Inhibitors of Cdc2-Like Kinase 4 to Elucidate the Mechanism and Controlling Gene Splicing
The Cdc2-like kinases (Clks) have specified roles in gene splicing. Specifically, the Clk class of enzymes has been shown to phosphorylate the SR proteins, which are a major component of the spliceosome. It has been hypothesized that inhibition of these targets may offer a mechanism to control splicing. This probe represents our continued examination of substituted 6-arylquinazolin-4-amines as Clk inhibitors. Several of the most potent inhibitors, including ML167 (CID_44968231), were validated as being highly selective within a comprehensive kinome scan. Appropriate aqueous solubility and stability were found for this agent.
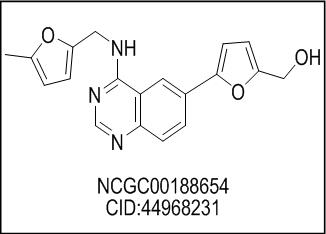
ML167
Key Investigators
National Center for Advancing Translational Sciences
Andrew S. Rosenthal, Ph.D.
Cordelle Tanega
Min Shen, Ph.D.
Bryan T. Mott
James M. Bougie
Dac-Trung Nguyen
Douglas S. Auld, Ph.D.
David Maloney, Ph.D.
Craig J. Thomas, Ph.D.
National Cancer Institute
Tom Misteli, Ph.D.
Public Health Impact
Clk4 plays a role in regulating gene splicing. This probe compound will be a valuable biological tool for the scientific community to use in exploring the specific functions of Clk4 in terms of gene splicing, which can influence many cellular pathways and events, and subsequently many aspects of health and disease.
Publications
Rosenthal AS, Tanega C, Shen M, et al. Potent and selective small molecule inhibitors of specific isoforms of Cdc2-like kinases (Clk) and dual specificity tyrosine-phosphorylation-regulated kinases (Dyrk). Bioorg Med Chem Lett, 2011;21(10):3152-3158.
Mott BT, Tanega C, Shen M, et al. Evaluation of substituted 6-arylquinazolin-4-amines as potent and selective inhibitors of cdc2-like kinases (Clk). Bioorg Med Chem Lett, 2009;19(23):6700-6705.
Social Media Links