine in whole blood used in clinical practice are enzyme-based biosensors with electrochemical detection. Like the more commonly used glucose sensors based on the same technology, creatinine sensors respond to the molality of creatinine in the sample (amount of creatinine per unit mass of water in the sample). It has been known for some time that molality of creatinine in erythrocyte fluid is equal to molality in plasma3, 4 and that creatinine is transported by passive diffusion through the lipid bilayer of the erythrocyte membrane.5 When these two sample phases are in equilibrium, the measured quantity in whole blood and its separated plasma by the direct biosensor method should be identical. The presence of erythrocytes may affect the whole blood measurement in other ways, for example, by hindering diffusion of analyte from bulk sample to the surface of the sensor, or by affecting the electrical conductivity between different elements of the electrochemical cell. Therefore, in practice, results obtained with whole blood and simultaneously collected serum or plasma often differ significantly, are usually sensitive to hematocrit, and correction for the presence of erythrocytes may be needed to force agreement between the whole blood and serum or plasma measurements.
This protocol assumes that interfering substances for the whole blood device have been characterized separately, and that samples used in Part 1 of the protocol are free of interfering substances. This protocol also assumes that the whole blood device has been shown to be linear toward creatinine concentrations over the manufacturer's claimed measurement range.
1. Calibration of whole blood creatinine method to a heparinized plasma IDMS-traceable method at variable hematocrit levels
A heparinized venous whole blood sample from a healthy volunteer should be collected in heparin anticoagulated tubes and determined to be within the adult reference ranges for the following analytes.
| US Units | SI Units | |
|---|---|---|
| Creatinine | 0.8 - 1.5 mg/dL | 70 - 132 µmol/L |
| Hematocrit | 35 - 47% (w) 40 - 52% (m) | 0.35 - 0.47 (w) 0.40 - 0.52 (m) |
| Total protein | 63 - 79 g/L | 63 - 79 g/L |
| Cholesterol | 150 - 250 mg/dL | 5.7 - 9.4 mmol/L |
| Triglycerides | 50 - 150 mg/dL | 0.56 - 2.83 mmol/L |
After adjustment of hematocrit and sample spiking to elevated creatinine concentrations, blood samples will be available according to the matrix below.
| Percent of Hematocrit | ||||
|---|---|---|---|---|
| Creatinine Conc. mg/dL |
20-30 | Not adjusted | 55-65 | |
| Not adjusted (Normal) |
||||
| Normal-mid | ||||
| Mid | ||||
| Mid-High | ||||
| High | ||||
2. Verification of agreement with an IDMS-traceable plasma method using patient samples
Patient specimens should be used to verify the above standardization procedure. It is recommended that at least 5 different whole blood creatinine measurement devices be used as part of this verification protocol in order to show device-to-device consistency.
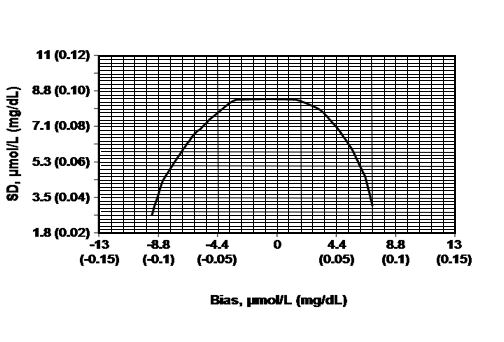
Figure 1: Total error budget for creatinine measurement in the range 88.4-133 µmol/L (1.00-1.50 mg/dL). The line represents the limit of systematic biases and random imprecisions that produce a relative increase of less than 10 percent in the root mean squared error when estimating GFR using the MDRD Study equation. Adapted from: Myers et al. Recommendations for Improving Serum Creatinine Measurement: A Report from the Laboratory Working Group of the National Kidney Disease Education Program. Clinical Chemistry. 2006;52:5-18.
Table 1: Number of replicates required based on the targeted agreement between the whole blood and IDMS-traceable methods and the known imprecision of the whole blood method (derived from Figure 1).
| Known within run standard deviation of the whole blood method (mg/dL) | Targeted bias between whole blood and IDMS-traceable plasma method (mg/dL) | Number of replicates required to detect targeted bias |
|---|---|---|
| 0.09 | -0.045 > x < 0.035 | 52 |
| 0.08 | -0.06 > x < 0.05 | 21 |
| 0.07 | -0.075 > x < 0.06 | 11 |
| 0.06 | -0.085 > x < 0.07 | 6 |
| 0.05 | -0.095 > x < 0.075 | 4 |
| 0.04 | -0.10 > x < 0.08 | 2 |
1. ISO 17511. In vitro diagnostic medical devices – measurement of quantities in biological samples – metrological traceability of values assigned to calibrators and control materials. International Organization for Standardization, 2003.
3. Miller BF, Dubos R. The Journal of Biological Chemistry. 1937;121:457-64.
4. Nolph K, Felts J, Moore R, Van Stone J. International Urology and Nephrology. 1978;10:59-64.
5. Langsdorf LJ, Zydney AL. Biotechnology and Bioengineering. 1994;43:115-21.
6. D'Orazio P, Conant J, Cervera J. Measurement of creatinine in whole blood samples supplemented to achieve elevated creatinine concentrations. Clinical Chemistry, 2008;54:451-2.
7. If the required degree of replication is excessive (e.g., 21 or greater), the suggested number of replicates from the Appendix may be run with the unadjusted, normal creatinine samples only, with a lesser degree of replication at the elevated creatinine concentrations.
8. Because the IDMS-traceable method is normalized using NIST SRM 967, it is assumed that the imprecision of the method will be equal to or better than the whole blood method.
9. Most devices that measure whole blood creatinine include a simultaneously measured hematocrit to allow for this correction. If total hemoglobin is reported instead, hematocrit may be estimated (Hct = tHb x 3).
Page last updated: March 1, 2012